J. Laamech, J. El-hilaly*, H. Fetoui1, Y. Chtourou1, A. Tahraoui and B. Lyoussi
Laboratory of Physiology, Pharmacology and Environmental Health.Faculty of Sciences DM, USMBA University, BP 1976, Atlas Fez, 30000, Morocco
1Laboratory of Toxicology Microbiology and Environmental Health (11ES70), Life Science Department, Sciences Faculty of Sfax, University of Sfax, BP 1171, 3000 Sfax, Tunisia.
- *Corresponding Author:
- J. El-hilaly
Laboratory of Physiology, Pharmacology and Environmental Health.Faculty of Sciences DM
USMBA University, BP 1976, Atlas Fez, 30000, Morocco
E-mail: elhilaly.jaouad@gmail.com
Received date: 28 Sep 2015; Revised date: 29 May 2016; Accepted date: 1 Jun 2016
Abstract
This study aimed to investigate the potential effects of Berberis vulgaris L. against lead acetate-induced nephrotoxicity in Swiss albino mice. Mice were exposed chronically to lead acetate, at a dose of 5 mg/kg, alone or in conjunction with B. vulgaris extract (25, 50, 100 and 150 mg/kg). Lead acetate administration increased lead burden in blood and kidneys, altered renal biomarkers (serum creatinine, urea and uric acid), increased malondialdehyde and protein carbonyls levels, and decreased reduced glutathione, glutathione peroxidase, catalase, and superoxide dismutase. Histological studies showed glomerular degeneration and hypercellularity. However, B. vulgaris extract co-treatment significantly and dose-dependently restored the renal parameters, antioxidant enzymes, and histopathological changes near to the normal status, mainly via its antioxidant properties and/or by preventing lead bioaccumulation. Therefore, B. vulgaris extract showed nephroprotective effects against lead-induced nephrotoxicity mainly through its antioxidant and metal chelating properties.
Keywords
Lead acetate, Berberis vulgaris, mice, nephrotoxicity, antioxidant enzymes, lipids peroxidation
Many heavy metals exist in our environment both naturally and from pollution. Some of them are very toxic and ranked as human carcinogens. Accordingly, lead (Pb) is a systemic toxic metal known for multiple industrial, domestic, agricultural, medical and technological applications that contribute to its wide distribution in the environment. Exposure to lead occurs mainly through intake of lead-contaminated food, water, dusts and paints[1-2]. This triggers generation of reactive oxygen species (ROS), and depletes the cellular antioxidant capacity. An imbalance of antioxidant pool affects cellular organelles, antioxidant enzymes, and damages membranes, DNA, proteins, and finally destroys the tissues[3-4]. Therefore, exogenous administration of antioxidant substances would have a beneficial effect on the cells’ antioxidant system to combat lead intoxication. In accordance, there are growing interests in using natural compounds to treat lead nephrotoxicity[5-6].
Berberis vulgaris L. (Berberidaceae) is a medicinal plant widely distributed in the Atlas and Rif mountains of Morocco. The species is commonly known as ??rys in Moroccan pharmacopoeia, and used to cure renal disorders and other diseases[7]. Moreover, it is the most widely used drug in homeopathic system of medicine for kidney pain as well as removal of kidney stones[8]. Many studies report protective effects of B. vulgaris and some of its bioactive molecules, especially berberine, which is the most important alkaloid claimed to be responsible for beneficial effects of B. vulgaris. Moreover, many studies report antioxidant effects of B. vulgaris, and several polyphenols with antioxidant activities have been isolated from root, bark, leaf and fruit[8-9]. To our knowledge, this is the first study to evaluate B. vulgaris effects against leadinduced nephrotoxicity in mice. Therefore, this study aims to investigate the potential protective effects of B. vulgaris against kidney damage induced by subchronic administration of lead acetate.
Materials And Methods
Lead acetate (PbAc), formalin (10%), ethanol (90%), nitric acid (HNO3, 65%), Triton X-100 (0.2%), sulfosalicylic acid (4%), Ellman’s reagent (dithionitrobenzene, DTNB), 0.1 M Tris–HCl, 0.001 M EDTA buffer (pH 7.4), 5% TCA, thiobarbituric acid reagent (TBA, 0.67%), 1,1,3,3-tetraethoxypropane, 2,4 dinitrophenyl hydrazine (DNPH), ascorbic acid (1%), palladium chloride (PdCl2), toluene, paraffin, hematoxylin and eosin solutions. All reagents and chemical products used in this study were of analytical grade and were purchased from Sigma Chemical Co. (St. Louis, France).
Plant material and preparation of the lyophilized aqueous extract:
Samples of B. vulgaris were collected in April-May, 2013 from mountain areas of Imouzzer Marmoucha (1713 m, 33° 28' 37 N, 4° 17' 44 W), a province of Fez-Boulemane region. Mature whole plant was authenticated at the herbarium of the Scientific National Institute (Rabat), where a voucher specimen (C3) is kept. The BV stem bark was separated and frozen until extraction. The plant collection was conducted in accordance with the ethical standards outlined in the international, national and institutional rules concerning the biodiversity rights.
50 g of dried powder of B. vulgaris stem bark was decocted with 500 ml of water. The mixture was heated and boiled under reflux for 30 min. The decoction obtained was centrifuged, filtered, frozen at −20°, and then lyophilized (FreeZone® Dry 4.5, USA). This yielded a total of 7.5 g of extract (15% w/w).
60 adult Swiss albino mice (25-30 g) were used in this study. The mice were kept in standard polypropylene cages. Animals were maintained under standard laboratory conditions of temperature (25±2°), relative humidity (50±15%), 12 h light-dark cycle, standard diet and water ad libitum. The care and handling of the animals were in accordance with the internationally accepted standard guidelines for use of animals, and the protocol was approved by our institutional committee on animal care following the French Technical Specifications for the Production, Care and Use of the Laboratory Animals.
Experimental procedure:
After 2 w of acclimatization to the laboratory conditions, the animals were randomly divided into six groups of 10 mice each and treated orally by force-feeding as follows[10]: Group 1: received double distilled water as vehicle during the whole course of study; served as normal control (CT); Group 2, received lead acetate (5 mg/kg/day) dissolved in double distilled water for 40 days; served as toxic control (PbAc); Groups 3-6: received B. vulgaris aqueous extract at doses of 25, 50, 100 and 150 mg/kg, respectively, once daily for 30 days from 11 days after beginning of lead acetate exposure (5 mg/kg/day) to the end of the experiment.
The B. vulgaris-aqueous extract doses were chosen according to previous studies on the plant or on its main constituent (berberine), and on the basis of acute toxicity study of the plant[11-12]. After 42 days, the animals were given rest overnight, and then on the next day they were sacrificed by cervical dislocation under light ether anesthesia. Before sacrificing the animals, blood samples were collected from retroorbital venous plexus in Eppendrof tubes rinsed with EDTA anticoagulant for lead bioaccumulation assay (5 mice from every experimental group), and other tubes without anticoagulant were used to determine biochemical parameters. The blood was centrifuged at 2200 g for 15 min at 4°, serum samples were drawn and kept at −20° until biochemical assays (5 mice from every experimental group). The kidneys were excised, cleaned and washed with ice cold normal saline. The portions of kidneys were homogenized in 0.1 M Tris HCl–0.001 M EDTA buffer (pH 7.4) and centrifuged at 12 000 g for 30 min at 4°. The supernatant was collected and used for determining biochemical parameters (n=5). Other kidneys were kept in 10% formalin (n=5) until histological assay.
Estimation of blood and kidneys lead burden:
The accumulation of lead in blood (Pb-B) and kidneys (Pb-K) has been measured by flame atomic absorption spectroscopy[13].
Serum creatinine, urea and uric acid levels:
Serum creatinine, urea and uric acid levels were estimated by spectrophotometry using Commercial kits Biomaghreb Diagnostics Casablanca, Morocco (refs. 20043, 20147, 20154; respectively).
Reduced glutathione (GSH):
Kidney GSH content was determined by Ellman’s method[14], modified by Jollow et al.[15], based on the development of a yellow color when DTNB is added to compounds containing sulfhydryl groups. Briefly, 3 ml of sulfosalicylic acid (4%) were added to 500 ml of homogenate tissues for deproteinization. The mixture was centrifuged at 2500 g for 15 min. Then Ellman’s reagent was added to 500 ml of supernatant. The absorbance was measured at 412 nm after 10 min. Total GSH content was expressed as μmol/g of tissue.
Glutathione peroxidase (GPx) activity:
GPx was measured according to Flohe and Gunzler[16]. The enzyme activity was expressed as nanomoles of GSH oxidized/min/mg protein.
Catalase activity:
Catalase (CAT) activity was assayed by the decomposition of hydrogen peroxide according to the method of Aebi[17]. Decrease in absorbance due to H2O2 degradations was monitored at 240 nm for 1 min and the enzyme activity was expressed as μmol H2O2 consumed/min/mg protein.
Superoxide dismutase (SOD) activity:
The total SOD activity was evaluated by measuring the inhibition of pyrogallol activity[18]. One unit (U) corresponds to the enzyme activity required to inhibit half of the oxidation of pyrogallol. Then SOD activity was expressed as U/mg of protein.
Assay of lipid peroxidation:
Lipid peroxidation in the renal tissue was estimated calorimetrically by measuring thiobarbituric acid reactive substances (TBARS) which were expressed in terms of malondialdehyde content[19]. Briefly, Aliquots of kidney homogenates were mixed with 1ml of 5% TCA and centrifuged at 4000 g for 10 min. one milliliter of thiobarbituric acid reagent (TBA, 0.67%) was added to 500 ml of supernatant and heated at 95° for 15 min. The mixture was then cooled and was measured for absorbance at 532 nm. The MDA values were calculated using 1,1,3,3-tetraethoxypropane as the standard and expressed as nanomoles of MDA/g of tissue.
Protein carbonyl assays:
Protein oxidation was determined based on the reaction of the carbonyl groups with 2,4-dinitrophenylhydrazine (DNPH) to form 2,4-dinitrophenylhydrazone, as described by Reznick and Packer[20]. Samples were read at 370 nm and carbonyl content was calculated using the molar absorption coefficient for aliphatic hydrazones (22 000 M-1cm-1) and expressed as nmol carbonyl/mg protein.
Histopathological study of kidney:
For qualitative analysis of kidney histology, the tissue samples were fixed for 48 h in 10% formalin solution, dehydrated in ascending graded series of ethanol, cleared in toluene and embedded in paraffin. Sections of the tissue (5-6 mm thickness) were prepared by using a rotary microtome and stained with hematoxylin and eosin (H and E) for microscopic observations.
Statistical analysis:
The data were analyzed using the statistical package program GraphPad Prism 5.03, USA. Data were statistically calculated by utilizing one-way ANOVA and expressed as mean±standard error of the mean (SEM) followed by Tukey’s test. Moreover, Student’s unpaired t-test was used when comparison between two groups was required. The values were considered significant when P<0.05.
Results And Discussion
Before treatment, the initial body weight of mice was similar in all animals groups. A non-significant decrease in the level of the final body weights (BW) was observed in PbAc-treated rats when compared with control rats (CT). Administration of B. vulgaris at doses of 100-150 mg/ kg body weight along with lead significantly (P<0.05) raised the values of final body weight when compared to normal control group. Likewise, B. vulgaris treatments increased significantly and dose-dependently (P<0.05) body weight in comparison with toxic control group.
Kidneys weight values were not significantly lowered in toxic control group as compared to normal control group. However, B. vulgaris-treated groups depicted significant (P<0.05) and dose-independent weight intake in comparison with toxic control group.
Mice exposure to PbAc significantly (P<0.05) raised the bioaccumulation of lead in blood and kidneys when compared with control group. Compared to Pbacetate treated group, B. vulgaris extract decreased significantly (P<0.05) at any dose tested the blood and kidney lead burden (Table 1).
| Parameters | CT | PbAc | PbAc+25 | PbAc+50 | PbAc+100 | PbAc+150 |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 28.33±0.67 | 28.26±0.52 | 27.79±0.29 | 27.73±0.66 | 28.37±0.4 | 27.67±0.42 |
| Final body weight (g) | 30.5±0.81 | 28.55±0.56 | 31.18±0.36* | 31.39±0.27* | 32.77±0.44*,# | 32.8±0.25*,# |
| Kidneys weight (g) | 0.45±0.003 | 0.43±0.01 | 0.46±0.003* | 0.46±0.004* | 0.46±0.004* | 0.46±0.01* |
| Pb-B (µg/ml) | 0.009±0.01 | 0.18±0.01# | 0.16±0.01# | 0.09±0.01*,# | 0.04±0.02* | 0.02±0.01* |
| Pb-K (µg/g wet tissue) | 0.009±0.00 | 0.17±0.01# | 0.08±0.003*,# | 0.03±0.004* | 0.03±0.003* | 0.02±0.003* |
Body and kidneys weights, blood and kidney lead burden of mice control group (CT), lead acetate-treated mice (PbAc), and Berberis vulgaris-treated mice (0, 25, 50, 100, 150 mg/ kg BW) in combination with PbAc (PbAc+BV). Values are expressed as mean±SEM for five animals in each group. #Values differs significantly (P<0.05) from normal control group (CT). *Values differ significantly (P<0.05) from toxic control group (PbAc).
TABLE 1: BODY AND KIDNEYS WEIGHTS, BLOOD AND KIDNEY LEAD BURDEN OF MICE
Daily treatment with lead resulted in significant (P<0.05) increase in serum levels of creatinine and urea in PbAc-treated group when compared to normal control (+64% and +75%, respectively). Coadministration of B. vulgaris-aqueous extract at doses of 100-150 mg/kg BW decreased significantly (P<0.05) the levels of these renal markers when compared to the toxic control group (PbAc); the highest dose (150 mg/ kg BW) restored these parameters levels to normal values. Toxic control (PbAc) exhibited a significant increase (P<0.05) in serum uric acid in comparison with normal control (CT). B. vulgaris extracts used in combination with PbAc significantly and dosedependently (P<0.05) decreased the serum uric acid levels nearly to their normal values (Table 2).
| Parameters | CT | PbAc | PbAc+25 | PbAc+50 | PbAc+100 | PbAc+150 |
|---|---|---|---|---|---|---|
| Creatinine (mg/dl) | 1.16±0.03 | 1.9±0.03# | 1.85±0.02# | 1.84±0.04# | 1.66±0.03# | 1.17±0.02* |
| Urea (mg/dl) | 43.26±1.98 | 75.9±1.54# | 73.46±1.08# | 71.5±2.53# | 54.14±0.72*,# | 45.96±1.34* |
| Uric acid (mg/dl) | 2.34±0.11 | 1.26±0.03# | 1.27±0.05# | 1.84±0.04*,# | 1.93±0.05*,# | 2.21±0.05* |
Plasma levels of creatinine, urea and uric acid of mice control group (CT), lead acetate-treated mice (PbAc), and Berberis vulgaris-treated mice (0, 25, 50, 100, 150 mg/ kg BW) in combination with PbAc (PbAc+BV). Values are mean±SEM, for five animals in each group. #Values differs significantly (P<0.05) from normal control group (CT). *Values differ significantly (P<0.05) from toxic control group (PbAc).
TABLE 2: PLASMA LEVELS OF CREATININE, UREA AND URIC ACID OF MICE
Daily dosing with lead induced a significant decrease of kidney GSH content (-60%; P<0.05) in PbAc group compared to normal control. A significant and dose-dependent recovery (P<0.05) in kidney GSH content was noticed in both 100 and 150 mg/kg-treated groups in comparison with toxic control group (Table 3). Glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD) activities were significantly decreased (about -60%) in kidney tissue of lead-treated mice as compared to their respective control group (P<0.05). Co-treatment of lead-treated mice with B. vulgaris extract, at doses higher or equal to 100 mg/kg BW, improved significantly the activities of GPx and CAT in kidneys of B. vulgaris?treated mice to a level similar to that of the normal control group. Whereas, the renal SOD activity was restored significantly to normal status by co-administration of BV at a dose of 150 mg/kg BW as compared to the toxic control group (PbAc) (Table 3).
| Parameters | CT | PbAc | PbAc+25 | PbAc+50 | PbAc+100 | PbAc+150 |
|---|---|---|---|---|---|---|
| GSH(µmol/g wet tissue) | 4.31±0.23 | 2.6±0.11# | 2.68±0.33# | 2.9±0.24# | 3.54±0.2* | 4.04±0.34* |
| GPx(nmol GSH/min/mg protein) | 63.9±0.28 | 40.12±1.89# | 41.69±2.36# | 39.24±1.66# | 55.13±3.87* | 61.14±5.19* |
| CAT(µmole H2O2/min/mg protein) | 37.45±0.61 | 23.43±1.6# | 23.46±1.48# | 26.45±0.82# | 33.31±0.81* | 34.79±1.92* |
| SOD(U/mg protein) | 15.37±0.4 | 10.37±1.04# | 10.19±0.35# | 11.88±0.53# | 12.73±0.56 | 14.48±1.04* |
| MDA (nanomoles of MDA/g of tissue) | 36.42±1.64 | 59.83±3.36# | 62.37±2.94# | 60.67±2.27# | 41.63±4.00* | 36.33±2.97* |
| PCO(nmolcarbonyl/mg protein) | 0.69±0.06 | 1.43±0.16# | 1.375±0.09# | 1.33±0.09# | 1.17±0.10# | 0.98±0.06* |
GSH, GPx, CAT, SOD, MDA and PCO contents in Kidneys of mice control group (CT), lead acetate-treated mice (PbAc), and Berberis vulgaris-treated mice (0, 25, 50, 100, 150 mg/ kg BW) in combination with PbAc (PbAc+BV). Values are expressed as mean±SEM for five animals in each group. #Values differs significantly (p<0.05) from normal control group (CT). *Values differ significantly (P<0.05) from toxic control group (PbAc).
TABLE 3: GSH, GPX, CAT, SOD, MDA AND PCO CONTENTS IN KIDNEYS OF MICE
PbAc exposure caused a significant increase (P<0.05) in the level of lipid peroxidation (+64%) and protein carbonyls (+96%) in the kidney tissue. Coadministration of B. vulgaris extract at doses of 100 and 150 mg/kg BW significantly (P<0.05) lowered the levels of MDA and PCO in comparison with their respective control values, respectively (Table 3).
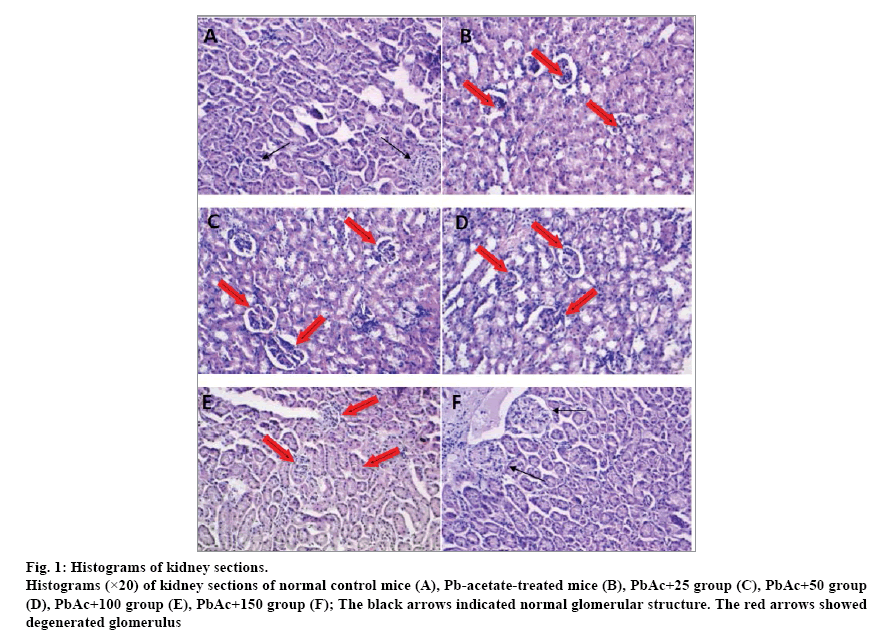
The histological examination of the kidney tissues of control mice showed normal cyto-architecture of glomerulus and tubules structure (fig. 1a). Mice treated with PbAc alone showed signs of glomerular degeneration as glomerular hypercellularity (fig. 1b), while those treated with lead-acetate in combination with B. vulgaris extract (150 mg/kg BW) restored more or less normal glomerular structures of renal tissues (fig. 1c-f).
Fig. 1: Histograms of kidney sections.
Histograms (×20) of kidney sections of normal control mice (A), Pb-acetate-treated mice (B), PbAc+25 group (C), PbAc+50 group (D), PbAc+100 group (E), PbAc+150 group (F); The black arrows indicated normal glomerular structure. The red arrows showed degenerated glomerulus
Chronic exposure to lead is known to induce abroad spectrum of toxicological effects and biochemical dysfunctions including those of the kidney[21-22]. Although the precise mechanism of lead toxicity is not yet entirely clear, it is evidenced that lead exposure generates reactive oxygen metabolites and causes oxidative stress in living systems[3-4]. Moreover, natural antioxidants can combat the over-production of free radicals and activated oxygen species or inhibit their reaction with biological structures. The destruction of most free radicals and activated oxygen species rely on the oxidation of endogenous antioxidant mainly scavenging and reducing molecules.
In the present study, PbAc orally administrated to mice did not affect markedly body and kidneys weights. This may probably due to the absence of effect on food intake by mice as well as tissues growth promoting. However, B. vulgaris co-treatment increased significantly body weight. These finding are congruent with those of Rajain et al[23], who indicates a significant positive effect of B. vulgaris on weight gain in chicken.
Moreover, lead exposure increased significantly blood and kidneys lead burden. This finding corroborates other studies of lead-induced toxicity in animals[24-25]. Co-administration of BV extract significantly reduced blood and renal intracellular lead levels in a dosedependent manner. Previous studies reported that B. vulgaris contains polyphenolics[8,26], which are known for their metal-chelating ability[27-28]. This might be one of the mechanisms by which B. vulgaris decreases lead content in blood and kidney, and therefore potentiates its body clearance.
On the other hand, urea and creatinine are considered as biomarkers of the first and advancing stages of kidney damage, respectively[29]. In this study, PbActreated mice showed increase in plasma creatinine and urea levels, which may reflect the renal failure onset[30]. Kidney plays a major role in the clearance and biotransformation of metals[31], and therefore is considered as a primary target organ for metals induced toxicity[32]. PbAC could increase blood urea by more than one mechanism, including enhancement of proteins catabolism, conversion of ammonia to urea by induction of arginase-enzyme synthesis[33], and inhibition of amino acids incorporation in proteins[34]. The hyperuricemia induced by PbAc treatment might result from over-production and/or reduced renal excretion of uric acid[35], and elevation of endogenous oxygen species levels[36]. Co-treatment with BV extract inhibited PbAc- induced nephrotoxicity, as indicated by significant restoration of serum creatinine, urea and uric acid.
Administration of PbAc decreased significantly reduced glutathione (GSH).This is consonant with some reports asserting that lead induces GSH level depletion in Pb-acetate treated rats[37]. Oxidative stress occurs when generation of free radicals exceed the capacity of antioxidant defense mechanisms. Lead toxicity provokes free radical-induced cellular damage through two main mechanisms: the production of ROS such as O2-, H2O2 and peroxy radical, and the direct decline of antioxidant reserves[37]. GSH acts in conjunction with antioxidant enzymes in the decomposition of H2O2 and other organic hydroperoxides. In this context, depletion of intracellular GSH is a prognostic indicator of increased cytotoxicity of H2O2 in kidney cells[38-39]. Therefore, BV aqueous extract could mitigate oxidative stress by increasing GSH level through scavenging H2O2 as it was demonstrated by Gurer and Ercal[4]. BV polyphenols might be the potential agents in the chelating processes[40-41].
This study also elucidated that PbAc-treated mice exhibited decreased kidneys antioxidant enzymes activities such as Glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD). These enzymes are metalloproteins that detoxify peroxides (–OOH), H2O2, and O2.-, respectively[42]. These antioxidant enzymes depend on essential trace elements and prosthetic groups for their structure and activity, and therefore are the potential targets of lead toxicity[43].
Therefore, the decreased activities of these antioxidant enzymes observed in lead-treated mice indicated a failure of antioxidant defense system to overcome the influx of ROS induced by PbAc exposure. Furthermore, many reports depicted the inhibition of the antioxidant enzymes activities as the main mechanism of leadinduced cytotoxicity[44-45]. B. vulgaris administration increased antioxidant enzyme activities in PbActreated mice, which could be induced by lowering free radicals generation. In this concern, B. vulgaris extract could react with free radicals or with lipid peroxidation metabolites, and may also enhance tissue thiol contents, which lead finally to the reduction of oxidative modification of enzymes, and enhancement of antioxidants and glutathione- related enzymes activities. In this context, the beneficial antioxidative effect of B. vulgaris has been previously reported in some animal models[46-47].
In the current study, PbAc induced the increase of kidneys malondialdehyde (MDA) and protein carbonyls (PCO) levels in the toxic control group. Lead is known to have toxic effects on membrane structure and functions[48]. Hence, altered lipid and proteins composition of membranes due to lead exposure is associated with an increase in the concentration of MDA, and PCO that may result in altered membrane integrity, permeability, and function. These would increase the susceptibility to lipid peroxidation and generation of free radicals[48-49]. The increased lipid peroxidation and oxidative modification of enzymes contents, which are assessed by increase in PCO levels after Pb-acetate exposure, indicate the implication of oxidative stress in lead-induced nephrotoxicity. Co-administration of B. vulgaris restored MDA and PCO levels respectively to normal values. Therefore, it seems likely that the B. vulgaris-extract effect is mediated by scavenging free radicals and decreasing hydroxyl radical generation. These findings are congruent with previous reports that concern the antioxidant effects of the whole plant or its main component, berberine[11-41].
Mice chronic exposure to lead was associated with noticeable modifications of the biochemical parameters and significant alteration of the kidney architecture, which was confirmed by histopathological assessment. In fact, the renal histoarchitecture of the leadtreated mice presented signs of nephrotoxicity, such as glomerulus cells degeneration characterized by glomerular hypercellularity. However, the higher dose (150 mg/kg BW) of the B. vulgaris-extract improved significantly the histopathological alterations and restored the glomerular structure near to normal status. Similar histopathological changes were found by evaluation of the kidneys of lead-intoxicated rats revealed severe histopathological changes, and treatment with Corchorus olitorius restored the tissue architecture almost similar to their normal ultrastructure.
In conclusion, the current work demonstrates that B. vulgaris has a nephroprotective effect against leadinduced toxicity in the kidney of mice. The mechanisms of effectiveness involve the quenching of free radicals, antioxidant and metal chelating ability of the plant extract. Therefore, B. vulgaris extract appears to be a promising agent for protection against lead-induced nephrotoxicity. Dietary supplementation with B. vulgaris could be a useful method to protect populations at high risk of environmental and/or occupational lead chronic intoxication, as this medicinal plant is widely used as food in many countries.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- Lanphear BP, Matte TD, Rogers J, Clickner RP, Dietz B, Bornschein RL, et al. The contribution of lead-contaminated house dust and residential soil to children's blood lead levels: a pooled analysis of 12 epidemiologic studies. Environ Res 1998;79:51-68.
- Schwartz J, Levin R. The risk of lead toxicity in homes with lead paint hazard. Environ Res 1991;54:1-7.
- Liu C-M, Ma J-Q, Sun Y-Z. Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ ToxicolPharmacol 2010;30:264-71.
- Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free RadicBiol Med 2000;29:927-45.
- Abdel-Moneim AM, El-Toweissy MY, Ali AM, Awad Allah AA, Darwish HS, Sadek IA. Curcumin ameliorates lead (Pb(2+))-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 2015;168:206-20.
- Kumral A, Giris M, Soluk-Tekkesin M, Olgac V, Dogru-Abbasoglu S, Turkoglu U, et al. Beneficial effects of carnosine and carnosine plus vitamin E treatments on doxorubicin-induced oxidative stress and cardiac, hepatic, and renal toxicity in rats. Hum ExpToxicol 2016;35:635-43.
- Bellakhdar J, Claisse R, Fleurentin J, Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoeia. J Ethnopharmacol 1991;35:123-43.
- Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgarisand its active constituent, berberine. Phytother Res 2008;22:999-1012.
- Tomosaka H, Chin YW, Salim AA, Keller WJ, Chai H, Kinghorn AD. Antioxidant and cytoprotective compounds from Berberis vulgaris(barberry). Phytother Res 2008;22:979-81.
- Sharma V, Sharma A, Kansal L. The effect of oral administration of Allium sativumextracts on lead nitrate induced toxicity in male mice. Food ChemToxicol 2010;48:928-36.
- Hermenean A, Popescu C, Ardelean A, Stan M, Hadaruga N, Mihali CV, et al. Hepatoprotective effects of Berberis vulgaris L. extract/beta cyclodextrin on carbon tetrachloride-induced acute toxicity in mice. Int J MolSci 2012;13:9014-34.
- Thirupurasundari CJ, Padmini R, Devaraj SN. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. ChemBiol Interact 2009;177:190-5.
- Kostial K, Blanuša M, Piasek M, Restek?Samar?ija N, Jones MM, Singh PK. Combined chelation therapy in reducing tissue lead concentrations in suckling rats. J ApplToxicol 1999;19:143-7.
- Ellman GL. Tissue sulfhydryl groups. Arch BiochemBiophys 1959;82:70-7.
- Jollow D, Mitchell J, Zampaglione Na, Gillette J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974;11:151-69.
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984;105:114-21.
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469-74.
- Draper H, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1989;186:421-31.
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994;233:357-63.
- Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med 2002;162:2443-9.
- Rastogi SK. Renal effects of environmental and occupational lead exposure. Indian J Occup Environ Med 2008;12:103-6.
- Rajaian H, Jalaee J, Aghajani A. Berberis vulgaris as growth promoter in broiler chickens. Int J PoultSci 2006;5:395-7.
- Dewanjee S, Sahu R, Karmakar S, Gangopadhyay M. Toxic effects of lead exposure in Wistar rats: involvement of oxidative stress and the beneficial role of edible jute (Corchorusolitorius) leaves. Food ChemToxicol 2013;55:78-91.
- Skoczy?ska A, Smolik R, Jele? M. Lipid abnormalities in rats given small doses of lead. Arch Toxicol 1993;67:200-4..
- Mokhber-Dezfuli N, Saeidnia S, Gohari AR, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of berberis species. Pharmacogn Rev 2014;8(15):8-15.
- Amudha K, Pari L. Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. ChemBiol Interact 2011;193:57-64.
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J NutrBiochem 2002;13:572-84.
- El-Nekeety AA, El-Kady AA, Soliman MS, Hassan NS, Abdel-Wahhab MA. Protective effect of Aquilegia vulgaris(L.) against lead acetate-induced oxidative stress in rats. Food ChemToxicol 2009;47:2209-15.
- Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int 1999;55:1878-84.
- Matos RC, Vieira C, Morais S, de Lourdes Pereira M, de Jesus JP. Nephrotoxicity of CCA-treated wood: A comparative study with As(2)O(5) and CrO(3) on mice. Environ ToxicolPharmacol 2009;27:259-63..
- Patra R, Swarup D, Dwivedi S. Antioxidant effects of α tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 2001;162:81-8.
- Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009;256:128-34.
- Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 2005;212:116-23.
- Ruilope LM, Garcia-Puig J. Hyperuricemia and renal function. CurrHypertens Rep 2001;3:197-202.
- Hooper D, Spitsin S, Kean R, Champion J, Dickson G, Chaudhry I, et al.Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. ProcNatlAcadSci USA. 1998;95:675-80.
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 2001;1:529-39.
- Ercal N, Neal R, Treeratphan P, Lutz PM, Hammond TC, Dennery P, et al.A role for oxidative stress in suppressing serum immunoglobulin levels in lead-exposed Fisher 344 rats. Arch Environ ContamToxicol 2000;39:251-6.
- Gürer H, Özgünes H, Neal R, Spitz DR, Erçal N. Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 1998;128:181-9.
- Wang J, Zhu H, Yang Z, Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol 2013;45:395-8.
- Charehsaz M, Sipahi H, Celep E, Ustundag A, CemilogluUlker O, Duydu Y, et al. The fruit extract of Berberiscrataegina DC: exerts potent antioxidant activity and protects DNA integrity. DARU 2015;23:24.
- Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free RadicBiol Med 1994;17:235-48.
- Othman AI, El-Missiry MA. Role of selenium against lead toxicity in male rats. J BiochemMolToxicol 1998;12:345-9.
- Cabell L, Ferguson C, Luginbill D, Kern M, Weingart A, Audesirk G. Differential induction of hemeoxygenase and other stress proteins in cultured hippocampal astrocytes and neurons by inorganic lead. ToxicolApplPharmacol 2004;198:49-60.
- Fowler BA, Whittaker MH, Lipsky M, Wang G, Chen X-Q. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: an overview. Biometals 2004;17:567-8.
- El-Wahab AEA, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitrobiological assessment of Berberis vulgarisand its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218.
- Jyothilakshmi V, Thellamudhu G, Chinta R, Alok K, Anil K, Debadatta N, et al.Beneficial antioxidative effect of the homeopathic preparation of Berberis vulgarisin alleviating oxidative stress in experimental urolithiasis. ForschKomplementmed 2014;21:7-12.
- Donaldson W, Knowles SO. Is lead toxicosis a reflection of altered fatty acid composition of membranes? Comp BiochemPhysiol C Comp Pharmacol 1993;104:377-9.
- Yiin S, Lin T. Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biol Trace Elem Res 1995;50:167-72.