- *Corresponding Author:
- X. Tao
Key Laboratory of Industrial Dust Control and Occupational Safety and Health, Ministry of Education, Anhui University of Science and Technology, Huainan 232001, China
E-mail: xrtao1116@hotmail.com
| Date of Submission | 22 January 2019 |
| Date of Revision | 04 June 2019 |
| Date of Acceptance | 15 September 2019 |
| Indian J Pharm Sci 2019;81(6):1000-1010 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Dendritic cells are antigen-presenting cells that act as messengers between the innate and adaptive immune systems. Their major function is to process foreign material and present it to CD4+ T-cells. Previous studies have demonstrated that nicotine suppresses the immune function of dendritic cells. However, the role of nicotine on CD4+ T-cells differentiation remains elusive. In this study, the effect of nicotine was examined on the phenotype, activity and apoptosis of murine bone marrow derived dendritic cells, and dendritic cells -dependent differentiation of CD4+ T-cells. It was found that nicotine protected dendritic cells from apoptosis, reduced expression of MHC class II molecular on dendritic cells and the phagocytic, but not endocytic, ability. Moreover, nicotine increased surface co-stimulatory signal markers CD40, CD80 and CD86 in lipopolysaccharide (LPS)-treated dendritic cells. Dendritic cell-mediated induction of CD4+ T-cells differentiation was evaluated by flow cytometry and q-PCR. Up-regulation of Foxp3, IL-6, IL-10, and IL-13 expression and down-regulating of INF-γ were observed in dendritic cells/CD4+ T-cells co-cultured with nicotine. The nicotine and LPS treatment increased mRNA expression of IL-4, IL-6, IL-13 and decreased INF-γ production and IL-1β mRNA significantly. In contrast, losing sustained nicotine exposure during CD4+ T-cells differentiation, nicotine-pretreated dendritic cells failed to prime native CD4+ T-cells to T helper cells (Th1, Th2, or Treg) for adaptive responses. These findings provide new insights into the immunosuppressive properties of nicotine in dendritic cells, and demonstrate that sustained presence of nicotine protected dendritic cells from apoptosis and play an important role in CD4+ T-cell stimulation and differentiation.
Keywords
Dendritic cells, CD4+ T-cells, nicotine, antiapoptosis
Tobacco smoke or nicotine exposure affects innate and adaptive immunity in animals and humans[1]. Tobacco smoke increases the incidence of a number of respiratory diseases and cancers[2,3], interestingly, cigarettes smokers have a lower risk of inflammatory enteritis, ulcerative colitis, sarcoidosis[4] and neurodegenerative diseases, such as Parkinson disease and Alzheimer’s disease[5], suggesting that nicotine has distinct effect on the immune system. Dendritic cells (DCs) are derived from hematopoietic bone marrow progenitor cells. These progenitor cells initially transform into immature DCs in the bone marrow[6]. Immature DCs constantly sample the surrounding environment for pathogens such as viruses and bacteria via pattern recognition receptors such as the toll-like receptors (TLRs)[7]. TLRs recognize specific chemical signatures found on subsets of pathogens[8]. Once DCs have come into contact with a presentable antigen, they become activated into mature DCs and begin to migrate to the lymph nodes where they activate helper T-cells and killer T-cells, as well as B-cells by presenting them with antigens derived from the pathogen. Additionally, DCs present non-antigen co-stimulatory signals that further initiate and shape the adaptive immune response[9,10]. Mature DCs stimulate and differentiate native T-cells into T-helper 1 (Th1), T-helper 2 (Th2), T-helper 17 (Th17), and regulatory T-cells (Treg)[11,12].
The role of nicotine exposure on DC function remains controversial[13,14]. Some studies suggest that nicotine strongly suppresses DC-mediated adaptive immune responses[15-17]. However, Vassallo et al.[18] reported that cigarette smoke extract, but not nicotine, inhibited DCmediated adaptive immune responses. Aicher et al.[19] showed that nicotine activated DCs and augmented their capacity to stimulate T-cell proliferation and cytokine secretion. The inconsistency in reports may be attributed to limitations of DC isolation techniques and cell culture environment. Nicotine participates a large number of signaling pathways promoting cell proliferation, migration and invasion, and inhibited cell apoptosis[20-24]. Not many studies were reported about the antiapoptotic effect of nicotine in DCs. Here, mouse bone marrow-derived DCs were used to systemically examine the impact of nicotine on the activation and apoptosis of DCs, and subsequent CD4+ T-cell differentiation to Th1, Th2 and Treg cells.
Materials and Methods
The complete culture medium used was RPMI 1640 basic (1×) containing 1 % L-glutamine, 25 μM HEPES, and 1 % penicillin/streptomycin. All components were obtained from GIBCO (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from Kybio (Tianjin, China). Complete culture media containing 10 % FBS (R-10) was used to culture DCs. Recombinant murine GM-CSF, IL-4, and recombinant TGF-β1 were obtained from Peprotech (Rocky Hill, NJ). TRIzol came from Invitrogen Life Technologies (Carlsbad, CA). RevertAid First Strand cDNA synthesis kit, Maxima SYBR Green/ROX qPCR Master Mix (2×) were purchased from Molecular Probes (Eugene, OR). (-)-Nicotine, LPS, and FITC-dextran were purchased from Sigma-Aldrich (St. Louis, MI). FITC-OVA was obtained from Solarbio (Beijing, China). CytoTox96 nonradioactive cytotoxicity assay was obtained from Promega (Madison, WI). Bradford protein assay kit and caspase 3 activity assay kit were bought from Beyotime (Shanghai, China). EasySep™ Murine CD4+ T-cell isolation kit, recombinant murine IL-2, and cell detachment solution were obtained from StemCell Technologies (Vancouver, Canada). Brefeldin A, antimurine CD3e and monoclonal antibodies (mAbs) including CD40 (HM40-3), CD86 (GL1), CD80 (16-X10A1), CD197 (4B12), MHC-II (1-A/I-E), CD4 (RM4-5), CD25 (PC61.5), Foxp3 (FJK-16s) and IFN-γ (XMG1.2) were purchased from eBioscience (San Diego, CA). Antimurine CD11c was purchased from Biolegend (San Diego, CA). Red cell lysis buffer, with main composition of ammonia chloride, was obtained from Biosharp (BL503A, Shanghai, China).
Experimental animals:
Specific pathogen-free adult C57BL/6 mice were purchased from the Changzhou Cavion Experimental Animal Co, Ltd. (license number SCXY (Su) 2011-0003). Mice were housed in a vivarium maintained on a standard 12 h light-dark cycle (lights on at 07:00 AM) with constant temperature and humidity (22° and 50 %, respectively) and ad libitum access to food and water. All procedures were conducted in accordance with the guidelines as described in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH publication no. 8023, revised 1978) and were approved by the Institutional Animal Care and Use Committee at Anhui University of Science and Technology (#201718).
Preparation and immunophenotyping of mBMDCs:
Femurs were collected after C57BL/6 mouse was euthanized. The precursor DCs were derived from the bone marrow, which was flushed out with phosphate buffered saline (PBS) using a syringe with a 0.50× 20 mm needle. Clusters within the marrow suspension were dissociated by vigorously pipetting and filtered through a 70-μm cell strainer. Red cells were depleted using 1 ml red cell lysis buffer for 2 min at room temperature. Cells were washed with PBS. Then, the cells were cultured in R-10 and plated on 100 mm dishes at 37° in 5 % humidified CO2. R-10 supplemented with 20 ng/ml of recombinant murine GM-CSF and 10 ng/ml of recombinant murine IL-4 was used to culture primary immature DCs, and 10 ml culture medium were added 3 or 4 d later. DCs were harvested after 7-9 d when they grew to a high confluence. DCs derived from murine bone marrow (mBMDCs) were identified with CD11c[25]. Cell surface molecules (CD11c, CD40, CD86, CD80, CD197, MHC-II) were determined by flow cytometry.
DCs antigen uptake:
The DCs were pre-treated with 100 μg/ml nicotine for 24 h (mImNicDCs). mBMDCs or mImNicDCs (5×104) were suspended in a 2 ml R-10 containing FITCdextran (250 μg/ml) or FITC-OVA (25 μg/ml). After 30 min incubation at 37° or 4° (negative control), cells were washed three times with cold staining medium (PBS containing 0.09 % NaN3 and 1 % FBS) and analysed by flow cytometry. Images were captured with a Leica DFC7000T microscope and analysed by LASX 3.0.2.16120 imaging software.
Caspase-3 (CCP-3) activity assay:
Total proteins and cleaved CCP-3 were identified by using Bradford protein assay kit and caspase 3 activity assay kit, respectively. mBMDCs were plated on 12-wells and stimulated with various concentration of nicotine (25, 50, 100 μg/ml) for 72 h without or with LPS (1 μg/ml). After stimulation, cells were washed three times in 1 ml of cold physiological saline, lysed in 50 μl lysis buffer for 15 min on ice. The supernatants were then obtained after centrifuged at high speed (20 000×g) for 15 min. Assays of CCP-3 were performed on 96-well microtitre plates by incubating 25 μl protein of cell lysate per sample in 25 μl reaction buffer containing 5 μl CCP-3 substrate (Ac-DEVD-pNA; 2 mM). Lysates were incubated at 37° for 2 h. Samples were measured with an ELISA reader (ELx800) at an absorbance of 405 nm. The detail analysis procedure was followed by the manufacturer’s protocol. The unit of enzyme CCP-3 in a sample was calculated according to the formula: (CCP-3: protein )×100 %.
In vitro T-cell purification and differentiation:
Spleens from C57BL/6 mice were removed aseptically and kept on ice. Single cell suspensions were obtained by grinding the tissue between two frosted glass slides and filtered through a 70-μm cell strainer. Red cells were removed by resuspending the cell pellet in 1 ml red cell lysis buffer for 2 min and cell pellets were collected by centrifugation. CD4+ T-cells were purified using EasySep™ Murine CD4+ T-cell isolation kit according to the manufacturer’s protocol. The purity of the enriched CD4+ T-cell population was >86 %. mImNicDCs and LPS-stimulated mImNicDCs were developed by incubating mBMDCs with nicotine (100 μg/ml) or a combination of nicotine (100 μg/ml) and LPS (1 μg/ml) for 24 h. DCs were co-cultured at a ratio of 1:10 with native CD4+ T-cells for 3 d. Differentiation of CD4+ T-cells into Th1 and Treg was induced by LPS (1 μg/ml) or TGF-β1 (5 ng/ml, positive control)[26]. Antimurine CD3e (1 μg/ml) and IL-2 (20 U/ml) were used for regulatory T cells induction and maintenance. Cytokines were analysed by flow cytometry and Q-PCR.
Quantitative real-time PCR (Q-PCR):
Total mRNA was extracted with TRIzol reagent and stored at -80° until use. Total mRNA (100 ng) was used for cDNA synthesis in a 20 μl reaction volume containing oligo (dT)18 primer (100 μmol), 20 U of RiboLock RNase inhibitor, 200 U of RevertAid M-MuLV RT, and 10 mM dNTP Mix (RevertAid first strand cDNA synthesis kit) according to the manufacturer’s instructions. Q-PCR reactions were performed in a Bio-Rad iQ5 real-time thermo cycler using Maxima SYBR Green/ROX qPCR Master Mix (2×). Q-PCR reactions were set up in triplicate for each cytokine using murine GAPDH gene as a reference for normalization. The qPCR reaction volume was kept at 25 μl with 1 μl of cDNA, and reaction condition was initial 95° for 10 min, 95° for 10 s, 58° for 1 min (40 cycles) for amplification. Primers used to amplify murine INF-γ, Foxp3, IL-13, IL-4, IL-10, IL-6, IL- 12p40, IL-1β, and GAPDH genes are included in Table 1. The relative abundance of transcripts for each gene was calculated according to the comparative 2-ΔΔCT method.
| Primer | Gene | Primer | Region | Amplicon size (bp) | |
|---|---|---|---|---|---|
| Orientation | Sequence (5’-3’) | ||||
| 1 | IFN-γ | F | AAATCCTGCAGAGCCAGATTAT | 206–315 | 110 |
| R | GCTGTTGCTGAAGAAGGTAGTA | ||||
| 2 | Foxp3 | F | AGAAGCTGGGAGCTATGCAG | 807-906 | 108 |
| R | GCTACGATGCAGCAAGAGC | ||||
| 3 | IL-13 | F | TTGCTTGCCTTGGTGGTCTC | 100-327 | 228 |
| R | TCTGGGTCCTGTAGATGGCA | ||||
| 4 | IL-4 | F | AACGAGGTCACAGGAGAAGG | 171-357 | 187 |
| R | TCTGCAGCTCCATGAGAACA | ||||
| 5 | IL-10 | F | CCA AGC CTT ATC GGA AAT GA | 315-473 | 159 |
| R | AGG GGA GAA ATC GAT GAC AG | ||||
| 6 | IL-6 | F | TACCACTTCACAAGTCGGAGGC | 169–284 | 116 |
| R | CTGCAAGTGCATCATCGTTGTTC | ||||
| 7 | IL-12p40 | F | TTGAACTGGCGTTGGAAGCACG | 720–851 | 132 |
| R | CCACCTGTGAGTTCTTCAAAGGC | ||||
| 8 | IL-1β | F | TGG ACC TTC CAG GAT GAG GAC A | 894-1041 | 148 |
| R | GTT CAT CTC GGA GCC TGT AGT G | ||||
| 9 | GAPDH | F | CACTGAGCATCTCCCTCACA | 1707-1817 | 111 |
| R | GTGGGTGCAGCGAACTTTAT |
Primers are used in Q-PCR analysis. F stands for forward primer. R stands for reverse primer
Table 1: Cytokine primers
Flow cytometry:
Cells were washed with cold staining medium three times to remove reagent and incubated for 15 min on ice with CD16/CD32 antibodies at a concentration of 1 μg per test for blockade of FC receptors. Cells were then treated with surface molecules mAbs for 1 h on ice without light. To detect Foxp3 and IFN-γ expression, cells were stained with antiCD4 and antiCD25 mAbs and incubated overnight at 4° in a dark box in fixation/permeabilization buffer. PE-conjugated Foxp3 mAbs and PerCP-Cy5.5-conjugated IFN-γ mAbs were used to detect Foxp3 and IFN-γ expression in cells, respectively. Brefeldin A (3 μg/ml) was added to examine IFN-γ levels 5 h before staining. Flow cytometry was done with FACSCalibur (BD, America) and data were analyzed with CellQuest software. The effect of nicotine on the endocytic and phagocytic activity of DCs was examined by adding FITC-dextran (250 μg/ml) or FITC-OVA (25 μg/ml) for 30 min.
Statistical analysis:
Data were expressed as mean±SEM. Statistical differences were determined by analysis of variance (ANOVA), followed by post hoc multi comparisons. p<0.05 was considered statistically significant.
Results and Discussion
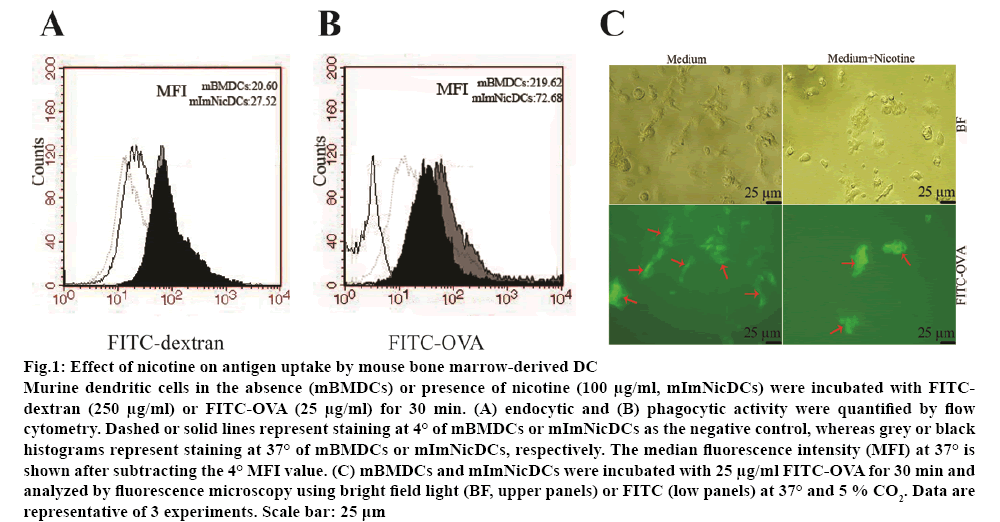
Nicotine reduced the antigen-presenting ability of DCs and subsequent stimulation of allogeneic T-cells. Flow cytometry and fluorescence microscopy were used to analyse the effect of nicotine (100 μg/ml) on endocytosis and phagocytosis. Nicotine at 100 μg/ml was not cytotoxic to DCs and CD4+ T-cells as tested by the lactate dehydrogenase-based cytotoxicity assay. There was no significant difference between the endocytic abilities of nicotine-treated and vehicletreated DCs (figure 1A). However, the phagocytic activity of DCs was dramatically inhibited by nicotine (roughly two-fold increase; figure 1B). Nicotine-treated mBMDCs also presented a lower amount of FITC staining (figure 1C).
Figure 1: Effect of nicotine on antigen uptake by mouse bone marrow-derived DC
Murine dendritic cells in the absence (mBMDCs) or presence of nicotine (100 μg/ml, mImNicDCs) were incubated with FITCdextran (250 μg/ml) or FITC-OVA (25 μg/ml) for 30 min. (A) endocytic and (B) phagocytic activity were quantified by flow cytometry. Dashed or solid lines represent staining at 4° of mBMDCs or mImNicDCs as the negative control, whereas grey or black histograms represent staining at 37° of mBMDCs or mImNicDCs, respectively. The median fluorescence intensity (MFI) at 37° is shown after subtracting the 4° MFI value. (C) mBMDCs and mImNicDCs were incubated with 25 μg/ml FITC-OVA for 30 min and analyzed by fluorescence microscopy using bright field light (BF, upper panels) or FITC (low panels) at 37° and 5 % CO2. Data are representative of 3 experiments. Scale bar: 25 μm
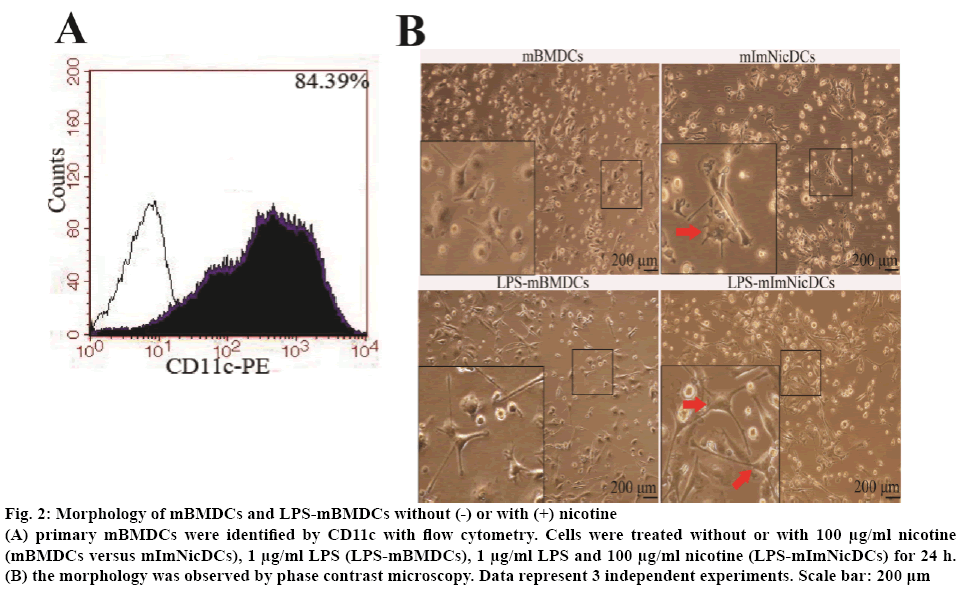
Nicotine exposure may alter the phenotype of DCs without or with LPS by changing the expression of stimulatory cell surface markers. To test this hypothesis, the DCs were set into 4 groups, R-10 medium (mBMDCs), 100 μg/ml nicotine (mImNicDCs), 1 μg/ml LPS (LPS-mBMDCs), 1 μg/ml LPS with 100 μg/ml nicotine (LPS-mImNicDCs), all groups were treated for 24 h. Flow cytometry results showed that CD11c positive cells went up to 84.39 % of the total (figure 2A). mImNicDCs and LPS-mImNicDCs presented more branched projections compared with DCs and LPS-mBMDCs respectively (figure 2B). The median fluorescence intensity of mImNicDCs have higher levels of CD40 and CD197, and lower levels of CD86 and MHC-II, but CD80 expression was not significantly difference between nicotine-treated and vehicle-treated groups (Table 2). LPS-mImNicDCs lead to an increase in expression of the co-stimulatory molecules CD40, CD86, and CD80, and a decreased expression of CD197 and MHC-II compared to LPSmBMDCs group (Table 2).
Figure 2: Morphology of mBMDCs and LPS-mBMDCs without (-) or with (+) nicotine
(A) primary mBMDCs were identified by CD11c with flow cytometry. Cells were treated without or with 100 μg/ml nicotine (mBMDCs versus mImNicDCs), 1 μg/ml LPS (LPS-mBMDCs), 1 μg/ml LPS and 100 μg/ml nicotine (LPS-mImNicDCs) for 24 h. (B) the morphology was observed by phase contrast microscopy. Data represent 3 independent experiments. Scale bar: 200 μm
Surface markers |
mBMDCs | mImNicDCs | T value | N | LPS-mBMDCs | LPS-mImNicDCs | T value | N |
|---|---|---|---|---|---|---|---|---|
| CD40 | 152.45 ± 7.68 | 296.69 ± 15.08* | -14.762 | 3 | 1632.81 ± 58.88 | 2444.60 ± 484.16* | -2.883 | 3 |
| CD86 | 74.70 ± 5.37 | 33.40 ± 2.84* | 11.784 | 3 | 74.66 ± 4.90 | 130.41 ± 6.90* | -11.412 | 3 |
| CD80 | 110.01 ± 4.33 | 103.10 ± 8.22 | 1.289 | 4 | 213.92 ± 15.15 | 412.22 ± 11.46* | -18.080 | 3 |
| CD197 | 13.61 ± 1.40 | 21.77 ± 3.17* | -4.079 | 3 | 20.73 ± 1.45 | 14.54 ± 1.20* | 5.696 | 3 |
| MHC-II | 902.9 ± 18.09 | 432.23 ± 11.58* | 37.985 | 3 | 1329.14 ± 64.54 | 969.44 ± 27.16* | 8.897 | 3 |
Flow cytometry analysis was performed to identify surface marker on murine dendritic cells. MFI is median fluorescence intensity. N is number of experiments. Data are representative of three or four experiments. Significant p value (*p<0.05) were obtained by Student’s t test analysis. Values were mean ± SEM
Table 2: Flow cytometry analysis of surface markers on nicotine or nicotine/LPS-treated murine dendritic cells
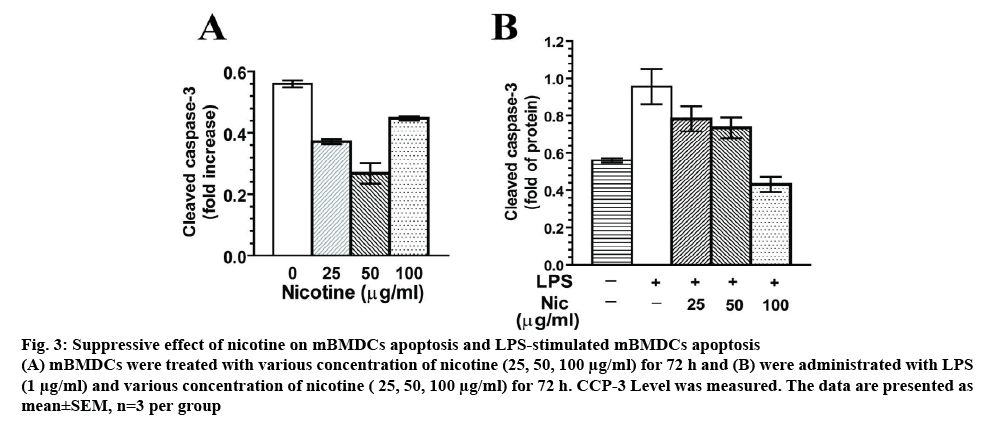
To explore the exact effect of nicotine on the apoptosis of DCs, mBMDCs were treated with nicotine (25, 50, 100 μg/ml) for 72 h. In figure 3A, cleaved CCP-3 level was significantly inhibited by nicotine ranging from 25 to 100 μg/ml compared with that in the blank groups (p<0.01). Nicotine treatment at 100 μg/ml showed an increase of CCP3 level relative to 50 μg/ml nicotine group, but lower level of CCP3 when compared to the control. To test whether nicotine influences LPSinduced apoptosis, LPS (1 μg/ml) combined with various concentration of nicotine (25, 50, 100 μg/ml) were tested in DC culture for 72 h. LPS-mImNicDCs showed significant reduction of CCP-3 release at 25, 50, 100 μg/ml compared with that in the LPS groups, as shown in figure 3B. Taken together, this revealed that nicotine treatment had antiapoptotic effects in mBMDCs.
Figure 3: Suppressive effect of nicotine on mBMDCs apoptosis and LPS-stimulated mBMDCs apoptosis
(A) mBMDCs were treated with various concentration of nicotine (25, 50, 100 μg/ml) for 72 h and (B) were administrated with LPS (1 μg/ml) and various concentration of nicotine ( 25, 50, 100 μg/ml) for 72 h. CCP-3 Level was measured. The data are presented as mean±SEM, n=3 per group
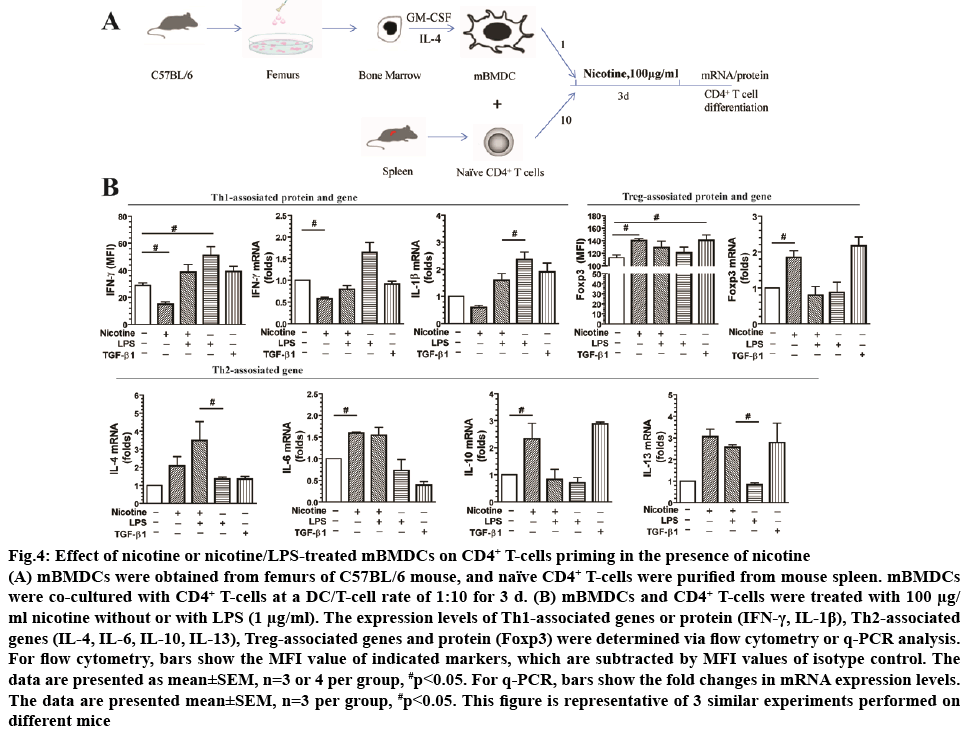
To investigate the effect of nicotine on DCs-induced CD4+ T-cell priming, mBMDCs were co-cultured with CD4+ T-cells at a DC/T-cell ratio of 1:10 for 3 d in the presence of nicotine (100 μg/ml), as shown in figure 4A. Nicotine alone increased Foxp3, a Treg associated maker, protein (1.24-fold) and mRNA levels (1.85-fold). mRNA levels of Th2 associated markers IL-10 (2.34- fold), IL-6 (1.59-fold), and IL-13 (3.06-fold) were increased as well. Additionally, nicotine treatment decreased protein (0.52-fold) and mRNA (0.58-fold) levels of Th1 marker IFN-γ. Similarly, nicotine treatment decreased mRNA levels of IL-1β (0.59-fold) compared to control cells. Treatment with TGF-β1 was used as a positive control and increased expression of Foxp3 (1.25-fold in protein level, 2.17-fold in mRNA level) compared with the control (p<0.05). There was no statistical difference in the IL-4 expression in mRNA levels (p>0.05; figure 4B).
Figure 4: Effect of nicotine or nicotine/LPS-treated mBMDCs on CD4+ T-cells priming in the presence of nicotine
(A) mBMDCs were obtained from femurs of C57BL/6 mouse, and naïve CD4+ T-cells were purified from mouse spleen. mBMDCs were co-cultured with CD4+ T-cells at a DC/T-cell rate of 1:10 for 3 d. (B) mBMDCs and CD4+ T-cells were treated with 100 μg/ml nicotine without or with LPS (1 μg/ml). The expression levels of Th1-associated genes or protein (IFN-γ, IL-1β), Th2-associated genes (IL-4, IL-6, IL-10, IL-13), Treg-associated genes and protein (Foxp3) were determined via flow cytometry or q-PCR analysis.
For flow cytometry, bars show the MFI value of indicated markers, which are subtracted by MFI values of isotype control. The data are presented as mean±SEM, n=3 or 4 per group, #p<0.05. For q-PCR, bars show the fold changes in mRNA expression levels. The data are presented mean±SEM, n=3 per group, #p<0.05. This figure is representative of 3 similar experiments performed on different mice
Nicotine may affect LPS-mediated DCs activation of CD4+ T-cells. To test this, mBMDCs were treated with LPS (1 μg/ml) or nicotine (100 μg/ml)/LPS and analysed by flow cytometry and qPCR. Compared to LPS treatment alone, the combination of nicotine and LPS reduced mRNA levels of Th1-associated markers IFN-γ (0.46-fold) and IL-1β (0.68-fold) and increased mRNA levels of Th2-associated markers IL-6 (2.11-fold), and IL-4 mRNA (2.57-fold). There was no statistical difference in IL-10 and Foxp3 mRNA levels or in IFN-γ and Foxp3 protein expression between treatment groups (figure 4B). LPS, as positive control, increased IFN-γ protein (1.76-fold) and mRNA (1.64-fold) expression (figure 4B) compared to control (p<0.01).
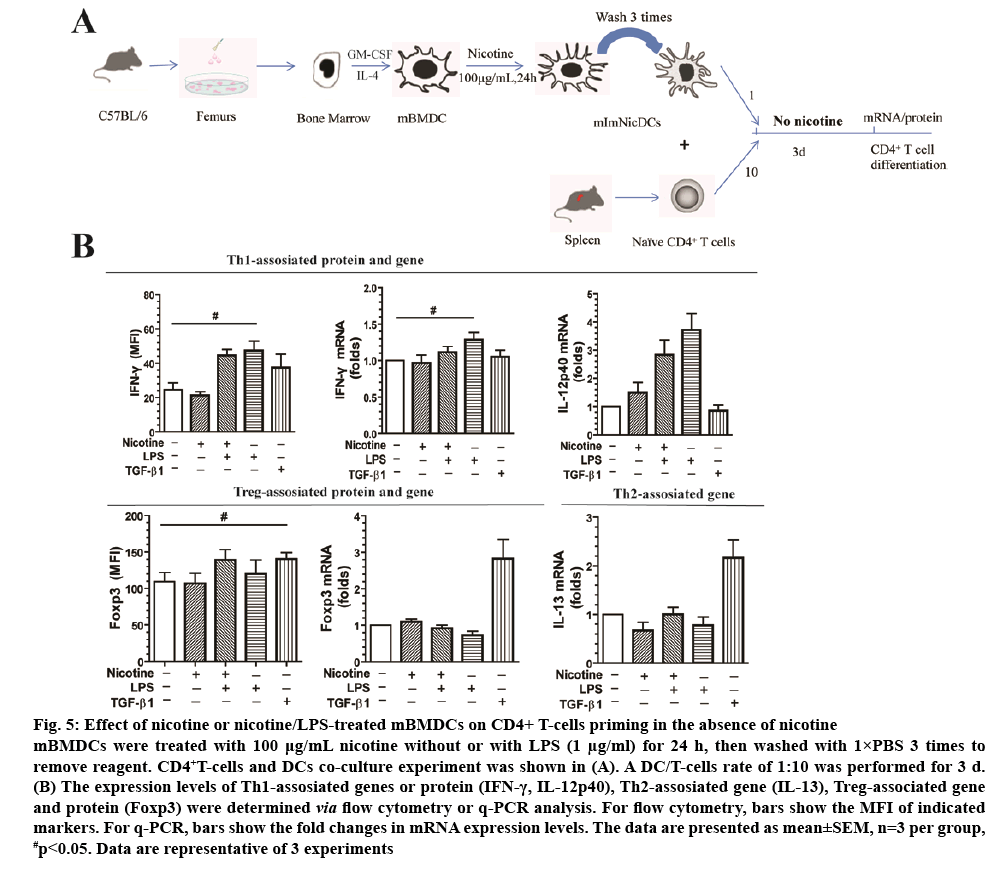
Native CD4+ T-cells have more than 3 different fates, Th1, Th2, and Tregs. The chosen path is determined primarily by signals and antigens presented by DCs in addition to the local microenvironment. To the extent that nicotine-treated DCs may be used in vaccines in the future, we examined how DCs affect CD4+ T-cell differentiation without nicotine in the culture medium (figure 5A). Nicotine or nicotine/LPS-treated DCs were cocultured with CD4+ T-cells at a DC/T-cell ratio of 1:10 for 3 d in the absence of nicotine. LPS, as positive control, induced CD4+ T-cells into Th1 cells with increased IFN-γ protein (1.85-fold) and mRNA (1.42-fold) expression compared with the control group (figure 5B). However, mRNA expression levels, or protein of Foxp3 and IFN-γ didn’t shown statistical difference (p>0.05) between nicotine group and LPS group (figure 5B). Additionally, IL-13 and IL-12p40 mRNA levels were not significantly different between groups either (p>0.05). TGF-β1, as positive control, increased Foxp3 mRNA expression 3.2-fold compared with the control (p<0.01, figure 5B), and 1.28-fold in protein levels (p<0.05, figure 5B).
Figure 5: Effect of nicotine or nicotine/LPS-treated mBMDCs on CD4+ T-cells priming in the absence of nicotine
mBMDCs were treated with 100 μg/mL nicotine without or with LPS (1 μg/ml) for 24 h, then washed with 1×PBS 3 times to remove reagent. CD4+T-cells and DCs co-culture experiment was shown in (A). A DC/T-cells rate of 1:10 was performed for 3 d. (B) The expression levels of Th1-assosiated genes or protein (IFN-γ, IL-12p40), Th2-assosiated gene (IL-13), Treg-associated gene and protein (Foxp3) were determined via flow cytometry or q-PCR analysis. For flow cytometry, bars show the MFI of indicated
markers. For q-PCR, bars show the fold changes in mRNA expression levels. The data are presented as mean±SEM, n=3 per group, #p<0.05. Data are representative of 3 experiments
Nicotine has antiinflammatory and neuron-protective effects[27-29]. In this study, it was confirmed that nicotine altered the functional activity of DCs. Moreover, nicotine impaired the phagocytic, but not endocytic activity of DCs. Nicotine increased the expression of co-stimulatory molecules on the membrane of DCs, protects DCs from apoptosis, leading to an increase in T-cell activation and differentiation.
DCs are the antigen processing cells (APCs) of the mammalian immune system. Their main function is to process foreign antigen material and present to T-cells. They act as messengers between the innate and the adaptive immune systems[30]. Nicotine reduced the antigen-presenting ability to stimulate T-cells.
It was observed that nicotine-treated DCs reduced antigen uptake and MHC-II molecular, which failed to efficiently induce type 1 interferon (IFN)-γ-producing T-cell polarization in DC/T-cell co-culture, whereas nicotine-treated DCs decreased CD86 expression while CD80 is not changed significantly. It also suggested that a decrease in CD86 expression, not CD80 expression on nicotine-treated DC was relative to nicotine-induced Treg differentiation.
DCs play a critical role in the development of a competent immune system by recognizing, processing, and presenting antigens to T-cells. DCs acquire exogenous antigens/pathogens by a variety of different endocytic processes including endocytosis and phagocytosis. Endocytosis requires antigen binding to a variety of receptors leading to internalization of the typically small foreign molecule; however, phagocytosis mediates the internalization of a widevariety of relatively large insoluble particulate antigens originating from necrotic/apoptotic cells and opsonized pathogenic organisms/viruses[31]. Data obtained in this investigation revealed that nicotine did not affect the endocytic abilities but did impair the phagocytic activity of DCs. This is consistent with the published data from Ween et al.[32], who showed that nicotine reduced phagocytosis of macrophages derived from THP-1 by affecting phagocytic recognition molecules. Considering that DCs are the most powerful APCs, its endocytic and phagocytic processes are principally responsible for uptake and presentation of pathogenic and pathogen-associated antigens. Dysregulation of these processes can be detrimental to the host defense[30,31].
Nicotine increased the expression of co-stimulatory molecules on the surface of DCs as well as increases cell priming and polarization[19,33] in addition to affecting DC-mediated immune responses[15]. Previous studies showed that nicotine enhanced DC maturation, reduced antigen uptake, and suppressed DC-mediated T-cell priming[15,34]. Present data revealed that nicotine-treated DCs did not alter CD80 levels, but showed increased expression of CD40 and CD197. Meanwhile, nicotinetreated DCs have a decreased expression of CD86 and MHC-II.
LPS can function as a microbial endotoxin deriving utility as a maturation stimulus for DCs and an activator of toll-like 4 receptor (TLR4) signaling. LPS treatment increased expression of co-stimulatory molecules CD80 (B7-1), CD86 (B7-2), CD40, and CD11c on the DC membrane[33,35] and induce DC-dependent priming of T-cells[36,37]. Current data indicated that LPS-treated DCs increased the expression of CD40, CD86, and CD80. However Nouri-Shirazi et al.[15] reported that nicotine (100 μg/ml) did not affect DCs maturation. Differences in the isolation, preparation, and treatment may account for these discrepancies. The concentrate of nicotine used in this work was chosen according to previous studies[12,15], and correlated with nicotine levels achievable in local tissues such as the oral cavity and the lungs, where tissue resident DCs encounter antigens[38].
Some reports showed that nicotine decreased cytotoxicity after oxidative stress occurred and have protective effect in primary cultured cortical and cerebellar granule cells through antiapoptotic effect[39-41]. In this study, it was found that nicotine protected DCs from apoptosis in the absence or presence of LPS. Studies showed that nicotine bidirectionally regulated the proliferation of human alveolar bone marrowderived mesenchymal stem cells[42], mouse embryonic stem cells[43], mouse hippocampal neuronal and glia cells[44]. The present study showed that antiapoptosis by nicotine in DCs might play an important role in T-cell priming, in addition to surface molecular alternation in DCs. Several signalling pathways regulating nicotinemediated antiapoptosis in different cells, such as Wnt/ beta-catenin[45], Notch[12], PI3K/AKT, JAK/STAT, Rb/E2F1[46]. Akt play vital role in nicotine-increased surface molecules expression in human peripheral blood mononuclear cell-derived DCs, whereas ribosomal protein S6 was involved in regulating growth and proliferation of cells[47].
Nicotine-treated DCs had been reported to have potential antitumor and antiviral effects[48-50] and a DC-based vaccine has been investigated in a clinical trial[51,52]. Moreover, nicotine treated-DCs have been reported to mediate cytotoxic T lymphocyte priming[53]; however, the role of nicotine stimulated-DC in CD4+ T-cell priming and the adaptive immune response had yet to be explored.
Guinet et al.[17] found nicotinic environment affects the differentiation and functional maturation of monocytes derived DCs. Nicotine-treated DC expressed low level of antigen-presenting molecule MHCs and chemotactic cytokine receptor CCR7 in respond to bacterial antigen LPS, which finally decreased Th1 differentiation in DC/T-cells co-culture[17]. When nicotine-pretreated DCs were cultured with CD4+ T-cell in the presence or absence of nicotine, it was surprising to find that neither nicotine-treated DCs or LPS and nicotine-treated DCs were able to prime CD4+ T-cells differentiate into Th1, Th2 or Treg cells in the absence of nicotine treatment, whereas CD4+ T-cells reduced Th1 differentiation and increase Treg and Th2 differentiation after co-cultured with DCs in the presence of nicotine. This suggested that direct impact of nicotine on CD4+ T-cells differentiation play a more important role than nicotine-pretreated DC. Indeed, Kawashima et al.[54] reported that nicotinic acetylcholine receptor (nAChR) was highly expressed in T-cells and B-cells and these receptors may induce nicotine response, but it needs further study in our future. Considering the small size and lipophilicity properties of nicotine, it can passively move across cell membranes; however, its primary effects are receptor mediated[55].
Nicotine produced profound effects on DCs and DC-mediated immune responses. In this study, the effect of nicotine on DCs, on CD4+ T-cell differentiation with or without LPS was examined. DCs express various nAChR subunits, including α2, α5, α6, α7, α10 and β2 subunits. Further studies are necessary to identify the role of individual nACh receptor and signalling pathway in nicotine-mediated immunity response.
Acknowledgements:
This work was supported by the National Natural Science Foundation in China, (81471161), Graduate student innovation fund (2017CX2115), Top Talent Project of Anhui Department of Education (gxbjZD16); Science and technology plan in Huainan (2015A2401), Natural Science Foundation of Anhui Province (108085MH247).
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- Johnson JD, Houchens DP, Kluwe WM, Craig DK, Fisher GL. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: a review. Crit Rev Toxicol 1990;20:369-95.
- Wei PL, Chang YJ, Ho YS, Lee CH, Yang YY, An J, et al. Tobacco-specific carcinogen enhances colon cancer cell migration through alpha7-nicotinic acetylcholine receptor. Ann Surg 2009;249:978-85.
- Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol 1998;83:148-56.
- Hoie O, Schouten LJ, Wolters FL, Solberg IC, Riis L, Mouzas IA, et al. Ulcerative colitis: no rise in mortality in a European-wide population based cohort 10 years after diagnosis. Gut 2007:56(4):497-503.
- Lieberman A, Lockhart TE, Olson MC, Smith Hussain VA, Frames CW, McCauley M, et al. Nicotine Bitartrate Reduces Falls and Freezing of Gait in Parkinson Disease: A Reanalysis. Front Neurol 2019;10:424.
- Zhang Y, Harada A, Wang JB, Zhang YY, Hashimoto S, Naito M, et al. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood 1998;92:118-28.
- Vendelova E, Ashour D, Blank P, Erhard F, Saliba AE, Kalinke U, et al. Tolerogenic Transcriptional Signatures of Steady-State and Pathogen-Induced Dendritic Cells. Front Immunol 2018; 9:333.
- Kane CM, Jung E, Pearce EJ. Schistosoma mansoni egg antigen-mediated modulation of Toll-like receptor (TLR)-induced activation occurs independently of TLR2, TLR4, and MyD88. Infect Immun 2008;76:5754-59.
- Ferreira I, Liberal J, Martins J, Silva A, Neves BM, Cruz MT. Inflammasome in dendritic cells immunobiology: Implications to diseases and therapeutic strategies. Curr Drug Targets 2017;18:1003-18.
- Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol 2005;42:535-39.
- Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol 2005;6:1069-70.
- Nouri-Shirazi M, Kahlden C, Nishino P, Guinet E. Nicotine exposure alters the mRNA expression of Notch ligands in dendritic cells and their response to Th1-/Th2-promoting stimuli. Scand J Immunol 2015;81:110-20.
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, et al. A. C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 2006;127:621-33.
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2:372-77.
- Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 2010;109:365-73.
- Tsoumakidou M, Demedts IK, Brusselle GG, Jeffery PK. Dendritic cells in chronic obstructive pulmonary disease: new players in an old game. Am J Respir Crit Care Med 2008;177:1180.
- Guinet E, Yoshida K, Nouri-Shirazi M. Nicotinic environment affects the differentiation and functional maturation of monocytes derived dendritic cells (DCs). Immunol Lett 2004;95:45-55.
- Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 2005;175:2684-91.
- Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: potential role for progression of atherosclerotic lesions. Circulation 2003;107(4):604-11.
- Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A 2006;103:6332-37.
- Yu W, Mechawar N, Krantic S, Quirion R. Alpha7 Nicotinic receptor activation reduces beta-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem 2011;119:848-58.
- Zhao J, Xin M, Wang T, Zhang Y, Deng X. Nicotine enhances the antiapoptotic function of Mcl-1 through phosphorylation. Mol Cancer Res 2009;7:1954-61.
- Jia Y, Sun H, Wu H, Zhang H, Zhang X, Xiao D, et al. Nicotine Inhibits Cisplatin-Induced Apoptosis via Regulating alpha5-nAChR/AKT Signaling in Human Gastric Cancer Cells. PLoS One 2016;11:e0149120.
- Copeland RL Jr, Das JR, Kanaan YM, Taylor RE, Tizabi Y. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Rese 2007;12(1):61-9.
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002;2:151-61.
- Edwards JP, Hodaka F, Zhou AX, John C, Derya U, Shevach EM. Regulation of the expression of GARP/latent TGF-β1 complexes on mouse T cells and their role in regulatory T cell and Th17 differentiation. J Immunol 2013;190:5506-15.
- Naddafi F, Reza Haidari M, Azizi G, Sedaghat R, Mirshafiey A. Novel therapeutic approach by nicotine in experimental model of multiple sclerosis. Innov Clin Neurosci 2013;10(4):20-5.
- Toledano A, Alvarez MI, Toledano-Diaz A: Variability in the effects of nicotine on different regions of the brain: changes in the concentration of superoxide dismutase isoforms. Cent Nerv Syst Agents Med Chem 2014:14(1):10-22.
- Revathikumar P, Bergqvist F, Gopalakrishnan S, Korotkova M, Jakobsson PJ, Lampa J, et al. Immunomodulatory effects of nicotine on interleukin 1β activated human astrocytes and the role of cyclooxygenase 2 in the underlying mechanism. J Neuroinflammation 2016;13:256.
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell 2001;106:255-58.
- Cardone M, Ikeda KN, Varano B, Belardelli F, Millefiorini E, Gessani S, et al. Opposite regulatory effects of IFN-β and IL-3 on C-type lectin receptors, antigen uptake, and phagocytosis in human macrophages. J Leukoc Biol 2014;95(1):161-68.
- Ween MP, Whittall JJ, Hamon R, Reynolds PN, Hodge SJ. Phagocytosis and Inflammation: Exploring the effects of the components of E-cigarette vapor on macrophages. Physiol Rep 2017;5:e13370.
- Hu SX, Sui HX, Jin HJ, Ni XY, Liu XX, Xue MQ, et al. Lipopolysaccharide and dose of nicotine determine the effects of nicotine on murine bone marrow-derived dendritic cells. Mol Med Rep 2012;5:1005-10.
- Nouri-Shirazi M, Tinajero R, Guinet E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs). Immunol Lett 2007;109:155-64.
- Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol 2004;5:678-84.
- Moscovis S, Hall S, Burns C, Scott R, Blackwell C. Development of an experimental model for assessing the effects of cigarette smoke and virus infections on inflammatory responses to bacterial antigens. Innate Immunity 2014;20(6):647-58.
- Wang F, Wang YY, Li J, You X, Qiu XH, Wang YN, et al. Increased antigen presentation but impaired T cells priming after upregulation of interferon-beta induced by lipopolysaccharides is mediated by upregulation of B7H1 and GITRL. PLoS One 2014; 9:e105636.
- Ebert RV, McNabb ME, McCusker KT, Snow SL. Amount of nicotine and carbon monoxide inhaled by smokers of low-tar, low-nicotine cigarettes. JAMA 1983;250(20):2840-42.
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res 2011;20:263-69.
- Chen J, Higby R, Tian D, Tan D, Johnson MD, Xiao Y, et al. Toxicological analysis of low-nicotine and nicotine-free cigarettes. Toxicology 2008;249:194-203.
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res 2005;7:319-22.
- Kim BS, Kim SJ, Kim HJ, Lee SJ, Park YJ, Lee J, et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci 2012;90:109-15.
- Qu Q, Zhang F, Zhang X, Yin W. Bidirectional Regulation of Mouse Embryonic Stem Cell Proliferation by Nicotine Is Mediated Through Wnt Signaling Pathway. Dose Response 2017;15:1559325817739760.
- Oliveira-da-Silva A, Manhaes AC, Cristina-Rodrigues F, Filgueiras CC, Abreu-Villaca Y. Hippocampal increased cell death and decreased cell density elicited by nicotine and/or ethanol during adolescence are reversed during drug withdrawal. Neuroscience 2010;167:163-73.
- Jiang DQ, Wei MD, Wang KW, Lan YX, Zhu N, Wang Y. Nicotine contributes to the neural stem cells fate against toxicity of microglial-derived factors induced by Abeta via the Wnt/beta-catenin pathway. Int J Neurosci 2016;126:257-68.
- Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res 2014;12:14-23.
- Wang YY, Yang YW, You X, Deng XQ, Hu CF, Zhu C, et al. Ex vivo nicotine stimulation augments the efficacy of human peripheral blood mononuclear cell-derived dendritic cell vaccination via activating Akt-S6 pathway. Anal Cell Pathol 2015:2015;741487.
- Gao FG, Wan da F, Gu JR. Ex vivo nicotine stimulation augments the efficacy of therapeutic bone marrow-derived dendritic cell vaccination. Clin Cancer Res 2007;13(12):3706-12.
- Gao FG, Li HT, Li ZJ, Gu JR. Nicotine Stimulated Dendritic Cells Could Achieve Anti-Tumor Effects in Mouse Lung and Liver Cancer. J Clin Immunol 2011;31:80-8.
- Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel RM. Dendritic cells efficiently induce protective antiviral immunity. J Virol 1998;72(5):3812-18.
- Conrad C, Nestle FO. Dendritic cell-based cancer therapy. Curr Opin Mol Ther 2003;5:405-12.
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B–cell lymphoma using autologous antigen–pulsed dendritic cells. Nat Med 1996;2:52-8.
- Jin HJ, Li HT, Sui HX, Xue MQ, Wang YN, Wang JX, et al. Nicotine stimulated bone marrow-derived dendritic cells could augment HBV specific CTL priming by activating PI3K-Akt pathway. Immunol Lett 2012;146(1-2):40-9.
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci 2004;9:2063.
- Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol 2001;167:6518-24.