- Corresponding Author:
- M. R. Mehta
Bombay College of Pharmacy, Kalina, Mumbai-400 098, India
E-mail: mohitmehta30@gmail.com
Abstract
In situ gelling systems based on temperature-dependent phase transition containing pheniramine and phenylephrine were developed using combination of Poloxamers, different cellulose polymers (HPMC) and xanthan gum. The formulations were tested for in vitro gelation, appearance, pH, drug content, in vitro drug release, mucoadhesive strength, preservative efficacy and stability. In vitro release studies revealed significant prolonged released of both drugs up to 24 h as against only 2 h with drug solution. Formulations were found to stable over period of 3 months. In vivo nasal residence time of in situ gel by gamma scintigraphy was found to be significantly higher (6 h) in comparison to drug solution (15 min). It can be concluded that Poloxamers in combination with HPMC are suitable to develop stable, safe in situ temperature-based mucoadhesive gelling systems with prolonged nasal residence time.
Keywords
Nasal in situ gelling systems, mucoadhesion, gamma scintigraphy, nasal residence time
Almost 15% of world?s population suffers from chronic sinusitis. Treatment for sinusitis is directed towards relief of symptoms; drugs used include antihistaminics, antibiotics, corticosteroids and decongestants. Several formulations for relief of sinusitis are approved for intranasal aerosol delivery by nasal spray pumps or by pMDI systems. These formulations require frequent administration due to nasal mucociliary clearance. Also, nasal decongestants may dry out the affected areas and damage tissues, and with prolonged use they often become ineffective. The tendency is to then increase the frequency of use to as often as once an hour which can cause rebound effect. The present work aimed to develop sustained release in situ gelling formulations of antihistaminic and decongestant combination for intranasal administration, for treatment of sinusitis, and there by overcome the frequent dosing required with conventional nasal formulations.
Materials and Methods
Poloxamer 407 and Poloxamer 188 (gift from BASF, Mumbai.), HPMC E15, HPMC E50 and HPMC K4M (gift from Colorcon, Mumbai), phenylephrine HCl (gift from Centaur Limited, Mumbai), pheniramine maleate (gift from Supriya Chemicals, Mumbai) Mucin (Type III) from porcine stomach (Aldrich, USA), xanthan gum (C P Kelco, USA), benzalkonium chloride, glycerine, sodium chloride (S. D. Fine-Chem Ltd., Mumbai).
In situ gelling systems based on temperaturedependent phase transition were developed using combination of Poloxamers. In order to reduce concentrations of above polymers and to develop bioadhesive property, different cellulose polymers (HPMC) and xanthan gum were tried in various concentrations. Glycerine (humectant), sodium chloride (tonicity adjuster) and benzalkonium chloride (preservative) were also included in the formulation. The formulations were tested for in vitro gelation to screen the suitable polymer combinations. Selected formulations were evaluated for appearance, pH, drug content, in vitro drug release, mucoadhesive strength, preservative efficacy and stability. In vitro drug release was studied in simulated nasal secretion at 370 for upto 24 h. Mucoadhesive strength was measured by modifi ed pan balance method with mucin (Type III) as a substrate. Preservative effi cacy testing was carried out as per IP method using E. coli, B. subtilis, C. albicans. Selected formulations were evaluated for in vivo nasal irritation in female Wistar rats and nasal mucosal residence time in New Zealand white rabbits by gamma scintigraphy.
Results and Discussion
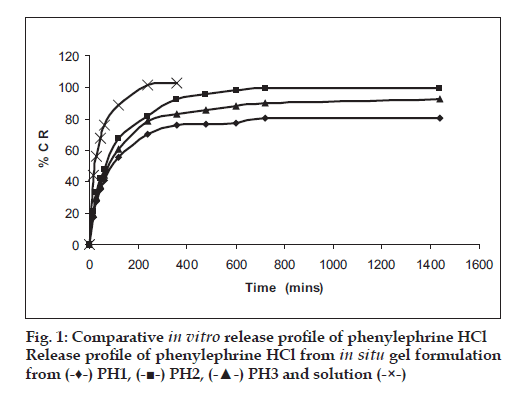
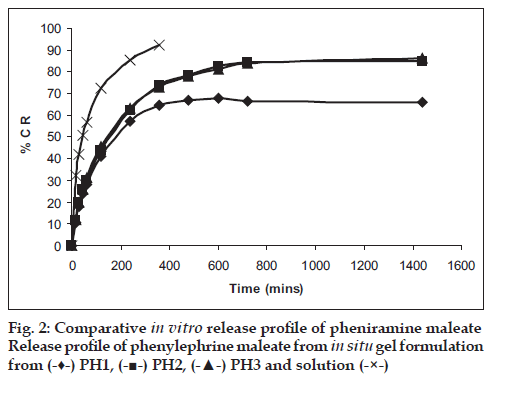
Among the various polymers tried, only HPMC E-15 was able to form a consistent gel at low concentration of Poloxamers (Table 1), and these three batches were selected for further characterization. pH of formulations was found to 4.8-5.0, suitable for nasal use. Mucoadhesive strength of in situ gel formulations was signifi cantly improved with inclusion of HPMC. In vitro release studies revealed signifi cant prolonged released of both drugs upto 24 h as against only 2 h with drug solution. Formulations were found to stable over period of 3 months (fi g. 1). In vivo nasal residence time of in situ gel by gamma scintigraphy was found to be signifi cantly higher (6 h) in comparison to drug solution (15 min) (fi g. 2). It can be concluded that Poloxamers in combination with HPMC are suitable to develop stable, safe in situ temperature-based mucoadhesive gelling systems with prolonged nasal residence time for the treatment of sinusitis.
| Batch | Gelling Temperature (°) |
Mucoadhesive strength (g) |
|---|---|---|
| PH 1 | 33 | 11.20 |
| PH2 | 34 | 7.89 |
| PH3 | 35 | 7.66 |
Table 1: In Vitro Gelation Time and Mucoadhesive Strength of Selected Batches
Acknowledgements
Authors wish to thank BASF ? Mumbai, Centaur Limited and Bombay Veterinary College, for their help and support.
References
- Talasaz AHH, Ali A. In Situ Gel Forming Systems of Poloxamer 407 and Hydroxypropylcellulose or Hydroxypropylmethylcellulose Mixtures for Controlled Delivery of Vancomycin, J Appl Polym Sci 2008;109:2369-4.
- Majithiya RH, Ghosh PK, Thermoreversible-mucoadhesive Gel for Nasal Delivery of Sumatriptan, AAPS PharmSciTech, 2006; 7(3):80-6.