- *Corresponding Author:
- L. Khantouche
Preparatory Institute for Scientific and Technical Studies, La Marsa 2075, Tunisia
E-mail: khantouchelinda@yahoo.fr
| Date of Submission | 11 January 2017 |
| Date of Revision | 12 May 2017 |
| Date of Acceptance | 21 January 2018 |
| Indian J Pharm Sci 2018;80(2):274-281 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This investigation dealt with the determination of the content of phenolic compounds, flavonoids, anthocyanins, tannins and in vitro antioxidant activity of the ethanol extract of the leaves of Globularia alypum. The physicochemical characteristics of the leaf powder of Globularia alypum such as metabolic energy, mineral element and organic matter were evaluated. Fatty acid content was determined using gas chromatography. This investigation appeared to be the first to report the analysis of the leaf powder, ethanol extracts of G. alypum L. In this study dried extracts, ashes, proteins, crude fat fibre, percent sugar, metabolic energy and minerals such as calcium, sodium and potassium were quantified. The ethanol extract of the leaf had shown antioxidant activity and a high percentage of polyphenols and flavonoids (180.5±2.1 mg Globularia alypum extracts/g and 20.7 mg QE/g). A high correlation was observed between the total phenolic and flavonoid content and the antioxidant activity (R2=0.943 and R2=0.910, respectively). Gas chromatographic analysis showed the presence of 13 acids in this extract, mainly linoleic acid (30.1 %) and linolenic acid (24.8 %). The leaf powder of this plant is considered as a source of mineral elements even though, it is full of fatty acids in comparison to proteins and sugar. The ethanol extract of the leaf of Globularia alypum could be considered as a source of polyphenols and as an extract with potential antioxidant activity.
Keywords
Globularia alypum L, physicochemical characteristics, phenolic compounds, antioxidants
Globularia alypum L is a wild plant belonging to Globulariaceae family. It is a perennial shrub found throughout the Mediterranean area. The plant is used for various purposes [1,2]. In the Tunisian traditional pharmacopoeia, G. alypum L. locally named as Zrigua, is one of the most used traditional plant remedies. Its leaves are traditionally used as hypoglycaemic, laxative, cholagogue, stomachic, purgative and sudorific agent [3,4]. It is also used in the treatment of cardiovascular and renal diseases as displayed in ethnobotanical surveys. An infusion of G. alypum exhibited no toxicological effects but produced a significant hypoglycaemic activity in rats both by oral and intra-peritoneal administrations [5,6]. The ethanol extract has been investigated for chemical composition and antioxidant activity.
This plant is widely used as a curative for many diseases in addition to the fact that only few studies have been reported on the Algerian and Moroccan G. alypum L strain [7]. This has prompted us to investigate the ethanol extract of this plant and their major compounds. So far, important chemical investigations of G. alypum were reported by Merghache et al. [8] and Amessis-Ouchemoukh et al. [9], where the presence of some glycosidic iridoids (globularin), phenolic acids, flavonoids and lignan diglucoside were reported. In addition, Touaibia et al. [7] have reported the presence of secondary metabolites such as poly-phenols, flavonoids and anthocyanins. However, an analysis of primary metabolites and minerals such as Ca, Na, P, K has yet to be reported. Therefore, an attempt has been made to analyse the above components in this plant. In the present work, the physicochemical characteristics of the leaf extract powder, gas chromatographic analysis, estimation of the phenolic content and antioxidant activity of the ethanol extract of the leaf of G. alypum is reported.
Materials and Methods
Fresh leaves of G. alypum were collected in March 2015 from the North East of Tunisia (Jbel Zaghouan). Specimens were identified in the National Institute of Applied Sciences and Technology (INSAT, Tunisia) and voucher specimens were deposited at the Herbarium of the Department of Molecular Physico-chemical in the Preparatory Institute of Scientific and Technical Studies.
Physicochemical analysis and mineral concentration of the leaves powder
The whole plants were shade-dried in a well-ventilated area for 20 d. Thereafter, the leaves were isolated from the plant and crushed. The powder was stored in dark glass boxes. To determine water or moisture content [10], a quantity of leaves (5±0.01 g, M0) dried at ambient temperature for 15 d was further dried in an oven at 105° for 24 h to constant weight (Md). The mass of the dried leaves was determined using a precise balance to 0.01 g. The water content in dry and wet bases was determined by the following Eqns.: X = 100×(M0/Md)/ Md (water/kg of the dry matter); Xh = 100×(M0/Md)/M0 (water/kg of the moist matter). The ash content was determined by combustion of 500 mg of the sample in a muffle furnace at 550° for 4 h [11].
The mineral content was determined by atomic absorption spectroscopy [11] based on the concept of atoms in the state E0 can absorb photons of energy. The estimating number of absorbed photons can be related to the concentration of elements in the solution to be analysed. The concentrations of calcium (Ca), sodium (Na) and potassium (K) were determined by atomic absorption (atomic adsorption spectrophotometer 1100, Perkin Elmer Instruments, Bois d’Arcy, French), powered by an air acetylene flame (C2H2). Total phosphorus (P) content was determined using a molybdenum blue colorimetric method.
The total sugar content was measured by colorimetric assay according to the phenol-sulphuric acid method. This method relies on the formation of furfural compounds by heating the neutral monosaccharide in sulphuric acid. Associated with phenols, furfural derivatives formed yellow complexes whose absorbance is spectrophotometrically determined at 490 nm [12]. To assess protein content in the leaves, the Kjeldahl method was followed. A factor of 6.25 was used for the conversion from total nitrogen into crude protein [13]. All assays were carried out in triplicate, and the results were calculated as the mean±standard deviation (SD).
Fifty grams of the leaf powder was macerated during 72 h at room temperature with 500 ml of ethanol under continuous magnetic stirring. The extract was filtered using a 0.45-1 μm Millipore filter and evaporated under rotavap. The volatile fatty acids of the ethanol extract were analysed using a Hewlett Packard 5890 series II Gas chromatograph equipped with flame ionization detector (FID) and HP-5 MS capillary column (5 % phenyl; 95 % dimethylpolysiloxane: 30 m×0.25 mm id, film thickness 0.25 μm). The injector and detector temperatures were set at 250 and 280°, respectively. Oven temperature was kept at 50° for 1 min then, gradually raised to 250° at 5°/min and subsequently, held isothermal for 4 min. Nitrogen was the carrying gas at a flow rate of 1.2 ml/min. The percent of the constituents were calculated by electronic integration FID peak areas, without the use of response factor correction. The peak identification of various fatty acids on a chromatogram is made by comprising of their retention times to those of a mixture of fatty acids previously injected indicator. The percent of the various fatty acids were calculated from the peak area represented in the chromatogram. The peak area was large and the percent of acids was important. The percent of fatty acids was estimated using the Eqn. 3: fatty acids (%) = S/Σs×100, where, S is the peak area corresponding to the considered fatty acid, Σs is the sum of the areas of all peaks of a chromatogram corresponding to the fatty acid mixture.
Determination of total phenolic content by the Folin-Ciocalteu method
The amount of total phenolics of the sample was determined with Folin-Ciocalteu reagent using the method of Lister and Wilson [14]. A curve of gallic acid (ranging from 0.005 to 0.05 mg/ml) was prepared, and the results determined by the regression equation of the calibration curve (y= 6.94x–0.67, R2= 0.99). In short, 100 μl of sample (diluted to obtain absorbance in the range of prepared calibration curve) were dissolved in 500 μl (1/10 dilution) of the Folin-Ciocalteu reagent and 1 ml of distilled water. After 1 min of reaction at room temperature, 1.5 ml of 20 % sodium carbonate (NaCO3) solution was added. The final mixture was shaken thoroughly and then incubated for 2 h in the dark at room temperature. Absorbance of all samples was measured at 760 nm using a HACH UVVis spectrophotometer [15]. Tests were presented as milligram gallic acid equivalents per gram dry weight of raw material (mg G. alypum extract/g dry weight).
Determination of total flavonoids
The total flavonoids were determined according to the Dowd method adopted by Arvouet-Grand et al. [16] In short, 1 ml of each sample or standard was mixed with 4 ml of aluminium trichloride solution (AlCl3, 2 %). The absorbance was read at 415 nm after 15 min against a blank sample consisting of methanol (4 ml) and an extract (4 ml) AlCl3 free. Quercetin was used as a standard for the construction of calibration curve. The results were expressed as milligram quercetin equivalent/gram of dry mass.
Determination of anthocyanins
Total anthocyanins are water soluble pigments. The amount of anthocyanins contents was determined using the method of Pamino-Duran et al. [17] The mixture composed with 1 ml of samples and 9 ml of HCl was incubated at room temperature for 5 min. The absorbance of samples was measured at 520 nm by UV/ Vis spectrophotometer. The anthocyanins content is displayed in terms of milligram equivalent of cyanine per gram of dried mass (mg E Cy/g dried mass).
Determination of tannin content
The total tannin content in the ethanol extract was determined according to the vanillin method [18]. One milliliter of each extract solution (1 mg/ml) was placed in a test tube with vanillin (1 % in 7 M H2SO4, 2 ml) in an ice bath, and then incubated at 25°. The absorbance was read at 500 nm after 15 min. The concentration was calculated as microgram tannic acid equivalent per milligram of dry mass from a calibration curve.
Quantification of total antioxidant activity
The antioxidant activities of G. alypum were investigated using three complementary colorimetric methods namely 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), β-carotene bleaching and 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assays and compared to those of butylated hydroxyl toluene (BHT), vitamin E and vitamin C. DPPH free radical scavenging activity was determined according to a slightly-modified version of the method reported by Barros et al. [19]. In short, 1 ml of DPPH (0.1 mM) was dissolved in methanol and added to a 0.5 ml solution of G. alypum extract at different concentrations. Absorbance was measured at 517 nm after 30 min of incubation in the dark at room temperature. The blank was prepared from the relation mixture without DPPH solution. BHT and vitamin E were used as positive controls. The decrease in the absorption of the DPPH solution was calculated by the following Eqn. 4: DPPH radical scavenging activity (%) = [(A0-A1)/A0]×100, where A0 refers to the absorbance of the control reaction containing all reagents except the tested compound, and A1 to the absorbance of the test compound. Results were expressed as IC50 (μg/ml) values, referring to the amount of a sample necessary to decrease by 50 % the absorbance of DPPH radicals.
The antioxidant activity of ethanol extract of the leaf was evaluated according to a slightly-modified version of the β-carotene bleaching method [15,20]. A stock of β-carotene linoleic acid mixture was prepared by dissolving 0.2 mg of β-carotene in 0.5 ml of chloroform; 20 μl of linoleic acid and 200 mg of Tween 40 were added, too. After the evaporation of the chloroform under vacuum, using a rotary evaporator (Heidolph, Germany) at 45°, the mixture was immediately diluted with 10 ml of triple-distilled water and was mixed well for 1-2 min. Then, 50 ml oxygenated distilled water was added and the mixture was vigorously shaken. Aliquots (4 ml) of this emulsion were transferred into different test tubes containing 0.2 ml of test samples. BHT was used for comparative purposes. A control, containing 0.2 ml of corresponding solvent and 4 ml of the above emulsion, was prepared. The tubes were placed, at 50°, in a water bath. Absorbance of all samples at 470 nm were taken at zero time (t0). The measurement of absorbance was continued, until the colour of the β-carotene disappeared in the control reaction (t=180 min), at 15 min intervals. The mixture prepared as above mentioned, without β-carotene, served as blank. The antioxidant activity of the extracts was evaluated in terms of bleaching of the β-carotene using the following Eqn.: % inhibition = 100× [1-(A0- At)/(A00-At0)], where A0 and A00 are the absorbance values measured at zero time of the incubation for test sample and controlled, respectively. At and At0 are the absorbance measured in the test sample and controlled, respectively, after incubation for 180 min [15].
ABTS.+ radical scavenging activity of extracts was determined according to Re et al. [21]. In this test, the relative capacity of antioxidants was measured to scavenge the ABTS.+ radical compared to the antioxidant potency of L(+) ascorbic acid (vitamin C) used as a standard. Thus, the ABTS.+ radical was freshly prepared by adding 5 ml of a 4.9 mM potassium persulfate (K2S2O8) solution to 5 ml of a 14 mM ABTS solution and kept for 16 h in the dark. Before usage, this solution was diluted to get an absorbance of 7.00±0.020 at 734 nm with PBS as a blank at pH 7.4 (5 mM NaH2PO4, 5 mM NaHPO4 and 154 mM NaCl). The final reaction mixture of standard group was made up to 1 ml with 950 μl of ABTS.+ solution and 50 μl of vitamin C. Similarly, in the test group, 1 ml of reaction mixture comprised 950 μl of ABTS.+ solution and 50 μl of the extract solution. The reaction mixture was vortexed for 10 s and the absorbance at 734 nm was recorded each minute after initial mixing. Appropriate solvent blanks were run in each assay, and all measurements were done within at least 6 min. The results, determined from regression equation of the calibration curve (y= 0.0446x-0.0076, R2= 0.987), were suggested as milligram ascorbic acid per gram of dry weight of raw material (mg EVC/g dry weight). All the determinations were performed in triplicates. The statistical differences between phenols, flavonoids and antioxidant activity values of the extracts were calculated out using the Excel program. The differences between measurements were considered significant at p<0.05. Correlations coefficients (R2) to determine the relationship between two variables were calculated using MS Excel software.
Results and Discussion
The physicochemical analysis of the leaves powder has never been realized (Table 1). Indeed, this first analysis showed that the leaves of G. alypum are rich in mineral elements such as calcium, sodium, potassium and phosphor (22.4; 302; 4.81 and 445 mg/kg of dry powder). In addition, the plant can supply an amount of energy of about 2405 kcal/kg of the dry powder. However, fat represents the lowest percent (3 %) in comparison with the organic material (protein: 6.2 % and sugar: 65.5 %). Due to its high energetic power and the presence of minerals in the G. alypum leaves, we can use it as food preparation or as additives in minerals and calories poor food. The fatty acid composition of G. alypum leaves was determined by GC. The results of this analysis are presented in Table 2. Indeed, Chromatographic analysis of the fat leaves of G. alypum identified 13 compounds that showed a total of 99.54 %.
| Analysis parameter | Values |
|---|---|
| Humidity (%) | 9.46±0.001 |
| Dry extract (%) | 90.54±0.13 |
| Ashes (%) | 5.5±0.001 |
| Protein (%) | 6.20±0.02 |
| Fat (%) | 3.05±0.03 |
| Crude fibre (%) | 10.28±0.02 |
| Non nitrogenous dry extract (sugar, %) | 65.5±0.031 |
| P (mg/Kg) | 445±0.21 |
| Ca (%) | 2.24±0.01 |
| Na (mg/Kg) | 302±0.32 |
| K (mg/Kg) | 4810±1.02 |
| Metabolic energy (Kcal/Kg) | 2405±1.23 |
Determination of the content of humidity, total solids, total ash, raw protein, total lipids, crude fibre and quantification of minerals (mg/kg), from the ash of G. alypum samples collected in Zaghouan
Table 1: The content of the ash of G. alypum samples collected in Zaghouan
In Table 2, the identification of compounds has allowed classifying in saturated fatty acids (SFA) and unsaturated fatty acids (UFA). This analysis has showed that UFA (69.31 %) is more abundant than SFA (30.23 %). The majority of UFA is linoleic (30.1 %) and linolenic (24.8 %). In other hand, the palmitic (15.9 %) and oleic (11.3 %) are the two major SFA. However, minor acids are palmitoleic, margaric (0.8 %), ginkgolic (0.84 %), cis vaccenic (0.95 %), gadoleic (0.9 %).
| Fatty acid (%) | Ethanol extract | |
|---|---|---|
| SFA | Lauric acid Myristic acid Palmitic acid Margaric acid Ginkgolic acid Stearic acid Arachidic acid |
3.54 2.94 15.91 0.82 0.84 3.62 2.56 |
| Total | - | 30.23 |
| UFA | Palmitoleic acid Gadoleic acid linolenic acid Oleic acid Cis vaccenic acid Linoleic acid |
1.26 0.9 24.8 11.3 0.95 30.1 |
| Total | - | 69.31 |
SFA: saturated fatty acid, IFA: unsaturated fatty acid
Table 2: Chemical composition of the ethanol extract of the leaf of G. alypum
The analysis of the chemical composition has revealed that the major compounds (linoleic and linolenic acid) have important biological activities. Therefore, linolenic acid eliminates chronic inflammation [22]. Other research studies showed the effectiveness of linolenic acid in the treatment of rheumatoid arthritis and other inflammatory disorders [23]. Numerous studies [22,24] have demonstrated that supplementation of linolenic acid tended to reduce pain, swelling and tenderness of the joints and reduce the need for antiinflammatory treatment.
It is worth noting that linoleic acid exhibits some interesting biological properties. According to Troegeler-Meynadier and Enjalbert [25], linoleic acids are capable of limiting the indication and the extension of malignant tumours stimulating immune functions and promoting growth, particularly bone development. Furthermore, they amplify peripheral tissue utilization of lipids and carbohydrates and simultaneously lower the corresponding metabolic pathways of synthesis and storage, as well as they decrease cholesterolemia and eicosanoid production. Therefore, the conjugated linoleic acid would be used for treatment or prevention of obesity, atherosclerosis and diabetes mellitus.
Despite the low lipid compounds (3.05 %) in the leaves of G. alypum, they are rich of fatty acids with biological wealth. Linolenic and linoleic acids are the major’s compounds of the leaves of G. alypum reveal the weightiness of the leaves with lipid fractions. Our study is an initial analysis of the lipid composition of G. alypum.
Total phenolics, flavonoids, anthocyanins and tannins present in the extract to the extent of 180.5±2.1 mg G. alypum extract/g of dry mass (Table 3). Our results are in agreement with those reported by Touaibia et al. [7] in which the amount of total phenol is 139 mg G. alypum extract/g of dry mass from the macerated ethanol extract of G. alypum leaves. In addition, Taghzouti et al. [26] have obtained 67.55 and 50.99 mg G. alypum extract/g of dry mass from macerated leaves methanol and ethyl acetate extracts, respectively. Djeridane et al. [27], have shown 21.54 mg G. alypum extract/g dry mass from a hydroethanol extract of G. alypum. The variability in phenolic between our result and other results could be explained by the variation between the extracting solvents. Indeed, many studies have reported that polar fractions have more phenolic amounts [28,29].
| Type of extract | Total phenolic mg GAE/g |
Flavonoids mg EQ/g |
Anthocyanins mg Cy/g |
Tannins mg TA/g |
Reference |
|---|---|---|---|---|---|
| Hydroethanol | 247.24 | 78.82 | 0.53 | - | [30] |
| Soxhlet water ethanol | 21.54 | - | - | - | [27] |
| Macerated methanol Macerated hydroacetate |
67.55 50.99 | 25.9 8.96 | - - | - - | [26] |
| Macerated ethanol | 139 | 19.26 | 35.10 | 5.64 | [7] |
| Macerated ethanol | 180.5±2.1 | 20.7±0.7 | 30.5±0.8 | 8.68±0.18 |
Milligram of gallic acid equivalent per g of dry plant extract (mg GAE/g); mg QE/g: mg of quercitin equivalent per g of dry plant extract; mg Cy/g: mg of cyanine equivalent per g of dry plant extract; mg TA/g: mg of tannic acid per g of dry plant extract
Table 3: Comparison of secondary metabolites of leaves of G. alypum
Concerning the content of flavonoids, the G. alypum exhibited 20.7 mg QE/g of dry mass. This result coincides with that of Touaibia et al. [7], (19.26 mg QE/g of dry mass) for the same sample using the same procedure. Taghzouti et al. [26], obtained 25.9 and 8.96 mg QE/g of dry mass from methanol and ethyl acetate extracts of leaves, respectively. However, the results of Ben Mansour et al. [30], showed that the amount of flavonoids in a leaves water-ethanol extract (20:80) was 78.82 mg QE/g of dry mass. This increase in concentration could be explained by the higher polarity of this extract.
The content of anthocyanins in G. alypum is 30.5 mg Cy/g dry mass. This concentration is lower than Touaibia et al. [7], (35.10 mg Cy/g dry mass). However, Ben Mansour et al. [30], obtained 0.53 mg Cy/g dry mass of hydroethanolic leave extracts. The amount of tannins in the G. alypum was higher than the results of Touaibia et al. [7], (8.68 and 5.64 mg TA/g of dry mass, respectively).
The variability in phenolic, flavonoid, tannin and anthocyanin amounts for our results and other studies [7,26,27,30] could be attributed to several biological factors, including genotypic differences, as well as other edaphical and environmental parameters, such as maturation stages, salinity, temperature, water stress and light intensity conditions.
The total antioxidant activities of vegetables cannot be evaluated by any single method, due to the complex nature of phytochemicals [31]. Two or more methods should always be used in order to evaluate the total antioxidative effects of vegetables. Accordingly, the antioxidant activities of the G. alypum were determined by three different methods: DPPH, ABTS and β-carotene bleaching test. The half maximal inhibitory concentrations (IC50) of the G. alypum, the BHT and the vitamin E are determined graphically at the concentration-dependent part of each plot. Results are presented in Table 4.
| IC50 (µg/ml)a | mg EVC/g dry weight | ||
|---|---|---|---|
| DPPH | β-carotenea | ABTSa | |
| GAp | 29.8±0.20* | 42.53±0.24* | 89.5±0.32* |
| BHTb | 31.7±0.32 | 35.87±0.21* | - |
| Vitamin Eb | 22.66±0.21* | - | - |
| Vitamin Cb | - | - | 25.76±0.23* |
The antioxidant activity was determined using DPPH, β-carotene bleaching and ABTS methods. Milligram equivalent of vitamin C/g dry weight: mg EVC/g dry weight. aValues were expressed as mean±SE (n=3), bPositive controls. Only the statistical differences between ethanol extract and BHT (DPPH) are shown as non-significant: p<0.05
Table 4: The antioxidant activity of ethanol extract of the leaf of Tunisian G. alypum
The presence of different antioxidant components in the plant tissues makes it relatively hard to quantify each antioxidant one separately. Therefore, in many studies, several intermediate extractions were used to ensure a maximum extraction of the available antioxidants [32]. The antioxidant activity of phenolics is mainly due to their redox properties, which make them act as reducing as agents, as hydrogen donors and singlet oxygen. They also may have a metallic chelating potential [33].
The determination of antioxidant activity in vitro with DPPH samples to donate hydrogen is checked by using the free radical DPPH. It is one of the known mechanisms by which antioxidants inhibit lipid peroxidation. The amount of sample needed to decrease the initial DPPH concentration by 50 % (IC50) is a parameter widely used to measure the antioxidant activity. A lower value of IC50 indicates a higher antioxidant power.
The investigated extract displays an ability to scavenge the stable DPPH free radical reaching 50 % of reduction with an IC50 value of 29.8 μg/ml, which is comparable to the IC50 determined by the BHT as a positive control (31.7 μg/ml). However, the scavenging activity of this extract is low when compared to the vitamin E reference standard (22.66 μg/ml) used at the same dose. Phytochemical analysis of the ethanolic extract (Table 3) reveals the presence of phenolic compounds and flavonoids to which are attributed the antioxidant properties, due to their hydrogen donation ability and their structural requirement considered to be essential for effective radical scavenging.
It has been shown by Ben Mansour et al. [30] that hydroethanol extract of G. alypum exhibited remarkable antioxidant effect, evaluated using the DPPH assay. In fact, the total phenolic and flavonoid contents in the hydroethanol extract of G. alypum were significantly higher (247.24 mg G. alypum extract/g dry matter and 78.82, respectively) than those of the ethanol extract (180.5 mg G. alypum extract/g dry matter and 20.7, respectively).
The variability observed in the antioxidant activities of G. alypum shown by many researchers could presumably be attributed to the differences in the methodology and the experimental conditions used, as well as to the use of vegetables from different geographical regions, grown in different climatic and storing conditions, differences in extraction procedures, sample processing or drying. During the processing of extracts, some active volatile compounds may have been destroyed or evaporated [34].
The potential of G. alypum to inhibit lipid peroxidation was evaluated using the β-carotene/linoleic acid bleaching test, which measures the extracts capacity for inhibiting the conjugated diene hydroperoxide formation upon linoleic acid oxidation. Results presented in Table 2 and 4 showed that G. alypum and BHT inhibit the linoleic acid oxidation. G. alypum is efficient to inhibit the linoleic acid oxidation with IC50 value of 42.53 μg/ml. Concentrations required for G. alypum to provide 50 % inhibition are compared to BHT as a reference standard. The results have indicated that G. alypum exhibits lower antioxidant activity than BHT. Many researchers have reported that BHT is more potent than the plant extracts [35-37].
The determination of antioxidant activity in vitro with ABTS method of G. alypum to scavenge free radicals is also assessed by their ability to quench ABTS.+. The antioxidant activity measurement of ethanol extract, expressed as mg equivalent of vitamin C/g dry weight is 89.5. This high value would imply greater antioxidant activity capacity to scavenge the ABTS.+ radical cation. Using the ABTS assay, our extract has shown a higher antioxidant activity compared to the aqueous ethanol extract of G. alypum (70:30, v/v) [27].
The analysis of the antioxidant activity by different methods can explain the strongly radical potential of G. alypum. For this reason, it has been reported those antioxidants possessed diverse biological activities such as anticarcinogenic, antidiabetic and antiinflammatory. On the other hand, the antiradical properties and the presence of secondary metabolites suggested that G. alypum might have several applications in human health care.
In food industry, antioxidants can also be used to retard the oxidative degradation of fats by inhibiting the formation of free radicals. Leaf powder G. alypum can replace synthetic antioxidants such as BHA and BHT. Therefore, the use of natural antioxidants as preservatives in food would have great potential due to the requirement of consumers to additive-free, fresher and more natural food.
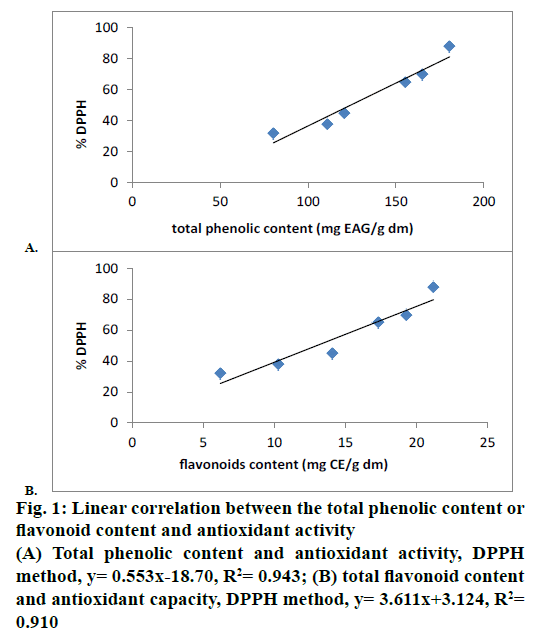
Furthermore, the content of phenols in G. alypum correlated well with the antioxidant activity leading to a possibility that phenols are likely to contribute to the radical scavenging activity of this plant extract. The correlation between the total phenolics, flavonoids and antioxidant activity ranges between R2= 0.943 and R2= 0.91, respectively (Figure 1A and B). This result suggested that between 94.3 and 91 % of the antioxidant capacity of extract is due to the contribution of total phenolics and flavonoids. The antioxidant effects, evaluated by the DPPH, correlate more with phenol contents than flavonoids.
It has been shown by Broadhurst et al. [38] that polyphenols, which are present in medicinal herbs, spices, vegetables, fruits and beverages have been used to treat many human diseases such as diabetes, cancer and coronary heart diseases. In other hand, flavonoids have been shown to exhibit the antioxidative, antiviral, antimicrobial and antiplatelet activities [39]. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which made them act as reducing agents, hydrogen donors, and singlet oxygen quenchers. They may also have a metallic chelating potential [33]. Therefore, the high contents of phenolic compounds and significant linear correlation between the value of phenolic concentration and flavonoid compounds and antioxidant activity indicated that these compounds contribute to the strong antioxidant activity.
In this study, the analysis of the leaf powder has shown it’s the richness in mineral elements such as calcium, sodium and phosphor. In addition, the ethanol extract of G. alypum leaves has a powerful ability to inhibit oxidation. The protective effect of this extract is higher with widely used synthetic antioxidants BHT, vitamin C and E. Thus, ethanol extract from G. alypum leaves could be prepared and added in food preparations, marketed in cosmetic and pharmaceutical industries as a natural antioxidant and a suitable alternative for some synthetic antioxidant.
Acknowledgements
The authors acknowledge the support of Dr. Boussaid Mohammed, National Institute of Applied Sciences and Technology (INSAT, Tunisia) in identifying the leaves of G. alypum.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Sezik E, Tabata M, Yesilada E, Honda G, Goto K, Ikeshiro Y. Traditional medicine in north-east Anatolia. J Ethnopharmacol 1991;35:191-6.
- Jouad H, Haloui M, Rhiouani H, El Hilaly J, Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J Ethnopharmacol 2001;77:175-82.
- Allali H, Benmahdi H, Dib MA, Tabti B, Ghalem S, Benabadji N. Phytotherapy of diabetes in west Algeria. Asian J Chem 2008;20:2701-10.
- Baba Aïssa F. Encyclopédie des plantes utiles. Flore d’Algérie et du Maghreb. Substances végétales d’Afrique, d’Orient et d’Occident". Alger: Librairie Moderne Rouiba, EDAS; 1999. p. 368.
- Skim F, Lazrek BH, El Amri H, Kaaya A, Jana M. Toxicological studies on Globumaria alypum and Zygophyllum gaetulum in rats. Phytother Res 1998;12:592-4.
- Skim F, Kaaya A, Jaouhari TJ, Lazrek HB, Jana M, El Amri H. Hypoglycaemic activity of Globularia alypum leaves in rats. Fitoterapia 1999;70:382-9.
- Touaibia M, Chaouch FZ. Global chemical composition and antioxidant effect of the ethanol extracts prepared from Globularia alypum leaves. Nat Technol 2016;14:2-6.
- Merghache S, Zerriouh M, Merghache D, Tabti B, Djaziri R, Ghalem S. Evaluation of hypoglycaemic and hypolipidemic activities of Globularin isolated from Globularia alypum L. in normal and streptozotocin-induced diabetic rats. J Appl Pharm Sci 2013;3:1-7.
- Amessis-Ouchemoukh N, Abu-Reidah IM, Quirantes-Piné R, Rodríguez-Pérez C, Madani K, Fernández-Gutiérrez A, et al. Tentative characterisation of iridoids, phenylethanoid glycosides and flavonoid derivatives from Globularia alypum L. (Globulariaceae) leaves by LC-ESI-QTOF-MS. Phytochem Anal 2014;25:389-98.
- AOAC Method 950.01. Official methods of analysis of the association of official analytical chemists.15th ed. Arlington: Association of Official Analytical Chemists; 1990.
- AOAC Method 960.52. Official Methods of Analysis.16th ed. Washington: Association of Official Analytical Chemists;1997.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for determination of sugars and related substances. Anal Chem 1956;28:350-6.
- Murica MA, Martinez-Tome M, Vera AM, Morte A, Gutiérrez A, Honrubia M, et al. Effect of industrial processing on desert truffles Terfezia claveryi and Picoa juniper vitt: Proximate composition and fatty acids. J Sci Food Agric 2003;83:535-41.
- Lister E, Wilson P. Measurement of total phenolics and ABTS assay for antioxidant activity (personal communication). Lincoln, New Zealand: Crop Research Institute; 2001.
- Hayouni EA, Abedrabba M, Bouix M, Hamdi M. the effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem 2007;105:1126-34.
- Arvouet-Grand A, Vennat B, Pourrat A, Legret PP. Standardisation d’un extrait de propolis et identification des principaux constituants. J Pharm Belg 1994;49:462-8.
- Pamino-Duran EA, Giusti MM, Wrolstad RE, Gloria MBA. Anthocyanins from Oxalis triangularis as potential food colorants. Food Chem 2001;75:211-16.
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Food Agric 1978;29:788-94.
- Barros L, Baptista P, Ferreira ICFR. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem Toxicol 2007;45:1731-7.
- Pratt DE. Natural antioxidants of soybean and other oil-seeds. In Simic MG, Karel M, editors, Autoxidation in food and biological system. New York: Plenum Press; 1980. p. 283-92.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:123-7.
- Zurier RB, Rossetti RG, Jacobson EW. Gamma-linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheumatol 1996;39:1808-17.
- DeLuca P, Rothman D, Zurier R B. Marine and botanical lipids as immunomodulatory and therapeutic agents in the treatment of rheumatoid arthritis. Rheum Dis Clin N Am 1995; 21:759-77.
- Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care 2001;4:115-21.
- Troegeler-Meynadier A, Enjalbert F. Les acides linoléiques conjugués: Intérêts biologiques en nutrition. Revue Méd Vét 2005;156:207-16.
- Taghzouti OK, Balouiri M, Ouedrhiri W, Ech chahad A, Rommane A. In vitro evaluation of the antioxidant and antimicrobial effects of Globularia alypum L. extracts. J Mater Environ Sci 2016;7:1988-95.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 2006;97:654-60.
- Harzallah JH, Neffati A, Skandrani I, Maaloul E, Chekir- Ghedira L, Mahjoub T. Antioxidant and antigenotoxic activities of Globularia alypum leaves extracts. J Med Plants Res 2010;4:2048-53.
- Khlifi D, Hamdi M, El Hayouni A, Cazaux S, Souchard JP, François Couderc, et al. Global chemical composition and antioxidant and antituberculosis activities of various extracts of Globularia alypum L. (Globulariaceae) Leaves. Molecules 2011;16:10592-603.
- Ben Mansour R, Gargouri B, Gargouri B, Elloumi N, Ben Haj Jilani I, Gharbi-Gammar Z, et al. Investigation of antioxidant activity of alcoholic extract of Globularia alypum L. J Med Plants Res 2012;6:4193-9.
- Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric 2000;80:561-6.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha J, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 1999;47:3954-62.
- Rice-Evans C, Miller NJ, Paganga G. Structure antioxydant activity relationshipi of flavonoids and phenolic acids. Free Radic Biol Med 1996;20:933-56.
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal 2006;41:1523-42.
- Sarikurkcu C, Tepe B, Daferera D, Polissiou M, Harmandar M. Studies on the antioxidant of the essential oil and methanol extract of Marrubium globosum subsp globosum (lamiaceae) by three different chemical assays. Bioresour Technol 2008;99:4239-46.
- Tosun M, Ercisli S, Sengul M, Ozer H, Polat T. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol Res 2009;41:175-81.
- Wu Z, Li C, Lv S, Zhou B. Pantothenate kinase-associated neurodegeneration: insights from a Drosophila model. Hum Mol Genet 2009;18:3659-72.
- Broadhurst CL, Polansky MM, Anderson RA. Insulin-like activity of culinary and medicinal plant aqueous extracts in vitro. J Agr Food Chem 2000;48:849-52.
- Fang Tian, Bio Li, Baoping Ji, Jinhua Yang, Guizhi Zhang, Yang Chen, et al. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinesis: the polarity affects the bioactivities. Food Chem 2009;113:173-9.