- *Corresponding Author:
- Meenakshi Patel
Babaria Institute of Pharmacy
BITS Edu Campus, Varnama
Vadodara-391240
Affiliated to Gujarat Technological University
Gujarat, India
E-mail: meenakshipatel.bip@bitseducampus.ac.in
| Date of Received | 10 September 2020 |
| Date of Revision | 07 February 2021 |
| Date of Acceptance | 28 March 2021 |
| Indian J Pharm Sci 2021;83(2):297-306 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present study was to prepare the floating matrix tablet of glipizide by applying Simplex lattice design using kappa carrageenan, Hydroxypropylmethylcellulose K15M and sodium bicarbonate as independent variable. The similarity factor (f2), time to release 50 % of drug and time to release 90 % of drug were taken as dependent factors. A total of 14 batches were prepared as given by Design-Expert® Software (version- 9.0.6, Stat-Ease) and the response obtained for the dependent variables was statistically evaluated. Radiological study was performed on healthy albino rabbits for checking the gastroretention for optimized floating tablet of glipizide. The results indicated that the formulation G-simplex lattice design 8 with 50 mg of Hydroxypropylmethylcellulose, 30 mg of kappa carrageenan and 10 mg of sodium alginate is the optimized formulation with highest desirability. The in vivo X-ray imaging study clearly revealed that the optimized formulations remained afloat in gastric fluid up to 12 h in the stomach of rabbit, which shows the possibility of the formulation to remain in the stomach of human being for the desired period. It was concluded that the simplex lattice design is suitable for efficiently optimizing the floating matrix tablet of glipizide.
Keywords
Simplex lattice design, Glipizide, gastroretentive, mixture design, floating tablet
The development of innovative pharmaceutical formulation by trial and error practise is very time consuming and requires huge amount of money. Due to these reason, the pharmaceutical industry has turned to investigate different methodologies in the advancement of novel drug delivery systems. The optimization techniques, by statistical analysis provide an economical and effective method for the prediction of the optimal composition of dosage form. That is the reason of tremendous increase in the use of Design of experiment (DoE), in research and development in pharmaceutical industries. Statistical designs, DoE, have been applied for the development and optimization of many pharmaceutical products[1]. Literature revealed that DoE has also been applied for the development of various gastroretentive formulations[2-4]. In the present study, optimization of Glipizide (GLP) gastroretentive matrix tablet has been done by simple lattice mixture design. Mixture design has been previously explored for the optimization of various types of pharmaceutical preparations[5-8]. A simplex lattice design (SLD) is a type of mixture design used to determine the relative proportion of ingredients that optimizes a formulation with respect to a specified variable(s) or outcome. This technique has been already employed for the design and optimization of floating dosage form of Dipyridamole[9].
The literature review suggested that GLP is weakly acidic in nature with pKa value equal to 5.9, which means that the drugs remains unionized at acidic pH[10,11]. The unionization is the prerequisite for the drugs to get absorbed by passive diffusion mechanism. Hence, the gastroretentive dosage form of GLP is desired. The elimination half-life of GLP is 2-4 h, which demands frequent administration of drug, to maintain its level in the body for extended period of time. Gastroretentive dosage form overcomes that demerit by releasing the drug continuously in the upper part of gastrointestinal tract, thereby achieving the better control of plasma glucose level[12,13].
The exhaustive literature research elucidates that gastro retentive formulations of GLP have been prepared using several approaches[14-16], but optimization by SLD has not been explored yet. In present research work, an attempt is made to prepare the floating matrix tablet of GLP by applying SLD. The formulation was prepared by effervescence mechanism using the combination of hydrophilic polymer Hydroxypropylmethylcellulose (HPMC) K15M with anionic polymer kappa carrageenan and sodium bicarbonate as gas forming agent. The combination of hydrophilic polymer and anionic polymer has been earlier explored by the authors for the metformin drug[17].
Materials and Methods
Materials
Glipizide was obtained as a gift sample from MicroLabs Mumbai. Sodium bicarbonate was procured from Sulab Reagents, Suvidhinath laboratories, Vadodara, kappa-carrageenan was procured from Rajesh Chemicals, Vadodara, Hydroxy Propyl Methyl Cellulose K15M was procured from Astron Chemicals, Ahmedabad. All other excipients were of analytical grade, obtained from the local market.
Experimental design
SLD Mixture design was used to optimize the gastroretentive floating matrix tablet of Glipizide. A simplex lattice is a system of equally spaced dots on a simplex (Lachman et al., 1970). Simplex designs provide an optimal distribution; hence the experiments should be well spread over the factor space. The design indicates the experimenting points in the factor space that allows an easy estimation of the parameters.
The preliminary studies suggested that HPMC K15 M and κ-Carrageenan can be successfully be used for the formulation of floating matrix tablets of GLP with desired release profile. These release retarding polymers have the capacity of releasing the drug for 12 h and had desired floating characteristics. Hence, these polymers were considered as independent variables along with sodium bicarbonate as gas forming agent for the final optimization of floating matrix tablet of GLP. The levels of the independent variable was decided based on the literature survey and by the experimentation done during the preliminary studies. SLD was used to optimize the gastroretentive formulation of GLP.
The SLD for three component system is presented by an equilateral triangle in two-dimensional space[6,7,18]. The general purpose of mixture design is to implement mathematical model to explain the response as a function of the composition of the mixture, by applying limited number of experiments[19]. The earlier studies have proved that SLD is a very efficient tool in optimization of gastroretentive matrix tablet[20]. In this study, the quantities of release retarding agents, hydroxypropylmethyl cellulose K15M (X1) and kappa- Carrageenan (X2), gas generating agent [sodium bicarbonate (X3)], were selected as independent elements with the total weight as 90 mg. Similarity factor f2 (%), time required for 50 % drug release (t50) and time required for 90 % drug release (t90) were claimed as dependent elements (Table 1). Design- Expert® Software (version- 9.0.6, Stat-Ease) was used to evaluate the design and total 14 experiments were run. In all the batches, the amount of other variables was kept constant with 10 mg Glipizide, 10 mg polyvinyl pyrrolidone (PVP) K 30, 38.5 mg microcrystalline cellulose and 1.5 mg magnesium stearate.
| Independent Variables/Levels | Quantity of HPMC K15M | Quantity of k-Carrageenan | Quantity of sodium bicarbonate |
|---|---|---|---|
| X1 (mg) | X2 (mg) | X3 (mg) | |
| Low | 50 | 20 | 10 |
| High | 60 | 30 | 20 |
| Dependent Variables | Y1 ? Similarity factor % Y2 ? Time required for 50 % drug release (t50) Y3 - Time required for 90 % drug release (t90) |
||
No. of replicates 4
Table 1: Variables and their Levels in Simplex Lattice Design
Preparation of GLP Floating Matrix Tablets
Tablets containing 10 mg of Glipizide were made by wet granulation technique with the concentrations given in Table 2[21,22]. The required quantities of HPMC K15M, kappa carrageenan and sodium bicarbonate were sieved through sieve number #80 and were thoroughly mixed in a mortar by following geometric order. Then, the required quantity of microcrystalline cellulose was added and the mixture was filled in plastic bottle. These bottles were placed in double cone blender and the equipment was run for 5 min. After the set time, the powder blend was put in mortar and the granulation was performed using PVP K30 as granulating agent. The mixture was blended properly with granulating fluid to form a dough mass. The mass was passed through mesh No. 10 to obtain wet granules. The wet granules were dried by keeping in hot air oven at 60º for an hour. The dried granules were passed through mesh No. 16 to break aggregates and then sieved through sieve no. 40 to separate granules and fines. The magnesium stearate (1 %) and (10 %) fines were added to dry granules and blended in double cone blender after enclosing into a closed plastic bottle. The granules were then compressed into tablets on rotary tablet compression machine, using 7 mm round and flat punches with the hardness of 5 kg/sq.cm.
| Runs | Batch code | Transformed Fractions of Variables* | Responses | |||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Lag time (s) |
Similarity factor f2 (%) | Time required for 50 % (h) | Time required for 90 % (h) | ||
| 1 | G-SLD 1 | 50 | 20 | 20 | 12.35±3.21 | 60 | 4.29±0.09 | 11.43±0.83 |
| 2 | G-SLD 2 | 56.6667 | 21.6667 | 11.666 | 39.16±2.54 | 61 | 4.95±0.17 | 9.47±0.16 |
| 3 | G-SLD 3 | 55 | 20 | 15 | 8.63±2.31 | 43 | 5.51±0.29 | 11.49±0.31 |
| 4 | G-SLD 4 | 55 | 25 | 10 | 90.43±4.52 | 56 | 4.61±0.18 | 9.77±0.49 |
| 5 | G-SLD 5 | 60 | 20 | 10 | 83.53±5.12 | 60 | 4.72±0.07 | 9.48±0.29 |
| 6 | G-SLD 6 | 60 | 20 | 10 | 85.53±4.21 | 61 | 4.63±0.08 | 9.21±0.34 |
| 7 | G-SLD 7 | 50 | 20 | 20 | 13.87±1.63 | 48 | 4.41±0.17 | 11.32±0.98 |
| 8 | G-SLD 8 | 50 | 30 | 10 | 20.42±1.12 | 70 | 3.92±0.09 | 10.01±0.72 |
| 9 | G-SLD 9 | 51.66 | 21.66 | 16.666 | 9.77±1.43 | 48 | 5.21±0.21 | 12.24±0.92 |
| 10 | G-SLD 10 | 50 | 25 | 15 | 22.40±2.19 | 47 | 5.36±0.15 | 9.59±0.59 |
| 11 | G-SLD 11 | 51.66 | 26.66 | 11.666 | 40.22±3.55 | 64 | 4.32±0.06 | 10.0±0.48 |
| 12 | G-SLD 12 | 55 | 25 | 10 | 88.46±5.21 | 55 | 4.43±0.17 | 9.71±0.44 |
| 13 | G-SLD 13 | 50 | 30 | 10 | 22.18±1.47 | 69 | 3.89±0.21 | 10.31±0.28 |
| 14 | G-SLD 14 | 53.33 | 23.333 | 13.333 | 31.96±2.63 | 58 | 4.88±0.18 | 11.2±0.28 |
Table 2: Composition and Results of GLP Matrix Tablets Formulated by Applying SLD
In vitro Buoyancy Studies
The floating behaviour of the tablets was observed visually, in triplicate, as per the floating lag time method explained by Rosa et al.[23]. Concisely, a tablet was put in a beaker, containing 200 ml of dissolution fluid, maintained in a water bath at 37±0.5º. The floating lag time, ‘‘the time between tablet was placed in a glass beaker with Hydrogen chloride (HCl) and its buoyancy” and total floating duration, ‘‘the time during which tablet remains buoyant”, were recorded.
In vitro Drug Release Studies
Drug release studies of the GLP floating tablets were performed, in triplicate, in a USP Dissolution Tester Apparatus, type- II (Paddle method) at 37±0.5º. The speed of rotation of paddles was kept as 100 rpm. The tablets were placed into 500 ml of 0.1N HCl solution (pH 1.2). At different time intervals, aliquots of 5 ml were withdrawn from the dissolution apparatus and filtered through a cellulose acetate membrane (0.45 μm). The drug content was determined at a wavelength of 275 nm by UV spectrophotometer. At each time of withdrawal, 5 ml of fresh medium was replaced into the dissolution flask, to maintain the sink condition. The release of the prepared gastroretentive formulations was compared with the sustained release marketed formulation (GLYTOP-SR) of the drug, using model independent method by calculating similarity factor[24,25]. Similarity factor means the comparison of resemblance in the release pattern of two comparative formulations. Generally a similarity factor in the range of 50-100 is acceptable according to the (United States Food and Drug Administration) USFDA. It can be calculated using the following equation:
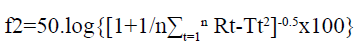
Where, n is the number of dissolution sample times, Rt and Tt are the individual or the mean percent dissolved at each time point, t, for the reference and test dissolution profiles, respectively.
Drug Excipient Compatibility Study
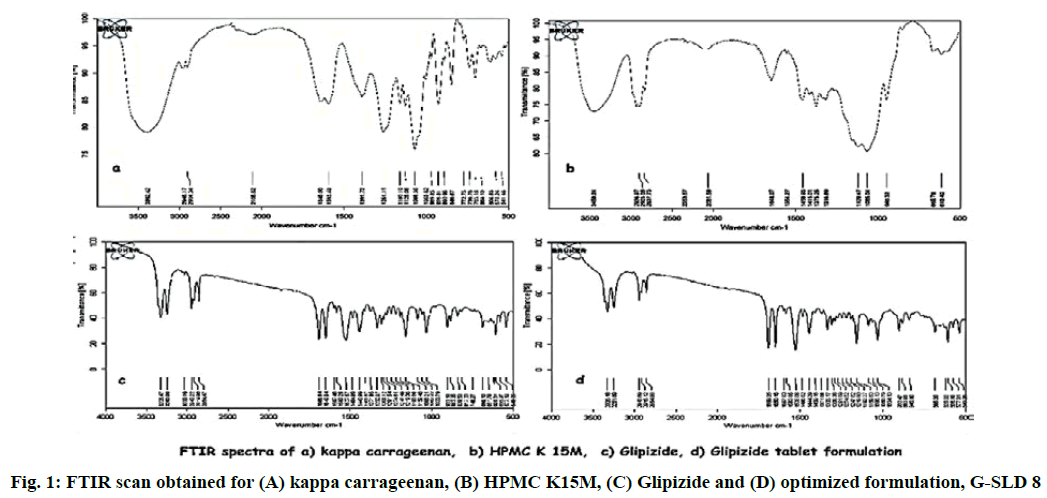
There is always the possibility of drug polymer interaction in any formulation. To check any such kind of interaction, Fourier-transform infrared spectroscopy (FTIR) study was conducted[26]. The FTIR scan of pure drug (Glipizide), polymers (HPMC K15M and kappa carrageenan) and physical mixture of drug-polymer were taken. The pure drug, polymer and physical mixture were separately mixed with IR grade KBr. This mixture was punched to form a disc, which was scanned over a wave number range of 4000 to 400 cm-1.
Validation of Model
To determine and validate the reliability of the mathematical models constructed in the present experiment, additional three formulations, suggested by the design expert, were developed. The anticipated results of the software were compared with the observational results obtained after the evaluation of check point batches, utilizing the mathematical models. Table 3, shows the values of the factors used for development of the validation batch, taken from the software. The amount of all other ingredients was kept constant.
| Factors | Composition | ||
|---|---|---|---|
| F 1 (mg) | F 2 (mg) | F 3 (mg) | |
| X1 : Amount of HPMC K15M | 52.03 | 55.81 | 58.51 |
| X2 : Amount of k-Carrageenan | 23.33 | 23.33 | 21.49 |
| X3 : Amount of sodium bicarbonate | 14.64 | 10.86 | 10.00 |
Table 3: Formula for Validation Runs of SLD design for the optimization of GLP Floating Matrix Tablets
To validate the applied Simple Lattice design, the experimental values of the responses were quantitatively compared with predicted values and the relative error (%) was estimated using the following equation.
Relative error (%) =Predicted value-Experimental value/Predicted value×100
In vivo Radiographic Studies
The gastroretentive formulation has to be evaluated for its gastroretentive property in vivo. There are various techniques like, radiographic study, gastroscopy, gamma scintillography, magnetic marker monitoring, etc. available to confirm the gastroretention of the formulation[27]. The in vivo radiographic studies were conducted on healthy albino rabbits (n=3) weighing 2.0 kg to 2.2 kg. The protocol (BIP/IAEC/2015/05) for this study was approved by the Institutional Animal Ethical Committee (IAEC) in accordance with guidance of Committee for the purpose of control and supervision of experiments on animals (CPCSEA). Gastroretentive floating matrix tablet was prepared by incorporating the X-ray opaque material in the optimized formula by replacing MTG with barium sulphate and keeping all other ingredients constant[28]. The quantity of barium sulphate was kept sufficient to ensure visibility by X-ray, but at the same time its amount was low enough to allow the formulation to float. After overnight fasting, the formulation was given to albino rabbit for in vivo X-ray imaging study. A radiograph was taken just before the administration of the tablet, at 0 h, to ensure the absence of radio opaque material in the stomach. During the study, the rabbit was not allowed to eat, but water was available freely and the X-ray images were snapped after 4 h and 12 h to monitor the gastroretention of optimized floating matrix tablets[29,30].
Result and Discussion
The gastroretentive floating matrix tablets of GLP were prepared with HPMC K15M as release retarding polymer in combination with kappa carrageenan by wet granulation technique. It is proven that tablets prepared with wet granulation technique offers delayed release. Chowdary et al., did a comparative study of the effect of methods for the preparation of tablets on the dissolution rate of the drug[11]. They established that the disintegration rate of the tablets prepared by direct compression technique was a lot more eminent than the tablets prepared by of wet granulation technique. This proves that wet granulation increases the cohesiveness between the particles and hence delays the release of the drug from the polymeric matrix. The granulation makes the polymeric matrix more compact and increases the adhesion between the particles. It also ensures the proper distribution of the contents in all the batches.
The FTIR scan of drug, polymers and physical mixture of drug and polymer was taken. FTIR scan of glipizide showed characteristic peaks at 1649, 2943.22, 3325.47, 1527.57, 1688.84 cm-1 corresponding to C═N aliphatic group, C─H2 aliphatic, N─H stretching of NH2, C─H aliphatic and C═O stretching respectively. No such peaks were observed in the FTIR scan of polymers. All these peaks were observed in the infrared spectra obtained from drug polymer blend, which demonstrates that there is no significant incompatibility between the drug and the other polymers (fig. 1).
SLD is a reliable method to optimize the process or the formulation where the total of the independent variables is to be kept fixed[31,32]. This technique was applied for the optimization of GLP matrix tablet. The quantities of release retarding agents, hydroxy propyl methyl cellulose K15M and kappa-Carrageenan, gas generating agent (sodium bicarbonate), were selected as independent elements in optimization of floating matrix tablet of GLP by simplex lattice design.
The result of all the dependent variables is given in Table 2. A statistical model, including 14 interactive terms was used to measure the effects.
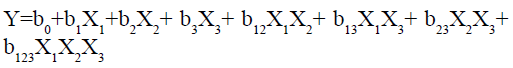
Where, Y is the dependent variable, b0 is arithmetic mean response of the 14 runs and b1 is the estimated coefficient for the factor X1 and so on. The primary effects (X1, X2 and X3) represent the intermediate effect of varying one element at a time from its low to high value. The interaction terms (X1X2, X2X3, X1X3 and X1X2X3) give the information about how the response changes when two or more elements are simultaneously modified. The values for Similarity factor f2 (Y1), Time required for 50 % drug release (t50) (Y2), Time required for 90 % drug release (t90) (Y3) 14 batches (G-SLD1- G-SLD14) is presented in Table 2. The results showed that the values of the dependent variable are getting significantly affected by the independent variables.
All the prepared formulations offered satisfactory floating lag time in the range of 8 to 90 s, which indicated that the selected independent variables had no substantial impression on the dependent variables. The formulations released 50 % of the drug in the time range of 3.89 to 5.51 h and released 90 % of the drug in the time range of 9.48 to 12.24 h.
Using analysis of variation (ANOVA), the implication (p˂0.05) of the ratio of mean square variation due to the regression coefficient, and the residual error were tested (Table 4). The Special Cubic Mixture model was found to be imperative for Y1 and Y2 responses, whereas Special Quartic Mixture model was followed by Y3 [21]. The high values of correlation coefficients for similarity factor f2 (R2=0.9443), t50 (R2=0.9643) and t90 (R2=0.9887) indicated a good agreement between the dependent and independent variables. The desired irrelevance of Lack of Fit was proved by F-value for Y1, Y2 and Y3 as 0.5410, 0.1048 and 0.2216 respectively.
| Source | Sum of Squares | Degree of freedom | Mean Square | F-Value | P-value | |
|---|---|---|---|---|---|---|
| Similarity factor % (f2) | ||||||
| Model | 643.69 | 6 | 107.28 | 6.33 | 0.0042 | significant |
| Linear Mixture | 301.37 | 2 | 150.68 | 8.89 | 0.0120 | |
| X1X3 | 173.23 | 1 | 173.23 | 10.22 | 0.0151 | |
| X2X3 | 158.15 | 1 | 158.15 | 9.33 | 0.0185 | |
| X1X2X3 | 187.16 | 1 | 187.16 | 11.04 | 0.0127 | |
| Residual | 118.67 | 7 | 16.95 | |||
| Lack of Fit | 45.67 | 3 | 15.22 | 0.83 | 0.5410 | not significant |
| Corrected Total | 762.36 | 13 | ||||
| Time to release 50 % of drug (t50) | ||||||
| Model | 3.05 | 6 | 0.51 | 31.54 | 0.0001 | significant |
| Linear Mixture | 0.96 | 2 | 0.48 | 29.88 | 0.0004 | |
| X1X3 | 0.91 | 1 | 0.91 | 56.31 | 0.0001 | |
| X2X3 | 1.22 | 1 | 1.22 | 75.59 | < 0.0001 | |
| X1X2X3 | 0.25 | 1 | 0.25 | 15.47 | 0.0057 | |
| Residual | 0.11 | 7 | 0.016 | |||
| Lack of Fit | 0.085 | 3 | 0.028 | 4.06 | 0.1048 | not significant |
| Corrected Total | 3.16 | 13 | ||||
| Time to release 90 % of drug (t90) | ||||||
| Model | 11.95 | 8 | 1.49 | 54.93 | 0.0002 | significant |
| Linear Mixture | 7.01 | 2 | 3.50 | 128.79 | < 0.0001 | |
| X1X3 | 0.99 | 1 | 0.99 | 36.38 | 0.0018 | |
| X2X3 | 1.16 | 1 | 1.16 | 42.76 | 0.0013 | |
| X12X2X3 | 0.54 | 1 | 0.54 | 19.79 | 0.0067 | |
| X1X2X32 | 1.71 | 1 | 1.71 | 62.92 | 0.0005 | |
| Residual | 0.14 | 5 | 0.027 | |||
| Lack of Fit | 0.047 | 1 | 0.047 | 2.09 | 0.2216 | not significant |
| Corrected Total | 12.09 | 13 | ||||
Table 4: ANOVA Table for Response Parameters for Simple Lattice Design Model for GLP Gastroretentive Floating Matrix Tablets
Similarity Factor f2
The similarity factor f2, given by Scale Up and Pose Approval Changes (SUPAC) guidelines for modified release dosage form was applied to compare dissolution profiles of developing GLP floating matrix tablets with marketed sustained release formulation of GLP[33]. The dissolution profiles are similar when f2 is between 50 and 100. The method was first reported by Moore and Flanner[34].
The statistical analysis of the results obtained for the similarity factor, of all the prepared formulations, was done by Design Expert. The result can be expressed for model investigation by Scheffe’s special cubic Mixture model, which is an extension of quadratic model. After observing the results of F statistics, it was detected that model probability was greater than F value i.e. 6.33, which approves the significance of the model. The model was proved to be significant as the p-value was found to be less than 0.0500. In this case X1, X2, X3, X1X3, X2X3, X1X2X3, are critical model terms.
The fitted equation for the responses is given as follows:
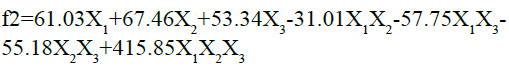
As the value of correlation coefficient was found to be high, the obtained polynomial equations can be used to get the implications after considering the scale of coefficient and the mathematical sign that it clutches. The conclusions can be drawn after perceiving the value of coefficient and the mathematical sign it carries (i.e. positive or minus). After looking into the above equation, it is obvious that the chosen independent factors, Amount of HPMC K15M (X1), kappa-carrageenan (X2) and sodium bicarbonate (X3) show significant optimistic effects on similarity factor (f2) of the designed floating matrix tablets of glipizide. It was detected that X2 had more significant effect on the similarity factor. This means, more the concentration of k-Carrageenan, more the drug release pattern of prepared floating matrix tablets of glipizide was matching the release pattern of marketed sustained release table of GLP. The interaction was found to be substantial and an appropriate combination of the three variables is necessary to get the maximum f2 value.
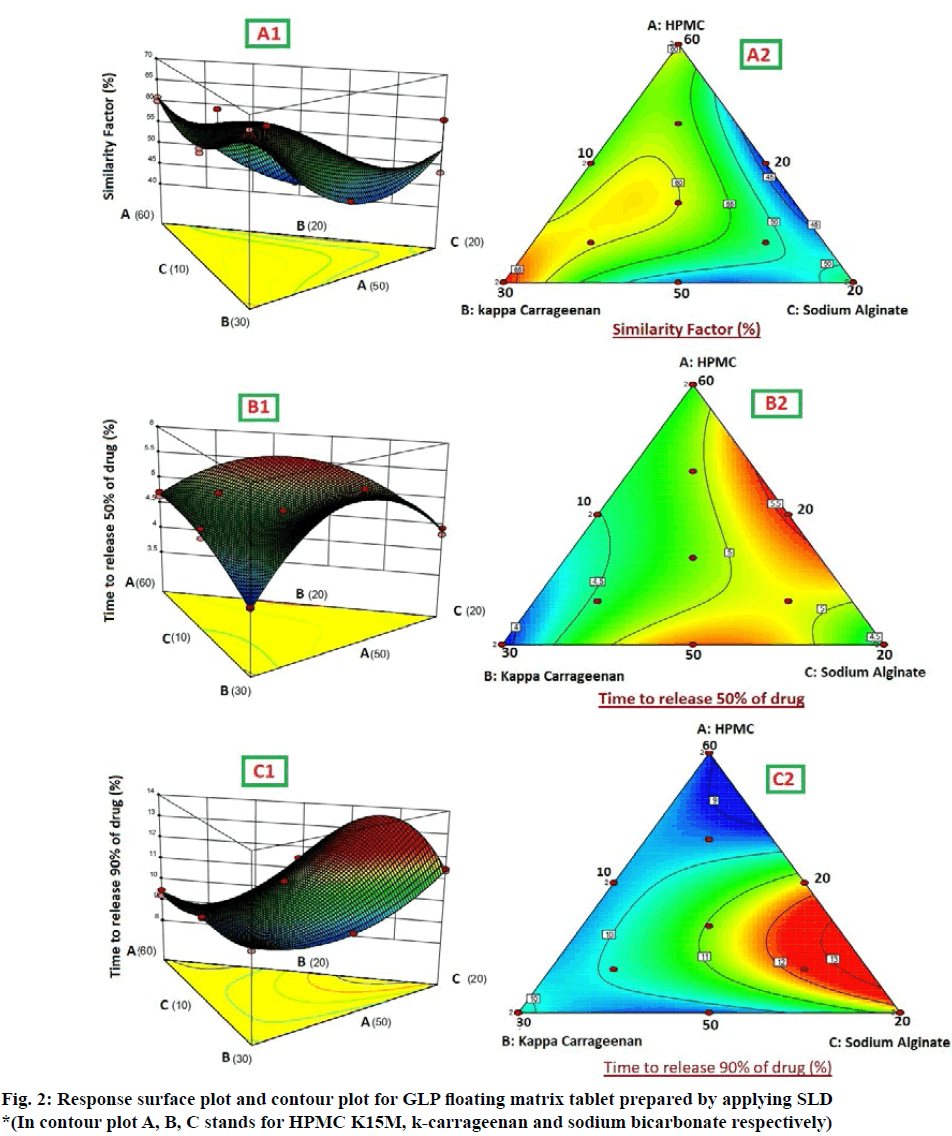
There was antagonistic effect of variables in two-dimensional plane indicating the significant interaction between the variables. This means that on changing the two variables simultaneously, the interaction was observed and that decreased the similarity factor value. However, the most significant coefficient with highest magnitude was when all the three factors were modified simultaneously; it had agonistic effect on Y1. The predicted and observed values of the similarity factor were identified to be similar, which further authenticates the fitness of the model. The three-dimensional response surface graphs for similarity factor given in fig. 2, shows the acquired contour plot (A2) and response surface plots (A1). This gives the information about the main and interaction effects of the independent components. It can be clearly seen that maximum similarity value, above 65 % is obtained in the portion with highest value of k-carrageenan. The results for Y3 could have been better if the higher value of X2 variable would have been increased beyond the existing level.
The ANOVA results for the applied model for time to release 50 % of drug are shown in Table 4. The results of F statistics detected that model probability was greater than F value i.e. 31.54, which approves the significance of the model. The significance of the model was also confirmed by the p-value less than 0.0500. In this case X1, X2, X3, X1X3, X2X3, X1X2X3, are significant model terms. The result can be presented for model analysis by Scheffe’s special cubic model using following equation:
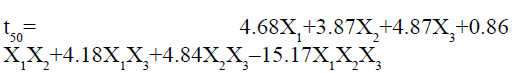
It is evident from the above equation that the chosen factors, Amount of HPMC K15M (X1), kappacarrageenan (X2) and sodium bicarbonate (X2) have optimistic effects on time to release 50 % of drug of the prepared gastroretentive floating matrix tablets of glipizide. The agonistic effect of the factors on the time for 50 % drug release was in the order of X2˃X1˃X2. The most significant factor was the amount of gas forming agent. The positive interaction effect was detected between the independent factors. There was a synergistic effect shown by X2 and X2 and there was strongest antagonistic effect observed when all the three factors were changed simultaneously. The three dimensional surface response graphs for time to release 50 % of drug are given in fig. 2. It shows the obtained response surface plots (B1) and contour plot (B2). This gives the information about the main and interaction effects of the independent factors. The contour plot indicated that maximum value for Y2 is obtained with minimum quantity of k-carrageenan and HPMC K15M. However, the sweet spot will be obtained only after putting constrains for the responses.
The ANOVA results for the applied model, on time to release 90 % of drug, are shown in Table 4. The results of F statistics showed that model probability was greater than F value i.e. 54.93, which approves the importance of the model. Also, the p-value was less than 0.0500, which further confirms the significance of the model. In this case X1, X2, X2, X1X2, X2X2, X1 2X2X2, X1X2X22, are significant model terms. As the cubic model was aliased, the result can be expressed by Special quartic model using following equation:
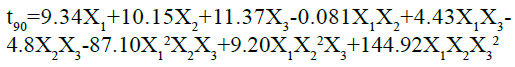
As the value of correlation coefficient was found to be high, the obtained polynomial equations was used to find the inferences after considering the scale of coefficient and the sign it carries. It is evident from the above equation that amount of HPMC K15M (X1), kappa-carrageenan (X2) and sodium bicarbonate (X2) shows optimistic effects on time to release 90 % of drug of the prepared floating matrix tablets of GLP. The agonistic effect of the factors on the time for 90 % drug release was in the order of X2˃X2˃X1. The interaction values in the equation indicated that there is non-significant effect when X1 and X2 are modified simultaneously. However, there was strong ternary antagonistic interactions observed at higher level of HPMC K15M and sturdiest synergistic effect was shown by a ternary interaction of X1X2X2 at higher level of sodium bicarbonate (X2). The dependent factor, time to release 90 % of drug, shown by three-dimensional response surface graphs (fig. 2), shows the obtained contour plot (C2) and response surface plots (C1). This gave the information about the key and interaction effects of the independent components by giving different color codes.
To authenticate the mathematical models built here with Simple Lattice design, additional three formulations, suggested by the design expert, were formulated. These check point batches were prepared according to the formula given by design expert and then evaluated for getting the experimental values of responses. The comparison between the experimental and predicted values was done and the relative errors (%) between the values for each response were calculated. The values were found to be within 5 % relative error, which confirms the validity of the model.
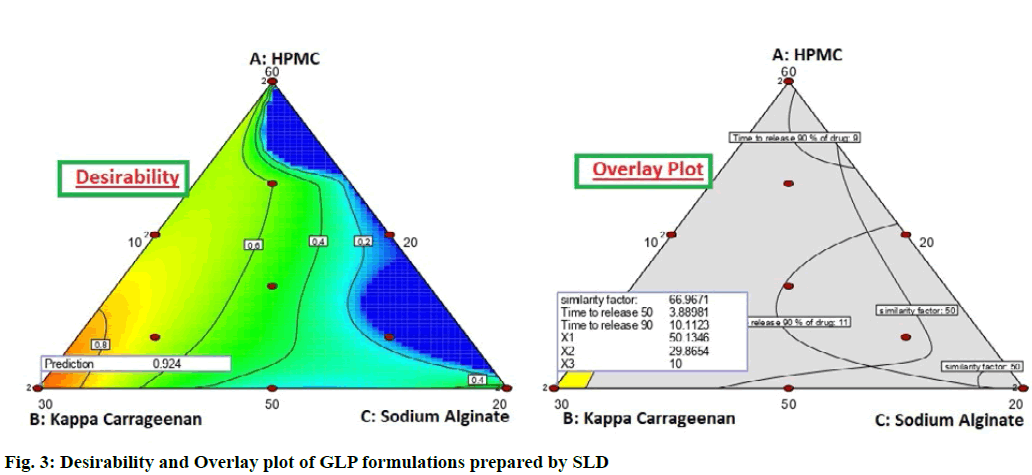
To optimize all the above responses with different targets, a numerical optimization technique by the desirability function and a graphical optimization technique by the desirability and overlay plot were used. The overlay plot distinguished between the operating window and undesired region by applying different colour codes as yellow and grey region respectively (fig. 3). The operating window means that the concentrations of the independent factors, within the yellow region are perfect to develop the formulation with a desired release profile of the drug. It is evident from the overlay plot that the minimum amount of HPMC K15M and gas generating agent, sodium bicarbonate is sufficient to give the desired effect. The results indicated that fomulation G-SLD 8 and G-SLD 13 (with same composition) fullfilled the desiarablity criteria and hence were considered as optimized formulation of floating matrix tablet of GLP.
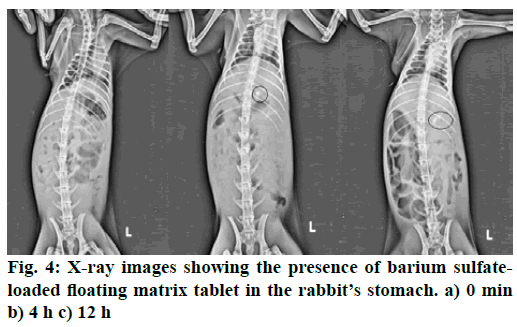
To determine the retention time of the optimized floating matrix tablets of GLP inside the body, radiographic studies were conducted. The barium Sulfate loaded tablets, prepared with optimized formula of matrix tablet, were given to rabbits. The X-ray photomicrographs were taken before and after administering the barium sulphate tablet to rabbits. Fig. 4 shows the X-ray images taken at 0, 4 and 12 h time period. The images clearly indicated that the tablets remained afloat in gastric fluid for up to 12 h in the stomach of rabbit. Hence, it can be predicted that the developed floating matrix tablet of GLP would show the gastroretentive behavior for 12 h when taken orally.
Floating matrix tablet of glipizide was also prepared using the combination of hydrophilic polymer HPMC K15M with anionic and non-ionic polymers. The final optimization of floating glipizide formulation was done by applying SLD using kappa carrageenan, HPMC K15M and sodium bicarbonate as independent variable. The levels of the variables were decided from preliminary studies and the tablets were prepared by wet granulation technique using PVP K30. The similarity factor (f2), time to release 50 % (t50) of drug and time to release 90 % (t90) of drug were taken as dependent factors. The design was employed and evaluated using the Design-Expert® Software (version- 9.0.6, Stat-Ease) by running 14 experiments. The overlay plot depicted that the minimum amount of gas generating agent is sufficient to give the desired effect with minimum concentration of HPMC K15M, whereas the amount of kappa carrageenan should be maximum. The optimum values of selected variables were found to be 50 mg of X1, 30 mg of X2 and 10 mg of X2 and this formulation showed highest desirability. The obtained result was contradictory to that of the results attained with the metformin gastroretentive floating tablet, prepared with the same combination of independent variable, where the minimum quantity of k-carrageenan was required to get the desired effect. The radiographic studies confirmed the gastroretentive behaviour of optimized formulation for 12 h.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Singh B, Chakkal SK, Ahuja N. Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS PharmSciTech 2006;1;7(1):E19-28.
- Gaur PK, Mishra S, Kumar A, Panda BP. Development and optimization of gastroretentive mucoadhesive microspheres of gabapentin by Box–Behnken design. Artif Cells Nanomed Biotechnol 2014;42(3):167-77.
- Jagdale S, Kurhe P, Kuchekar B, Chabukswar A. Application of design of experiments to optimizing novel gastroretentive drug delivery of simvastatin. Curr Drug Deliv 2013;10(5):527-41.
- Venkateswarlu K, Chandrasekhar KB. Development and statistical optimization of sustained release gastro retentive floating tablets of cephalexin. Marmara Pharm J 2016;20(2):172-83.
- Duangjit S, Mehr LM, Kumpugdee-Vollrath M, Ngawhirunpat T. Role of simplex lattice statistical design in the formulation and optimization of microemulsions for transdermal delivery. Biol Pharm Bull 2014;37(12):1948-57.
- Gohel MC, Parikh RK, Aghara PY, Nagori SA, Delvadia RR, Dabhi MR. Application of simplex lattice design and desirability function for the formulation development of mouth dissolving film of salbutamol sulphate. Curr Drug Deliv 2009;6(5):486-94.
- Suhesti TS, Fudholi A, Martien R. Application of Simplex Lattice Design for the Optimization of the Piroxicam Nanosupensions Formulation using Evaporative Antisolvent Technique. Int J Pharm Clin Res 2016;8(5):433-9.
- Aatish T, Nishith P, Patel MB. Formulation and optimization of acyclovir floating tablet. J Pharm Sci Bio Sci Res 2014;4:216-21.
- Mandlik SK, Adhikari S, Deshpande AA. Application of Simplex Lattice Design in Formulation and Development of Buoyant Matrices of Dipyridamole. J Appl Pharm Sci 2012;2(12):107-11.
- Foster RH, Plosker GL. Glipizide. Pharmacoeconomics 2000;18(3):289-306.
- Chowdary KR, Rao NK, Malathi K. Ethyl cellulose microspheres of glipizide: Characterization, in vitro and in vivo evaluation. Indian J Pharm Sci 2004;66(4):412.
- Robinson JR, Li HK, Lee VH. Influence of drug properties and routes of administration on design of sustained and controlled release systems. Controlled drug delivery: Fundamentals and applications. 2nd ed. New York: Marcel Dekker Inc 1987;29:5-12.
- Patel JK, Patel RP, Amin AF, Patel MM. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS PharmSciTech 2005;6(1):E49-55.
- Prabhu P, Harish NM, Gulzar AM, Yadav B, Narayana CR, Satyanarayana D, Subrahmanayam EV. Formulation and in vitro evaluation of gastric oral floating tablets of glipizide. Indian J Pharm Educ Res 2008;42(2):174-83.
- Tripathi M, Radhika PR, Sivakumar T. Formulation and evaluation of glipizide hollow microballoons for floating drug delivery. Bull Pharm Res 2011;1(1):67-74.
- Mallikarjun V, Ravi P, Babu VR, Kiran G, Kumar MS. Design and evaluation of Glipizide floating tablets. J Pharm Res 2009;2(4):691-3.
- Patel MB, Shaikh F, Patel V, Surti NI. Application of simplex centroid design in formulation and optimization of floating matrix tablets of metformin. J Appl Pharm Sci 2017;7(04):023-30.
- Bolton S. Statistical applications in the pharmaceutical sciences. The Theory and Practice of Industrial Pharmacy. 3rd ed. Bombay: Varghese Publishing House 1987;243-289.
- Huisman R, Van Kamp HV, Weyland JW, Doornbos DA, Bolhuis GK, Lerk CF. Development and optimization of pharmaceutical formulations using a simplex lattice design. Pharm Weekbl Sci 1984;6(5):185-94.
- Patel DM, Patel NM, Pandya NN, Jogani PD. Gastroretentive drug delivery system of carbamazepine: formulation optimization using simplex lattice design: a technical note. AAPS PharmSciTech 2007;8(1):E82-6.
- Prajapati ST, Patel LD, Patel DM. Studies on formulation and in vitro evaluation of floating matrix tablets of domperidone. Indian J Pharm Sci 2009;71(1):19.
- Garg R, Gupta GD. Preparation and evaluation of gastroretentive floating tablets of silymarin. Chem Pharm Bull 2009;57(6):545-9.
- Jiménez-Castellanos MR, Zia H, Rhodes CT. Design and testing in vitro of a bioadhesive and floating drug delivery system for oral application. Int J Pharm 1994;105(1):65-70.
- Yuksel N, Kanık AE, Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and-independent methods. Int J Pharm 2000;209(1):57-67.
- Costa P, Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13(2):123-33.
- Pani NR, Nath LK, Acharya S, Bhuniya B. Application of DSC, IST, and FTIR study in the compatibility testing of nateglinide with different pharmaceutical excipients. J Therm Anal Calorim 2011;108(1):219-26.
- Parikh DC, Amin AF. In vitro and in vivo techniques to assess the performance of gastro-retentive drug delivery systems: a review. Expert Opin Drug Deliv 2008;5(9):951-65.
- Patel A, Modasiya M, Shah D, Patel V. Development and in vivo floating behavior of verapamil HCl intragastric floating tablets. AAPS PharmSciTech 2009;10(1):310-5.
- Sharma OP, Shah MV, Parikh DC, Mehta TA. Formulation optimization of gastroretentive drug delivery system for allopurinol using experimental design. Expert Opin Drug Deliv 2015;12(4):513-24.
- Shakya R, Thapa P, Saha RN. In vitro and in vivo evaluation of gastroretentive floating drug delivery system of ofloxacin. Asian J Pharm Sci 2013;8(3):191-8.
- Cornell JA. Experiments with mixtures: an update and bibliography. Technometrics 1979;21(1):95-106.
- Gorman JW, Hinman JE. Simplex lattice designs for multicomponent systems. Technometrics 1962;4(4):463-87.
- US Food and Drug Administration, 1997. Guidance for industry SUPAC-MR: Modified release solid oral dosage forms scale-up and post approval changes: Chemistry, manufacturing and controls. in vitro Dissolution Testing and in vivo Bioequivalence Documentation.
- Moore JW. Mathematical comparison of dissolution profiles. Pharm Technol 1996;20:64-75.