- *Corresponding Author:
- Yuan Zhao
Department of Foreign Language, Shaanxi University of Chinese Medicine, Xianyang 712044, China
E-mail: zhaoyuan029@outlook.com
| Date of Received | 28 February 2022 |
| Date of Revision | 23 November 2022 |
| Date of Acceptance | 10 March 2023 |
| Indian J Pharm Sci 2023;85(2):369-375 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Chronic subdural hematoma is identified as an intracranial hemorrhagic disorder of human. Presently, neither surgical nor symptomatic treatment of Western medicine has achieved good curative effects. This study aimed to clarify efficacy of Peiyuan-Huayu formula on neovascularization and explore associated mechanisms. Rats were intragastricly administrated with Peiyuan-Huayu formula and drug containing serum was harvested. Cell counting kit 8 assay was used to determine proliferation of human microvascular endothelial-1 cells. Human microvascular endothelial-1 cells were divided into negative group, blank-serum group and drug-serum group. In vitro scratch healing assay was employed to evaluate migration distance of human microvascular endothelial cells-1. Tube-like structures formation based on human microvascular endothelial-1 cells was conducted using tube formation assay on matrigel. Expression of vascular endothelial growth factor A, mitogen-activated protein kinase kinase, phosphorylation of mitogen-activated protein kinase kinase and phosphorylation of extracellular signal-regulated kinase was determined with western blotting. The 15 % drug-serum is optimal concentration for inhibiting human microvascular endothelial cells-1 proliferation. Peiyuan-Huayu formula drug-serum remarkably inhibited migration of human microvascular endothelial cells compared to Blank-serum group (p<0.05). Peiyuan-Huayu formula drug-serum obviously suppressed angiogenesis compared to blank-serum group (p<0.05). Peiyuan-Huayu formula drug-serum down-regulated vascular endothelial growth factor A expression, activated phosphorylation of mitogenactivated protein kinase kinase and induced phosphorylation of extracellular signal-regulated kinase 1/2 in human microvascular endothelial-1 cells. In conclusion, Peiyuan-Huayu formula inhibited proliferation, migration and angiogenesis of human microvascular endothelial-1 cells. Effects of Peiyuan-Huayu formula were mediated by reducing expression of mitogen-activated protein kinase kinase 1/2, phosphorylation of mitogen-activated protein kinase kinase 1/2, phosphorylation of extracellular signal-regulated kinase 1/2 and vascular endothelial growth factor molecule.
Keywords
Chronic subdural hematoma, Peiyuan-Huayu formula, angiogenesis, vascular endothelial growth factor A, traditional Chinese medicine
Chronic Subdural Hematoma (CSDH) is identified to be an intra-cranial hemorrhagic disorder of human, especially for the elderly human[1,2]. According to the previous report, the incidence for CSDH has been achieved to about 1-13 out of 100 000 individuals annually, with higher incidence of 127.1 out of 10 000 in individuals aged more than 80 y old[3]. The symptoms for CSDH are usually non-specific. For CSDH patients, the headache is the most common discomfort and may be accompanied by weakness of limbs, difficulty in balance and memory disorder[4]. Some CSDH patients have no obvious postoperative discomfort, however, this doesn't rule out the possibility of recurrence[5]. Therefore, it is necessary to consider the complex pathogenesis and a variety of clinical factors for comprehensive treatment of CSDH patients. Presently, neither surgical treatment nor symptomatic treatment of Western medicine has achieved good curative effect and recovery, therefore, the multi-target treatment of Chinese medicine has been widely concerned[6,7]. In recent years, the combination of Traditional Chinese Medicine (TCM) and postoperative treatment has also experienced clinical tests, which has inhibited the recurrence of CSDH and has been greatly affirmed by the industry.
TCM formula has been extensively applied in the clinical practice in China for a few thousands of years because of the obvious efficacy and lower side-effects[8]. TCM formula is mainly characterized by the multiple ingredients, multiple disorder targets and synergy effects[9,10]. Peiyuan- Huayu (PYHY) formula, as a classical TCM arrests bleeding, improves blood circulation, benefits vital energy (Qi) and control blood[1]. Therefore, PYHY has been applied for treating the hemorrhagic disorders and also acted as a necessary ingredient in adjuvant treatment for the intracranial hemorrhage[11].
To our best known, there are even no study focusing on the therapy of CSDH using TCM and exploring the associated mechanisms. Therefore, this study attempted to clarify the efficacy of PYHY treatment on neovascularization in Human Microvascular Endothelial Cells (HMEC) and explore associated mechanisms.
Materials and Methods
Animals and cells:
Total of thirty Specific-Pathogen Free (SPF) male Wistar rats (License No: scxk2019-0014), aging 8 w old and weighing 250-300 g, were purchased from Xi'an Keao biological Co., Ltd. (Xi'an, China). The rats were fed in animal room of Experimental Center of Shaanxi University of TCM (Xi'an, China), at 25° and 35 % humidity with illumination lasted for 12 h. The rats freely accessed to the food and water. HMEC (Cat No. zq0456) were purchased from Shanghai Zhongqiao Xinzhou Company (Shanghai, China). HMEC-1 cells were cultured in the medium (Cat. No. zq-1304, Shanghai Zhongqiao Xinzhou Company) at 37°, gas phase and 5 % CO2.
This study has been approved by Ethical Committee of Shanxi University of Chinese Medicine, Xi'an, China. This study was also conformed to the NIH guidelines for usage of laboratory animals. This study was performed in animals in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Preparation for drug-containing serum:
PYHY was provided by the Chinese Medicine Room of Affiliated Hospital of Shaanxi University of TCM. The dosage of PYHY met the requirements of Chinese Pharmacopoeia. According to body weight control table of human and rat, the dosage of the drug was converted and prepared.
Intragastric dosage of experimental rats=clinical dosage of human×rat/adult equivalent dose coefficient (according to body surface area).
PYHY was concentrated and decocted with TCM, and the final concentration was 2 g/ml and stored at 4° for obtaining drug-containing serum. Thirty rats were numbered by random number table method and divided into 5 groups with 6 rats per group, including group A (drug-serum deriving from 0.5 h drugtreated), group B (drug-serum deriving from 1 h drugtreated), group C (drug-serum deriving from 1.5 h drug-treated), group D (drug-serum deriving from 2 h drug-treated) and group E (serum deriving from mice without drug treatment). After 1 w of adaptive feeding, mice in A, B, C and D group were administrated with PYHY at dosage of 12 g/kg/d, twice a day. While, the mice in group E was administrated with the same amount of normal saline for 3 d. The mice were fasting for 12 h before the last PYHY administration on the 3
d. The peripheral blood was collected from abdominal aorta at 0.5, 1, 1.5 and 2 h post the last administration. After collecting the arterial blood, the blood was kept at 4° for 30 min and centrifuged at 4000 r/min for 15 min. The supernatants of blood (serum) post centrifugation were collected in sterile eppendorf tube and inactivated in 56° water bath for 30 min. Then, the serum was filtered with a 0.22 μm microporous membrane and stored at -20° for following experiments.
Cell Counting Kit 8 (CCK-8) assay for determining cell proliferation:
The HMEC-1 cells were seeded at density of 5×105 HMEC-1 cells/ml and cultured in the 96-well plates for 24 h at 37°. Then, the HMEC-1 cells were incubated with a series of concentrations of PYHY drug-serums (5 %, 10 %, 15 %, 20 %, 25 %, 30 %, 35%, 40 %, 45 % and 50 %) for 24 h. The cell viability of HMEC-1 cells was determined using CCK-8 kit (Cat. No. AR1199, Boster Bio. Tech. Co. Ltd., Wuhan, China) as instructed by protocol of manufacturer. The absorbance for the 96-well plates was directly examined with a microplate reader (Model: ELX- 800, Bio-Tek Inc., Winooski, VT, USA) at 450 nm. All the CCK-8 experiments were repeated for more than three times.
Experiment grouping:
For the HMEC-1 cells, which were divided into three groups, including negative group, blank-serum group and drug-serum group. The cells in negative group were treated with only cell culture medium. The cells in blank serum group and drug-serum group were treated with serum deriving from mice without drug treatment and serum deriving from mice treating with drug.
In vitro scratch healing assay:
Migration ability is one of the main markers of cell activity. In vitro scratch healing assay was carried out as described by a previous study[12]. Briefly, HMEC-1 cells were seeded at density of 5×105 cells/ ml, incubated with 100 ng/ml VEGF, and cultured in 6-well plates at 37° overnight. When the HMEC- 1 cells were fully adhered to the plates, the excess medium was removed, and the horizontal line was vertically marked with a 100 μl sterile gun head to scratch. Then, cells were washed with PBS twice to remove the dropped cells. Finally, photographs for scratch wound were captured under an inverted microscope (Model: CX40M, Shunyu Bio., Ningbo, China), at 12 h and 24 h post the cell culture and the scratch areas were measured with an Image J software.
Tube formation assay:
The tube-like structures formation based on HMEC- 1 cells on matrigel was conducted as described by a form study by Lee et al.[13]. In brief, the 24-well plates were coated using the matrigel as the described by the instruction of manufacturer. The HMEC-1 cells were cultured in medium containing 1 % fetal bovine serum at 37° for 6 h. Then, the HMEC-1 cells were incubated in the matrigel coated plates and cultured for 24 h at 37 ?. The tube formation in HMEC-1 cells was observed with an inverted microscope. The numbers of tube (lumens) and tube areas were observed and recorded at 3 h post cultures, and analyzed using the Image J software.
Western blot assay:
The HMEC-1 cells were cultured in medium for 24 h at 37° and harvested for obtaining the total protein of cells in negative group, blank-drug group and drugserum group. HMEC-1 cells were lysed with the radioimmunoprecipitation assay buffer (containing 0.5 % deoxycholic acid, 50 mM Tris-HCl, 0.1 % sodium dodecylsulphate, 1 % Nonidet P-40, adjusting pH 8.0). The protein concentration was determined using bicinchoninic acid detection Kit (Cat. No. WLA004, Wanlei Bio., Beijing, China) as instructed by manufacturer. Total of 25 μg lysates were separated using Sodium dodecyl-sulfate polyacrylamide gel electrophoresis and electro-transferred onto the Polyvinylidene Fluoride (PVDF) membranes. The PVDF membranes were incubated using the antibodies against the Vascular Endothelial Growth Factor A (VEGFA), Mitogen-activated protein kinase kinase (MEK), phosphorylation of MEK (p-MEK) and phosphorylation of extracellular signal-regulated kinase (p-ERK) at 4° overnight. Post washing with PBS for 3 times, PVDF membranes were incubated with the Horse Radish Peroxidase (HRP)-conjugated secondary antibodies at room temperature for 2 h. All of the above antibodies were purchased from Wanlei Bio. (Beijing, China). Then, the signal intensities for the above proteins were examined using the ECL kit (Cat. No. WLA003, Wanlei Bio.), as described by the instruction of manufacturer.
Statistical analysis:
The data in this study were defined as mean standard deviation and analyzed using the SPSS software. The differences among groups were carried out using the Tukey's post hoc test validated one-way ANOVA test; p<0.05 was assigned as the statistical significance.
Results and Discussion
In order to verify the optimal drug-serum concentration, the CCK-8 assay was conducted. The results indicated that administration with 15 % drugserum triggered obviously lower cell proliferation compared to the other concentrations of drug-serum (fig. 1). Therefore, we believed that the 15 % drugserum is the optimal concentration for inhibiting the cell proliferation of HMEC-1. In the following experiments, the HMEC-1 were treated with 15 % drug-serum.
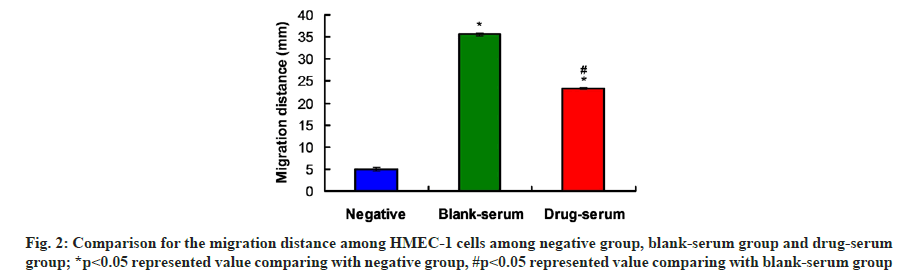
According to the migration assay findings, the migration distances of HMEC-1 in blank-serum and drug-serum group were significantly farther compared to that in negative group (fig. 2, p<0.05). Moreover, PYHY drug-serum administration (Drugserum group) remarkably decreased the migration distance of HMEC-1 cells compared to that in blankserum group (fig. 2, p<0.05). These results suggest that PYHY drug-serum could inhibit the migration of HMEC-1.
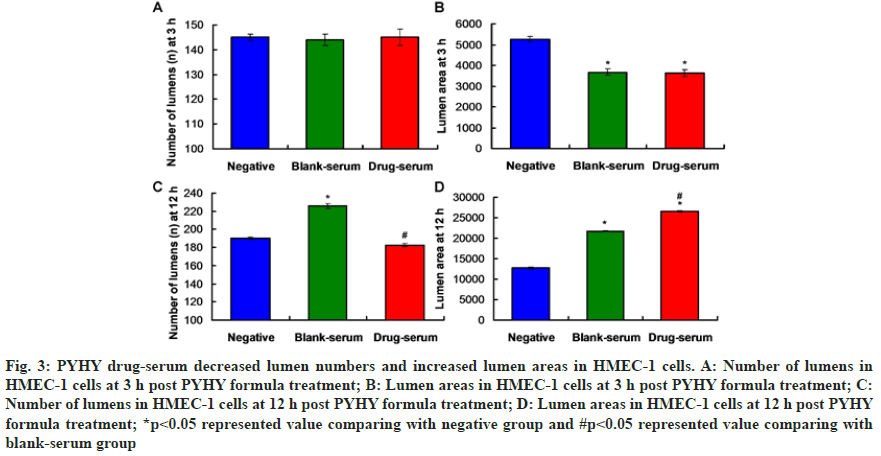
Tube formation assay results showed that there were no significantly differences for the lumens numbers (fig. 3A) and lumen area (fig. 3B) at 3 h post treatment between blank-serum group and drug-serum group (p>0.05). However, the number of lumens at 12 h post treatment in drug-serum group were significantly less compared to that in blank-serum group (fig. 3C, p<0.05). Meanwhile, the lumen area at 12 h post treatment in Drug-serum group was remarkably larger compared to that in drug-serum group (fig. 3D, p<0.05). Therefore, the PYHY treatment could obviously suppress the angiogenesis.
Fig. 3:PYHY drug-serum decreased lumen numbers and increased lumen areas in HMEC-1 cells. A: Number of lumens in HMEC-1 cells at 3 h post PYHY formula treatment; B: Lumen areas in HMEC-1 cells at 3 h post PYHY formula treatment; C: Number of lumens in HMEC-1 cells at 12 h post PYHY formula treatment; D: Lumen areas in HMEC-1 cells at 12 h post PYHY formula treatment; *p<0.05 represented value comparing with negative group and #p<0.05 represented value comparing with blank-serum group
In this study, the VEGFA expression in HMEC-1 cells was determined using western blot assay (fig. 4A). The results indicated that both of blank serum and drug-serum markedly reduced the VEGFA expression compared to that in negative group (fig. 4B, p<0.05). What's most important is that the VEGFA expression in HMEC-1 cells of Drug-serum group was significantly lower compared to that of Blank-serum group (fig. 4B, p<0.05). Thus, the PYHY drug-serum could down-regulate the VEGFA expression in the HMEC-1 cells.
Fig. 4: PYHY drug-serum triggered the reduction of VEGFA expression in HMEC-1 cells; A: Western blot bands for VEGFA expression; B: Quantification for VEGFA expression deriving from western blot analysis; *p<0.05 represented value comparing with negative group; #p<0.05 represented value comparing with blank-serum group
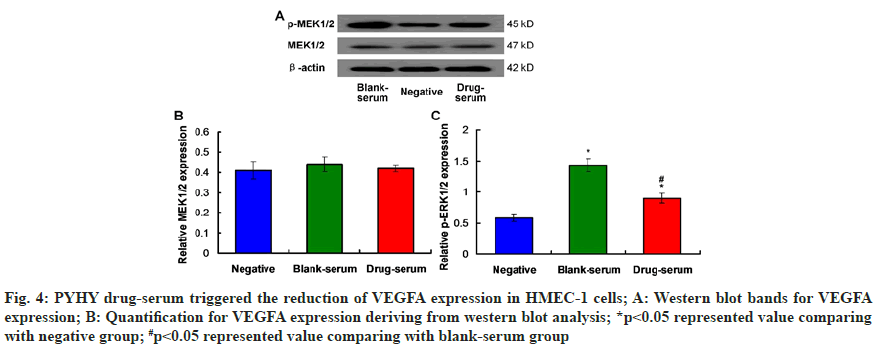
According to the western blot images (fig. 5A) for expression of p-MEK and MEK in HMEC-1 cells, there were no obvious differences for MEK1/2 expression among negative group, blank-serum group and drug-serum group (fig. 5B, p<0.05). The results also identified that PYHY treatment (drug-serum group) markedly decreased the p-MEK1/2 expression in HMEC-1 cells compared to that in blank-serum group (p<0.05). Therefore, the p-MEK1/2-MEK1/2 signaling pathway was involved in the functions of PYHY treatment.
Fig. 5: p-MEK1/2-MEK1/2 signaling pathway was activated in HMEC-1 cells undergoing PYHY drug-serum treatment; A: Western blot bands for p-MEK1/2 and MEK1/2 expression; B: Quantification for MEK1/2 expression deriving from western blot assay; *p<0.05 represented value comparing with negative group, #p<0.05 represented value comparing with blank-serum group
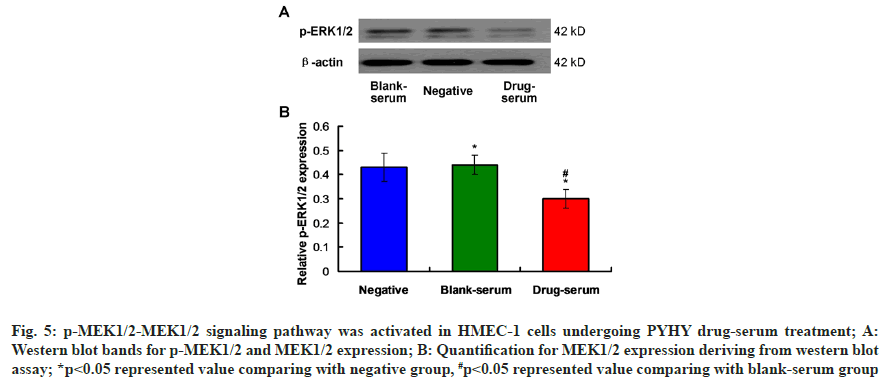
Due to the relationship between extracellular signal-regulated kinase (ERK) 1/2 and the embryo angiogenesis[14], effects of PYHY treatment on the phosphorylation of ERK1/2 was evaluated using western blot assay (fig. 6A). The findings showed that drug-serum (drug-serum) treatment down-regulated the p-ERK1/2 expression in HMEC-1 cells in drugserum group compared to that in blank-serum group, with a significant difference (fig. 6B, p<0.05). These results suggest that PYHY treatment induces the phosphorylation of ERK1/2 in HMEC-1 cells.
In the recent years, the CSDH has been prevalent with increased incidence rate because of the aging population, increased application of anti-platelet drugs and anti-coagulant drugs[1]. Due to the highhospital costs, recurrence rate post-surgery and psychologic burden of surgery, the neurological operation is not the appropriate strategy for treating CSDH[1,15]. Therefore, it critical and urgent to discover a therapeutic strategy treating CSDH with higher efficacy and relatively lower costs. In this study, HMEC-1 cells were selected for generating the in vitro cell model, under the simulating condition of growth environment for the human brain microvascular diseases. Under the intervention of serum containing PYHY formula, the cell proliferation, migration and angiogenesis were evaluated. Meanwhile, to explore the associated mechanism, the expression of VEGF, MEK1/2, p-MEK and p-ERK were evaluated using western blot assay.
The findings for the cell proliferation assay showed that 15 % PYHY drug-serum group demonstrated the strongest inhibitive effects on the cell proliferation compared with other concentration groups and the blank serum group, with the lower optical density value. Therefore, 15 % drug-serum was considered to be the ideal serum concentration for the following experiments. The cell migration experiment showed that cell migration was inhibited under the intervention of PYHY treatment. Therefore, we speculated that the angiogenesis of brain microvessels[16] might be inhibited post administrating with this PYHY formula. In the angiogenesis experiment, the results identified that the amount of angiogenesis in drugserum group was significantly less than that in blankserum group at 12 h post PYHY treatment, however, the lumen area was larger than that in blank-serum group. These results hint that under the intervention of PYHY formula, the generation of new blood vessels was reduced, the new blood vessels were more stable, and the newly generated blood vessels were more mature. Therefore, PYHY formula reduced the angiogenesis and promoted the stability and mature of blood vessels[17].
VEGFA is an important molecule of VEGF protein family, which could activate downstream signaling pathways by interacting with VEGF Receptor 2 (VEGFR-2), leading to normal angiogenesis or abnormal lesions[18,19]. VEGF could induce ERK molecule and further activate the endothelial cells after primary cerebral hypoxia and glucose deprivation, resulting in cell damage[20]. MEK/ERK signaling pathway may play a role by destroying the endothelial cell gap, therefore, increasing the possibility of CSDH[14]. Activation of MEK usually leads to phosphorylation of ERK[21], which can control the proliferation and migration of endothelial cells during embryonic angiogenesis. VEGF induced cell proliferation, migration and tubular formation could be inhibited by inhibiting MEK/ERK pathway in endothelial cells[22].
The western blot findings showed that PYHY formula decreased the protein expression of MEK1/2, p-MEK1/2, p-ERK1/2 and VEGF in HMEC-1 cells compared with that in Blank-serum group. PYHY formula inhibited the MEK in upstream of MEK/ ERK pathway, phosphorylated the MEK and then activated the phosphorylation of ERK in plasma membrane, therefore, inhibited the proliferation and migration of HMEC-1 cells. VEGF, as an upstream factor, can activate MEK/ERK signaling pathway[23]. Our results showed that PYHY formula could break the "CSDH cycle"[24] and prevent the recurrence of CSDH by blocking the MEK/ERK signaling pathway and inhibiting the angiogenesis induced by VEGF. There are also a few limitations. First, although this study involved the MEK1/2, p-MEK1/2, p-ERK1/2 and VEGF molecule in the angiogenesis of HMEC-1 cells, the specific mechanism hasn't been completely clarified. Second, the findings of this study have not been verified with the animal experiments. The present findings demonstrated that PYHY formula could inhibit proliferation, migration and angiogenesis of HMEC-1 cells. Meanwhile, PYHY formula reduced expression of MEK1/2, p-MEK1/2, p-ERK1/2 and VEGF molecule. Therefore, the inhibition of VEGF by PYHY formula is related to MEK/ERK signaling pathway. However, whether there are other targets and other signal pathways involved in this process is also needed to be discussed in the following experiments.
Author contribution:
Xiaoxuan Fan and Geting Liang contributed equally to this work.
Acknowledgements:
The present study was granted by National Natural Science Foundation of China (Grant No. 81873288) and Construction project of clinical research center of TCM encephalopathy in Shaanxi Province (Grant No. 201704). Shaanxi Province Key Industrial Innovation Project of China (2023-ZDLSF-55).
Conflict of interest:
The authors declared there is no conflict of interest.
References
- Tong Y, Liu W, Xu L, Ou Y, Li K, Yang T, Zhao T, Guan R, Fan Y. Nonsurgical treatment of chronic subdural hematoma with Chinese herbal medicine: A STROBE-compliant retrospective study. Medicine. 2020 Aug 8;99(33):e21674.
[Crossref] [Google Scholar] [PubMed]
- Xu C, Chen B, Xue L, Xia L, Yang X, Wei M, et al. Randomized controlled study on the curative effects of twist?drill craniotomy and burr?hole craniotomy in the treatment of chronic subdural hematoma. Exp Ther Med 2018;16(2):959-65.
[Crossref] [Google Scholar] [PubMed]
- Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Epidemiology of chronic subdural hematomas. No Shinkei Geka 2011;39(12):1149-53.
[Google Scholar] [PubMed]
- Ramachandran R, Hegde T. Chronic subdural hematomas causes of morbidity and mortality. Surg Neurol 2007;67(4):367-72.
[Crossref ] [Google Scholar] [PubMed]
- Huang GH, Li XC, Ren L, Dai RX, Sun ZL, Jiang XF, et al. Take it seriously or not: Postoperative pneumocephalus in CSDH patients? Br J Neurosurg 2020;34(3):284-9.
[Crossref] [Google Scholar] [PubMed]
- Shen HB, Zhou YN, Zheng J, Zhu RH. Multi-component-multi-target-multi-pathway mechanism of Kuihua Hugan Tablets based on network pharmacology. Chin J Chin Materia Med 2019;44(7):1464-74.
[Crossref] [Google Scholar] [PubMed]
- Song Z, Li X. Recent advances in molecular marker-assisted breeding for quality improvement of Traditional Chinese medicine. Curr Pharm Biotechnol 2021;22(6):867-75.
[Crossref] [Google Scholar] [PubMed]
- Xing X, Chen S, Li L, Cao Y, Chen L, Wang X, et al. The Active Components of Fuzheng Huayu Formula and their potential mechanism of action in inhibiting the hepatic stellate cells viability: A network pharmacology and transcriptomics approach. Front Pharmacol 2018;9:525.
[Crossref] [Google Scholar] [PubMed]
- Li S, Zhang B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chinese J Nat Med 2013;11(2):110-20.
[Crossref] [Google Scholar] [PubMed]
- Medfai H, Khalil A, Rousseau A, Nuyens V, Paumann-Page M, Sevcnikar B, et al. Human peroxidasin 1 promotes angiogenesis through ERK1/2, Akt, and FAK pathways. Cardiovasc Res 2019;115(2):463-75.
- Fan XX, Liang GT, Zhao XP. Intervention effect and action mechanism of Peiyuan-Huayu recipe in treating chronic subdural hematoma in rabbits. Pract J Card Cerebr Pneum Vasc Dis 2019;27:15-9.
- Zhang X, Kang X, Jin L, Bai J, Liu W, Wang Z. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int J Nanomed 2018;13:3897.
[Crossref ] [Google Scholar] [PubMed]
- Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, et al. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK-and PI3K/Akt/eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol 2006;291(6):H2836-46.
[Crossref ] [Google Scholar] [PubMed]
- Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Aoyama M, Niwa A, et al. Activation of Ras/MEK/ERK signaling in chronic subdural hematoma outer membranes. Brain Res 2012;1489:98-103.
[Crossref] [Google Scholar] [PubMed]
- Yadav Y, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg 2016;11(4):330-42.
[Crossref] [Google Scholar] [PubMed]
- Zhuang SF, Liu DX, Wang HJ, Zhang SH, Wei JY, Fang WG, et al. Atg7 regulates brain angiogenesis via NF-κB-dependent IL-6 production. Int J Mol Sci 2017;18(5):968.
[Crossref] [Google Scholar] [PubMed]
- Katsuta E, Qi Q, Peng X, Hochwald SN, Yan L, Takabe K. Pancreatic adenocarcinomas with mature blood vessels have better overall survival. Sci Rep 2019;9(1):1310.
[Crossref ] [Google Scholar] [PubMed]
- Ferrara N. VEGF-A: A critical regulator of blood vessel growth. Eur Cytokine Netw 2009;20(4):158-63.
[Crossref] [Google Scholar] [PubMed]
- Mostafa TM, El-Din MA, Rashdan AR. Celecoxib as an adjuvant to chemotherapy for patients with metastatic colorectal cancer: A randomized controlled clinical study. Saudi Med J 2022;43(1):37.
[Crossref] [Google Scholar] [PubMed]
- Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF Stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke 2009;40(4):1467-73.
[Crossref ] [Google Scholar] [PubMed]
- Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J 1994;13(7):1610-9.
[Crossref] [Google Scholar] [PubMed]
- Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol 2008;28(5):835-40.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Liu W, Zhang X, Wang Y, Liu H, Li H. MEK/ERK signaling is involved in the role of VEGF and IGF1 in cardiomyocyte differentiation of mouse adipose tissue-derived stromal cells. Int J Cardiol 2017;228:427-34.
[Crossref ] [Google Scholar] [PubMed]
- Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: Epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg 2020;141:339-45.
[Crossref] [Google Scholar] [PubMed]
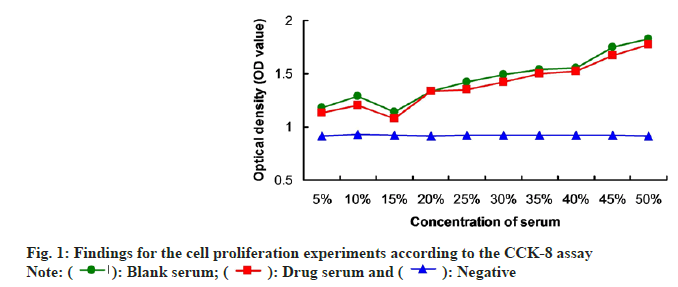
 ): Blank serum; (
): Blank serum; ( ): Drug serum and (
): Drug serum and ( ): Negative
): Negative