- *Corresponding Author:
- J. Bagyalakshmi
Sri Ramakrishna Institute of Paramedical Sciences, College of Pharmacy, Coimbatore-641 044, India
E-mail: bagi_972003@yahoo.co.in
| Date of Submission | 09 September 2004 |
| Date of Revision | 21 Ocgtober 2005 |
| Date of Acceptance | 13 August 2006 |
| Indian J Pharm Sci,2006, 68 (4): 540-541 |
Abstract
The purpose of this study was to examine the activity of ampicillin sodium developed as transdermal patch against Escherichia coli . In the present work, the efficiency of ampicillin sodium against E. coli was investigated in an in vitro infection model which simulates human pharmacokinetics. The E. coli stains were exposed to transdermal patch with different kinds of polymers such as sodium alginate, cellulose acetate phthalate, hydroxypropylmethylcellulose, chitosan and carboxymethylcellulose and the drug releasing capacity was studied through colony-forming units (CFU). The process was carried out for 24 h at 37°. It was found out that hydroxypropylmethylcellulose was the best polymer that gave less number of CFU, followed by carboxyl methyl cellulose, chitosan, cellulose acetate phthalate and sodium alginate.
Normal gastrointestinal micro flora forms a relatively stable ecosystem [1]. However, certain factors, like the administration of antibiotics, can profoundly disrupt this microbial balance [2]. The most common antibiotic-association adverse effects include overgrowth of preexisting microorganisms as a consequence of a systemic infection or severe diarrhea [3-5].
Although ß-lactam antibiotics are by far the most effective, safe and widely used antibiotics, they may alter the normal intestinal microflora [5]. The substance in bacterial composition reflects the antibacterial spectrum and the activity of the drug used [6]. After parenteral injection, ampicillin is distributed rapidly and widely, resulting in a high concentration of the drug in bile [7].From bile it is excreted into the gut and is known to cause disruption of the normal intestinal microflora [8], by diminishing the main flora and increasing the number of yeast [9] as well as inducing a high risk of Clostridium difficile colitis [10].
It is anticipated that these side effects could be overcome by the delivery of ampicillin sodium via the skin, since it has also been suggested [10] that the amino groups as confers an ability to cross cell wall barriers that are impenetrable to other penicillins. Hence the present study was aimed at investigating the activity of ampicillin from the patch against E. coli [13] in an in vitro infection model that simulates human pharmacokinetics.
Gift sample of ampicillin sodium IP was obtained form Karnataka Antibiotics Ltd., Bangalore. Hydroxypropylmethylcellulose (HPMC), sodium alginate, chitosan, carboxymethylcellulose (CMC), cellulose acetate phthalate (CAP) were obtained from Bharat Coats, Chennai. All other reagents and chemicals used were of analytical grade. The strain of Escherichia coli was NCIM 2665, obtained from The National Collection of Industrial Microorganisms (NCIM), Pune. Muller Hinton broth supplemented with calcium (25 mg/l) and magnesium (12.5 mg/l) (SMHB) was used as the medium for all experiments.
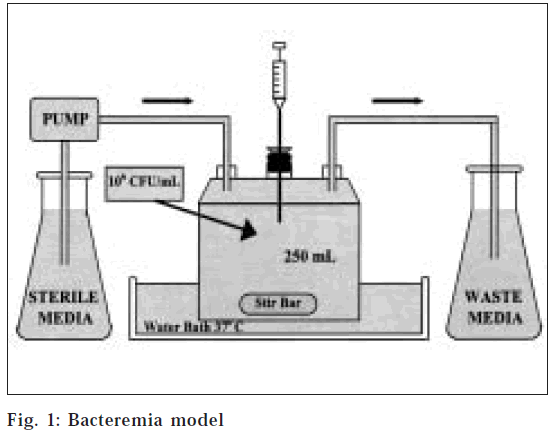
An in vitro infection model [14] consisting of a one-compartment glass chamber with ports for the addition and removal of SMHB, delivery of antibiotics and collection of bacterial samples and drug concentrations was utilized over 24 h (fig. 1). Before each experiment, bacterial colonies from an overnight growth on tryptic soy agar TSA) were added to the SMHB to obtain a 106CFU/ml suspension. Then 2.5 ml of this suspension was added to each of the infection models to produce a starting inoculum of 106CFU/ml. The model was placed in a 37° water bath during the duration of the experiment with magnetic stir bars in the media to allow for continuous mixing. A peristaltic pump was used to replace antibiotic- containing media with fresh SMHB.
Model experiments were assessed over 24 h in duplicate on different days. Ampicillin sodium developed as a transdermal patch with various polymers like 3% HPMC, 3% chitosan, 3% CAP, 3% sodium alginate, 3% CMC with 20 mg of the drug was used. Aliquots (0.1 ml) were removed for determination of bacterial counts at 24th h [15]. After suitable 10-fold dilutions with cold 0.9% sodium chloride, 20 μl was plated onto tryptic soysagar TSA in triplicate. The plates were incubated [15]and are as reported in Table 1. It was observed that ampicillin sodium transdermal patch prepared with 3% HPMC gave less number of colony-forming units among the formulations studied. It was followed by CMC, chitosan, CAP and finally sodium alginate.
| Formulation code | Colony count at 24h(log10FU/ml) |
|---|---|
| A | 1.6 |
| B | 1.7 |
| C | 1.9 |
| D | 1.8 |
| E | 1.9 |
Data obtained from evaluation of various formulations, A-hydroxypropyl methyl cellulose (HPMC), B-carboxymethyl cellulose (CMC), C-sodium alginate, D-chitosan, E-Cellulose acetate phthalate
Table 1: Pharmaco dynamic activity of ampicillin sodium patches made With various polymers against Escherichia coli Ncim2665
Hence from the present study, it can be concluded that ampicillin sodium can be developed as a transdermal patch using HPMC, among the formulation studies for further development of the transdermal patch type delivery system of ampicillin sodium.
References
- Batt,R.M.,Eur.J.ComparitiveGastroenterol.,1996,1,19.
- Nord,C.E.andEdlund,C.J.Chemother.,1990,4,218.
- Nord,C.E.VeterinaryMicrobiology.,1993,3,193.
- vandenBogaard,A.E.andStobberingh,E.E.Drugs.,199958,589-607.[ISI]
- Edlund,C.,Stark,C.andNord,C.E.,J.antimicro.chemother.,1994, 34, 12738.
- Nayaka, R.,Chida, T.andSibaoka,BifidobacteriaMicroflora,1981, 1,2537.
- Acred,P.,Brown,D.M.,Turner,D.H.andWilson,M.J.Brit.J.Pharmacol.,1962,18,35669.
- Sullivan,A.,Edlund,C.andNord,C.E.Lancet,2001,1,10114.
- Amtsberg,G.,Stock,V.,Treschnak,E.andRingel,U.AdvancesinAnimal Physiologyan Animal Nutrition.,1989, 19, 12030.
- Gorbach,S.L.,Veterinary and Human Toxicol.,1993,35,1523.
- Martin,R.A.,In;Delgodo,N.J.andWilliam,A.R.,Eds.,WilsonandGrisvold’s 9th Edn.,T.B.LippincottCompany,Philadelphia,1991,243.
- Carafa, M., Marianecci, C., Lucania, G., Marchei, E. and Santucci, E J.Control.Release,2004,95,67.
- Lamp, K.C. andVickers, M.K.,Antimicro AgentsChemother.,1998,42,231.
- AkinsL.R.andRybakJ.M.,Antimicro.AgentsChemother.,2000,44,1925.
- Bradford,P.A.andSanders,C.C.,Antimicro.AgentsChemother.,1993,37,259.