M. Rezvanian, Z. T. Ooi, Jamia A. Jamal*, K. Husain, J. Jalil, Z. Yaacob, H. F. Mohamad and A. A. Ghani
Drug and Herbal Research Centre, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
- *Corresponding Author:
- Jamia A. Jamal
Drug and Herbal Research Centre, Faculty of Pharmacy, Universiti Kebangsaan Malaysia
Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
E-mail: jamia@ukm.edu.my
| Date of Submission | 08 Aug 2015 |
| Date of Revision | 30 May 2016 |
| Date of Acceptance | 03 Jun 2016 |
| Indian J Pharm Sci 2016; 78(3): 334-343 |
Abstract
Dried Piper nigrum Linn. fruit, also known as black pepper, is an economically valuable medicinal plant that has diverse pharmacological activities. The current study was aimed to establish a research on P. nigrum fruits that can be used as a tool for quality control and standardisation of this plant in Malaysia. Black pepper specimens were collected from three different locations in Malaysia; namely Johor, Malacca and Sarawak. Various tests were conducted in the present work to obtain detailed pharmacognostic and chromatographic profiles of P. nigrum fruits, including plant morphology, taxonomy characteristics, phytochemical screening, physicochemical properties and chromatographic analysis. Using a validated high performance liquid chromatography method we quantified piperine in the samples and the highest content was obtained for the specimen from Malacca. It can be concluded that the proposed analytical methods could be applied for both qualitative and quantitative analysis of P. nigrum fruits to control the quality of Malaysian black pepper.
Keywords
Black pepper, chromatography, pharmacognosy, Piper nigrum, piperine
Medicinal herbs as a source of medication have been used throughout the globe in various cultures. Evidence shows that early humans relied upon natural resources including plants in order to treat various diseases [1]. According to the WHO, about 80% of world’s population use herbal and traditional medicines as a whole or part of the treatment. It is also stated that about 25% of all modern medicines are directly or indirectly obtained from higher plants [2,3].
Dried Piper nigrum Linn. fruits, commonly known as black pepper, is one of the most significant and oldest condiments. It is known as the king of spice and valuable for its pungency and flavor [4]. This plant is a flowering vine belonging to family Piperaceae and cultivated mostly for its fruits. It contains various medicinal properties and is valuable for numerous pathophysiological conditions [5]. P. nigrum fruits contains volatile oil, starch, proteins, alkaloids, saponins, carbohydrates and amygdalin but not tannins [6]. Piperine is reported to be the principle pungent alkaloid and the most abundant active constituent of Piper species [7,8]. Numerous biological and pharmacological properties are attributed to P. nigrum and it is used in traditional medicine system of Ayruvedic and Unani for the treatment of different diseases such as fever, pain and inflammation [9].
Standardization plays an essential role in herbal medicine as it helps to lessen variations and achieve consistency in the quality of herbal products [10]. P. nigrum is a well-known plant in Malaysia that is grown and used extensively across the country as a spice and in herbal preparations. Therefore, the aim of the present study was to develop a monographic specification for P. nigrum fruits by applying various pharmacognostical and chromatographic methods that can serve as a standard reference for quality control of this medicinal plant.
Materials And Methods
Dried P. nigrum fruits were sent to Herbarium of Universiti Kebangsaan Malaysia for authentication and deposition of the voucher specimens. Specimens were ground for further analysis. The detailed pharmacognostical tests were established for this plant based on the Malaysian Herbal Monograph [11] and other official publications. Dried fruit samples were obtained from three different locations in Malaysia, viz. Johor (Kluang), Malacca (Alor Gajah) and Sarawak (Kuching). The procedure for each of the corresponding tests is as follows.
Macroscopic/microscopic studies and color test:
The macroscopic and microscopic characteristics and colour tests of powdered P. nigrum, were evaluated as per the Malaysian Herbal Monograph [11].
Phytochemical screening:
Phytochemical screening was carried out to determine presence of alkaloids, triterpenes, saponins, phenolics, flavonoids and steroids. The procedure was adopted as previously described by Husain et al. and Sahu et al [12,13].
High performance thin layer chromatography (HPTLC):
In this test, 1 g of each powdered specimen was individually mixed with 10 ml of methanol as solvent and extracted by shaking the mixture for 10 min. The mixture was then filtered and the filtrate was dried in the oven. Weighed extracts were then reconstituted with 1 ml of methanol. The standard piperine solution (100 μg/ml; Sigma Aldrich, 97% purity) and specimen extracts (1 mg/ml) were spotted in the form of bands of 8.0 mm width by means of an automatic specimen applicator (Camag Linomat 5, Muttenz Switzerland), fitted with a Hamilton microliter syringe on a 10×10 cm precoated silica gel 60 F254 glass plate (E. Merck). Chromatographic development was carried out in a saturated developing chamber containing the mobile phase of hexane-ethyl acetate-dichloromethane (5:3:2) to a distance of 90 mm. The developed plate was heated on Camag HPTLC plate heater III at 60° for 5 min and then scannedunder day light, UV light at 254 nm and 366 nm using a Camag Scanner III densitometer before and after derivatisation with Dragendorff’s reagent.
Determination of water and ethanol soluble extractive values and loss on drying:
These tests were performed in accordance with the procedure given in the Malaysian Herbal Monograph [11].
Total and acid insoluble ash values:
The tests were carried out based on the methodology described by Kirk and Sawyer [14] and Association of Official Analytical Chemists [15].
Heavy metal and microbiological tests:
The heavy metal test was performed using an inhouse method (TPMB/TM/Lab/009) that was based on the methodology described by American Public Health Association [16]. The microbiological test was conducted by referring to European Herbal Infusions Association and British Pharmacopoeia [17,18].
Determination of moisture content:
The method used in this determination was based on the WHO quality control methods for herbal materials [19] by using the Dean-Stark apparatus. Whole specimen (50 g) was mixed with 150 ml toluene and anti-bumping agent. The heating process was carried out for 3 h and the amount of moisture content was recorded [20].
Preparation of essential oil and determination of yield:
The essential oil of every specimen was prepared by hydro distillation using the Clavenger apparatus. Powdered material (150 g) was mixed with 1000 ml of distilled water and anti-bumping agent. The hydro distillation process was carried out for 8 h and the essential oil was collected [20].
Determination of essential oil composition:
The chemical composition of the essential oil was determined using a gas chromatography with flame ionisation detector (GC-FID) and a gas chromatography coupled with mass spectrometry (GC-MS). For GCFID, the oil was diluted with ethyl acetate as a solvent before 1 μl of the solution was injected with nitrogen as the carrier gas. The column used was a DB-5 column, with the dimension of 30 m×0.25 mm and the temperature ranged from 75 to 250°. The process was carried out for 73.333 min. Standard hydrocarbons of C8 to C22 were used as the standard. GC-MS analysis of the oil was carried out and prepared in the same manner as the procedure for GC-FID. The Kovats index (KI) of a compound was determined based on the retention time of a peak in the oil specimen and the standard hydrocarbon being used [21].
Piperine assay using high-performance liquid chromatography (HPLC)
Powdered specimen (5 g) was extracted with 80 ml of ethanol (99.8%) using a reflux method at a temperature of 60° for 30 min. The extract was filtered and the solvent was evaporated to dryness using a rotary evaporator. The dried extract was reconstituted with HPLC-grade methanol to give a 1 mg/ml solution. The mixture was ultra sonicated for 15 min and then filtered through a 0.45 μm nylon membrane filter using the 4 mm syringe. The resultant solution was used for the HPLC analysis.
Preparation of standard solutions:
A stock standard solution of piperine (1000 μg/ml, Sigma Aldrich, USA) was prepared in HPLC-grade methanol, sonicated for 20 min and then filtered through a 0.45 μm nylon membrane filter. The solution was then diluted to construct a series of calibration working standard solutions in the range of 0.3 to 0.7 mg/ml.
Validation of HPLC system:
Validation method provided assurance and documented evidence that the applied analytical method was suitable for its intended use [22]. The developed HPLC method for piperine analysis was validated based on the ICH guidelines for linearity, accuracy, precision, system suitability, and limits of detection (LOD) and quantification (LOQ) [23]. A Shimadzu system (Kyoto, Japan), equipped with a liquid chromatography Model LC-20 AT, an autoinjector, a photodiode array detector (SPD-M20A) and LC solution software, was used in this study. Separation of piperine was achieved using a reversed phase XBridge-C18 (250×4.6 mm, 5 μm; Ireland) along with a guard column. Mobile phase containing methanol and water (76:24) was applied at a flow rate of 1.0 ml/min. Oven temperature was kept at 27°, injection volume was 20 μl, and detection wavelength used was 254 nm. All extracts were analysed for piperine under the same analytical conditions using a validated HPLC system.
Statistical analysis:
All quantitative data including extractive values, loss on drying, ash values, essential oil composition and HPLC analysis were expressed as the mean±standard deviation (SD). Tests were conducted at least two times to get the most precise result with a relative standard deviation within 2%.
Results And Discussion
The detailed morphological, taxonomical, pharmacognostical and chromatographic evaluation of P. nigrum fruits collected from three different locations in Malaysia were carried out and the results are shown in the Tables 1, 2 and figs. 1-3.
| Specimens | Water-soluble extractive values (%±SD) |
Ethanol-soluble extractive values (%±SD) |
||
|---|---|---|---|---|
| Cold method | Hot method | Cold method | Hot method | |
| Malacca | 10.89±1.54 | 30.47±0.66 | 6.97±1.42 | 10.49±0.69 |
| Johor | 18.46±0.71 | 27.45±0.63 | 11.43±0.64 | 15.50±0.71 |
| Sarawak | 12.46±0.72 | 32.90±1.27 | 8.44±0.80 | 11.95±1.37 |
TABLE 1: DETERMINATION OF EXTRACTIVE VALUESOF PIPER NIGRUM FRUITS OBTAINED FROM DIFFERENT SOURCES
| Compounds | RI* | Essential Oil Composition (%±SD) | ||
|---|---|---|---|---|
| Malacca | Johor | Sarawak | ||
| α-thujene | 930 | 0.13±0.00 | 0.21±0.05 | 1.05±0.04 |
| α-pinene | 939 | 4.74±0.17 | 2.27±0.56 | 5.33±0.32 |
| β-pinene | 979 | 8.71±0.20 | 4.59±0.63 | 7.56±0.67 |
| Trans-isolimonene | 985 | - | - | 8.10±0.64 |
| Myrcene | 991 | 2.08±0.07 | 1.08±0.07 | 1.86±0.07 |
| α-phellandrene | 1003 | 3.35±0.31 | 1.96±0.02 | 2.99±0.09 |
| α-terpinene | 1017 | 16.69±1.01 | 10.0±0.39 | 22.23±0.29 |
| Limonene | 1029 | 0.46±0.04 | 0.28±0.03 | 1.69±0.10 |
| Z, β-ocimene | 1037 | 13.97±0.53 | 8.05±0.13 | 13.78±0.57 |
| γ-terpinene | 1060 | 0.05±0.01 | 0.19±0.01 | 0.45±0.04 |
| Terpinolene | 1089 | 0.24±0.01 | 0.17±0.00 | 0.26±0.00 |
| Linalool | 1097 | 0.68±0.05 | 0.50±0.03 | 0.66±0.01 |
| cis, ρ-menth-2-en-1-ol | 1122 | - | - | 0.08±0.01 |
| cis, β-terpineol | 1144 | - | - | 0.05±0.02 |
| Camphor | 1146 | - | - | 0.18±0.02 |
| Terpinene-4-ol | 1177 | 0.02±0.00 | - | - |
| ρ-cymen-8-ol | 1183 | 0.13±0.02 | 0.29±0.02 | 1.11± 0.03 |
| α-terpenol | 1189 | - | - | 0.15± 0.01 |
| α-cubebene | 1351 | 3.51±0.07 | 4.61±0.43 | 1.14±0.10 |
| β-cubebene | 1388 | - | 3.46±0.09 | 0.54±0.09 |
| β-elemene | 1391 | 1.82±0.30 | - | - |
| E-caryophyllene | 1419 | 0.10±0.01 | - | - |
| γ-elemene | 1437 | - | 0.11±0.00 | - |
| Z, β-farnesene | 1443 | 30.79±1.90 | 40.61±1.42 | 10.57±1.29 |
| γ-gurjunene | 1477 | 1.97±0.16 | 2.31±0.07 | 0.84±0.08 |
| Germacrene D | 1485 | 0.13±0.01 | 0.21±0.01 | 0.15±0.03 |
| β-bisabolene | 1506 | 0.75±0.06 | 0.72±0.00 | 0.53±0.03 |
| γ-cadiene | 1514 | 0.83±0.06 | 2.02±0.05 | 1.30±0.15 |
| δ-cadiene | 1523 | - | - | 0.06±0.02 |
| Z-nerolidol | 1533 | 0.17±0.01 | 0.44±0.02 | 0.42±0.02 |
| α-calacorene | 1546 | 1.30±0.08 | 2.03±0.01 | 1.25±0.01 |
| Elemol | 1550 | 0.05±0.00 | 0.11±0.00 | 0.08±0.00 |
| Germacrene D-4-ol | 1576 | tr | - | 0.09±0.00 |
| Spathulenol | 1578 | - | 0.05±0.01 | 0.06±0.00 |
| β-copaen-4-α-ol | 1591 | - | - | 0.03±0.01 |
| 2-acetyl naphthalene | 1609 | 0.92±0.05 | 0.76±0.03 | 2.39±0.14 |
| Epoxy alloaromadendrene | 1641 | 0.07±0.03 | 0.10±0.00 | 0.13±0.00 |
| Cubenol | 1647 | 1.00±0.39 | 0.64±0.03 | 2.35±0.29 |
| α-cadinol | 1654 | - | - | 0.40±0.04 |
SD=standard deviation (n=2); (-) not detected; RI*=retention index of DB-5 column; MS=mass fragmentation; tr=trace compound (concentration<0.01%).
TABLE 2: PERCENTAGE COMPOSITION OF PIPER NIGRUM FRUIT ESSENTIAL OILS OBTAINED FROM DIFFERENT SOURCES
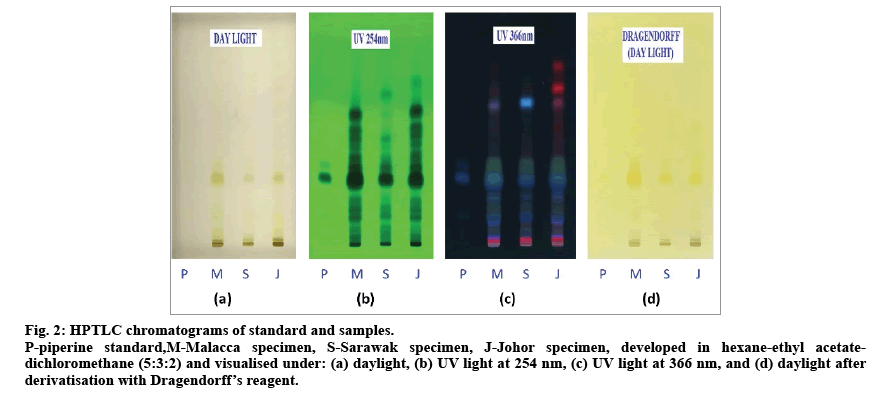
Fig. 2: HPTLC chromatograms of standard and samples.
P-piperine standard,M-Malacca specimen, S-Sarawak specimen, J-Johor specimen, developed in hexane-ethyl acetatedichloromethane (5:3:2) and visualised under: (a) daylight, (b) UV light at 254 nm, (c) UV light at 366 nm, and (d) daylight after derivatisation with Dragendorff’s reagent.
The P. nigrum fruits were all round or oval in shape of 3 mm in diameter that were smaller in dimension compared to the Indian P. nigrum (6.5 mm) [24]. The hard endocarp confined within the fleshy pericarp and enclosed a single seed. The fruit adhered to the stalk tightly without being covered. It was green in colour but turned red upon ripening.The findings were in accordance to the previous study by Ravindran et al [25]. The dried P. nigrum fruits used in this study were dark brown except for the specimen from Malacca which was grey brown. The powder gave a pungent smell, but it was less pungent for Malacca specimen.
Microscopic examination revealed presence of mostly broad and rectangular sclereids (fig. 1a); the cell body was elongated, bordered by thick and densely lignified cell wall that striated with funnel like fissures. They were found abundantly in the specimen and arranged laterally one to another and these findings were consistent with previous studies [11,26]. The morphology of sclereid is specific to each and different species of fruit therefore; it can serve as a diagnostic tool to identify P. nigrum fruits [27]. Two types of parenchymal cells were observed, that is, thin pentagonal cells (fig. 1b) and thick irregular cells (fig. 1c). Both types of cells were lignified and stained bright red when treated with phloroglucinol/HCl reagent, indicating the presence of lignin. The other common elements observed were annular (fig. 1d) and spiral (fig. 1e) vessels, and calcium oxalate crystal in the form of rosette cluster (fig. 1f) in the parenchyma cells. The starch granules (fig. 1g) were tiny and spherical, with hilum in the form of a point and no striation. The granules were either agglomerated or scattered around. The appearance of starch granules is also a very useful tool for identification. This is because the granules from any particular plant only vary within definite limits, so that it is possible to distinguish between the starches derived from different species. The characteristically different microscopic features can be used to differentiate P. nigrum fruits from other plants. The components of starch amylose and amylopectin can be distinctive in their reaction to iodine. Amylose reacts with iodine to form a deep blue complex while amylopectin gives a blueviolet or purple colour [28,29]. Such appearance was seen in the starch granules of P. nigrum fruits and it indicated that amylopectin was the major component in the starch of these specimens compared to amylose (fig. 1). The findings were in accordance to the previous studies [11,24]. The consistency of the result suggested that the specific characteristics of the starch granules provide diagnostic value to identify P. nigrum fruits.
Colour change during a reaction is determined by the chemical composition in the plant. Environmental factor will alter the chemical composition of a plant [28] , so it is the common cause of variation of result within plants of the same species but grew from different locations. From the study, potassium hydroxide (5%), sodium hydroxide (5%) and ammonium hydroxide (25%) could be used to chemically characterise P. nigrum fruits. Formation of yellowish green solution with ferric (III) chloride (5%) could indicate presence of phenolic compounds in the fruits that is somewhat similar to the previous findings [26]. However, concentrated hydrochloric and sulphuric acids were found to be more sensitive reagents to differentiate qualities of the fruits. Hydrochloric acid changed the colour to light yellow for Malacca specimen and dark yellow for both Johor and Sarawak specimens. On the other hand, sulphuric acid changed the colour to maroon for Malacca specimen and dark red for the other two specimens. This substantiates the different macroscopic characteristics of Malacca specimen compared with the others.
The knowledge of chemical constituents in herbal medicine is essential for understanding its pharmacological and toxicological profiles as well as for developing quality control methods [30]. All specimens of P. nigrum fruits were found to contain alkaloids, triterpenes, steroids, phenolics and flavonoids; comparable to the Indian P. nigrum fruits [26]. However, only Johor specimen was reported to have saponins.
Fig. 2 shows chromatograms of developed HPTLC plate comprising of the extracts of three different specimens along with a piperine standard. Good separation of phytochemical components of methanol extracts of P. nigrum fruits were obtained using hexane-ethyl acetatedichloromethane (5:3:2) mixture as compared to the previous reports [11,26]. Before derivatisation, piperine was detected at Rf value of 0.35 under UV light at 254 and 366 nm as dark and blue colour, respectively, but it was not seen under day light. After derivatisation with Dragendorff’s reagent, piperine appreared as yellowish orange band on TLC plate. Piperine was detected as a major component for all extracts with different band size and intensity, suggesting a variation in piperine content of the P. nigrum fruits obtained from different locations. The specimen from Sarawak was found to have the least intense band corresponding to piperine.
Temperature and types of solvent can give impact on the quantity of extractable matter of a plant. The extractive capacity (measured as extractive value) increases with the amount of extractive matter produced under a particular condition. The Malaysian Herbal Monograph [11] specified that the limits for water soluble extractive values for black pepper using cold and hot methods are not less than 23% and 8%, respectively, and that for cold ethanol-soluble extractive is not less than 1.5%. All specimens were found superior than the standard specifications. From this study, higher temperature and using water as solvent exhibited a better extractive capacity than in room temperature and alcohol based solvent. The water soluble extractive values were found to be two to three folds higher than that of the ethanol soluble ones; likewise, the extractive values recorded for hot method were fairly higher than that of the cold method. When yield of extractive matter is the key interest in case of P. nigrum fruits, employing high temperature and water as solvent to increase output can be applied in the pharmaceutical or herbal industry [29].
According to the Malaysian Herbal Monograph [11] , loss on drying value of P. nigrum fruits should not exceed 13%. Among all specimens, only specimen from Malacca (15.86±0.10%) did not meet the specification and those from Johor and Sarawak showed the loss on drying values of 9.73±0.02% and 11.73±0.53%, respectively. The failure of specimen to meet the standard can be either extrinsically due to the ineffective air drying procedure or internally due to high content volatile matter that evaporates together with moisture while heating. In order to overcome this issue the specimens should be air dried for a longer period or using a higher temperature to facilitate the drying process.
According to the European Pharmacopoeia [27] , limit of total ash and acid insoluble ash value for a crude drug, with respect to any species, should be a maximum of 14% and 2%, respectively. However, the Malaysian Herbal Monograph [11] stated the limits for P. nigrum fruits as not more than 3.5% total ash and 2% acid insoluble ash. Based on the latter limits, specimens from Malacca, Johor and Sarawak did not meet the specification for total ash (4.39±0.17%, 5.33±0.42% and 3.56±0.04%, respectively) but complied with the acid insoluble ash limit (0.52±0.08%,0.43±0.17% and 0.15±0.02%, respectively). High ash value is an indication that specimens were contaminated by extraneous matter in a great extent. Acid insoluble ash value is to further confirm that the high total ash value is derived from physiological or non-physiological ash. A plant of good quality should not contain high non-physiological ash, which is the residue of the extraneous matter such as sand and soil [14]. In this case, it was possible that the specimens used in this study had physiological ash due to the presence of numerous calcium oxalate crystals as evidenced from the microscopic analysis (fig. 1f). Results from this study were in fact comparable to the ash values reported for the Indian P. nigrum fruits [26]. Thus, limits set by the Malaysian Herbal Monograph may be revised.
The Malaysian National Pharmaceutical Control Bureau specified the maximum limit of heavy metal levels in herbal materials for arsenic as 5.0 ppm, cadmium (0.3 ppm), mercury (0.5 ppm) and lead (10.0 ppm) [31]. The results showed that all specimens in this study met the specification, whereby the content of heavy metals ranged from 0.03 ppm to 0.41 ppm. A main source of heavy metal contamination of plants is the usage of pesticides during cultivation [32]. Consumption of heavy metals could cause detrimental effects to human, hence its level in herbal is of safety concern.
Microbiological test is to ensure the safety profile of a medicinal plant. All specimens met the specifications stated in the British Pharmacopoeia [18]. The total viable aerobic count for bacteria were found to be 3.5×106 , 1.8×104 and 4.3×105 cfu/g for Malacca, Johor and Sarawak specimens, respectively. However, the total viable aerobic count for fungi were found to be 1300, 60 and 600 cfu/g for Malacca, Johor and Sarawak specimens, respectively. E. coli (in 1 g), Salmonella (in 10 g) and Staphylococcus aereus (in 1 g) were absent in all specimens.
The yield of essential oils of P. nigrum fruits from Malacca, Johor and Sarawak were 1.06%, 0.76% and 1.00%, respectively. The oils showed similar major components such as Z,β-farnesene (10.57-40.61%), α-terpinene (10.01-22.23%) and Z, β-ocimene (8.05- 13.97%, Table 2). The other compounds found in appreciable amount were β-pinene (4.59-8.71%), α-pinene (2.27-5.33%), α-cubebene (1.14-4.61%), α-phellandrene (1.96-3.35%), 2-acetyl naphthalene (0.92-2.39%), cubenol (0.64-2.35%) and γ-gurjunene (0.84-2.31%). Monoterpenes were the primary constituents in the oils that comprised about 57% of the total oil content. However, the results were inconsistent to the previous studies. Jirovetz et al. [33] reported that major compounds in P. nigrum fruits from Cameroon, West Africa were germacrone D (11.01%) and limonene (10.26%); while Menon and Padmakumari [34] identified sabinene (3.9-18.8%) and β-pinene (3.9-10.9%) as the major components in P. nigrum fruit oil from Kerala, India. The variation of results in different regions may suggest a phenomenon called chemical polymorphism, whereby the essential oil content variability is influenced by geographical and genetic factors.
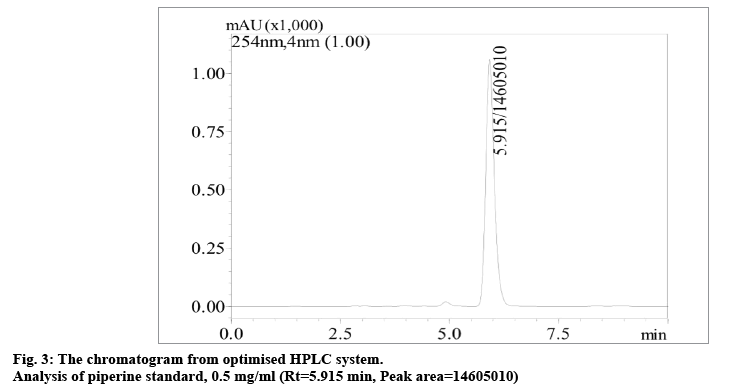
Various chromatographic conditions were applied in order to develop an optimum HPLC system that can provide adequate chemical information and separation of the assay. The chromatogram of the optimized HPLC method for the analysis of piperine is shown in fig. 3. There are several methods available for quantitation of piperine but dealing with herbal analysis necessitates the development of an ideal method under current condition of study. Different mobile phases in various ratios, columns, flow rates and detection wavelengths were investigated to determine the optimal condition for separation of compounds. From this study, the calibration curve gave a good linearity for piperine (r2>0.997) over the range of 0.3-0.7 mg/ml and the regression equation was used to determine the percentage content of piperine in the ethanol (% w/w) extracts of P. nigrum fruits. The limit of detection (LOD) and limit of quantitation (LOQ) were found to be 0.14 and 0.42 mg/ml, respectively. Based on the results obtained, it was revealed that the method was able to detect and quantify analyte below the minimum concentration of piperine used in the calibration curve. For system suitability testing, the results obtained for the chromatographic performance of peak area and retention time were taken into account for eight determinations over one concentration level of piperine standard (0.5 mg/ml). The relative standard deviation (RSD) value obtained for retention times and peak areas were 0.07 and 0.05%, respectively, that were within the acceptable limits (RSD<2%). This clearly indicated that the analytical system was performing well for the analysis of the specimens and suitable for its intended purpose [23]. The analytical method showed to be precise by both intraday (RSD 0.97-1.39%) and interday (RSD 0.20-0.55%) analysis of the piperine standard under the same analytical condition for three different concentration levels. The obtained results were satisfactory and below the recommended limit of 2% by ICH Harmonised Tripartite Guideline [23] which demonstrated that the developed method of analysis for piperine was highly precise. Accuracy of the developed method was evaluated by recovery test. A known amount of piperine standard solution was added in an ethanol extract of specimen from Malacca. The spiking was performed using three different standard concentrations of 0.05, 0.1 and 0.15 mg/ml. The mean recovery of piperine was 98.11% with an RSD value of 4.90%. The RSD value was lower than the maximum limit of 5% [23,35], indicating that the proposed method was accurate for quantitative analysis of piperine in P. nigrum fruits.
Analysis of piperine in P. nigrum fruits based on the validated HPLC method showed that the Malacca specimen contained the highest amount of piperine (5.85%) compared to that of Johor (4.93%) and Sarawak (4.75%). Moreover, piperine content had been reported in the range of 2-7% for Indian and Malaysian P. nigrum fruits, 7-15% for Sri Lankan specimen [36] and 3.2% for commercial P. nigrum fruits [37]. In this study, the HPLC result revealed the impact of geographical location on variation of piperine content in the P. nigrum fruits and suggested that Malacca could be a potential cultivation site of P. nigrum in Malaysia so as to obtain high yield of piperine for medicinal purposes. Analysis of P. nigrum fruits by HPLC could be used as a standard fingerprint technique for the purpose of authentication, identification, purification, quality control assessment and to differentiate its adulterants in order to guarantee the therapeutic efficacy of herbal products [38].
In conclusion, the qualitative information and quantitative data of the current study can serve as a reference for the standardization and the establishment of herbal monograph of the P. nigrum fruits in Malaysia. Identification tests such as macroscopy, microscopy, colour test, phytochemical analysis and thin layer chromatography provided information about characteristic features of each specimen that enables quick and precise identification of related crude drugs. Limit tests including determination of extractive values, loss on drying, ash values, heavy metals and microbial content are useful to determine the quality and purity of the specimens. Quantitative analysis of essential oils of P. nigrum fruits revealed information related to the chemical composition in the specimens and the major compounds that might contribute to therapeutic value for human. A simple RP-HPLC-PDA method that was successfully developed and validated for the analysis of piperine can help to compare and distinguish P. nigrum fruits from various locations in Malaysia based on the presence of piperine. Interestingly for the Malacca specimen, despite of not having the pungency, gave the highest yield of essential oil and piperine content. To the best knowledge of authors, this is the first report concerning complete pharmacognostic analysis of P. nigrum collected in Malaysia for the purpose of quality control. Thus, it is concluded that the proposed analytical methods could be applied for both qualitative and quantitative evaluation of P. nigrum fruits by the pharmaceutical or herbal industries and the regulatory authorities.
Acknowledgement:
The authors would like to thank Emeritus Professor Dato’ Dr. Abdul Latiff Mohamad for plant authentication.
Financial support and sponsorship:
The authors thank Ministry of Agriculture and Agro Based Industry (NF-2012-001) for the financial support provided for this project.
Conflicts of interest:
There are no conflicts of interest.
References
- Kunle, OF, Egharevba HO, Ahmadu, PO. Standardization of herbal medicines- A review. Int J BiodiversConserv2012;4:101-12.
- World Health Organization, Monographs on Selected Medicinal Plants, Geneva, 2001.
- Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents).Braz J Med Biol Res 2000;33:179-89.
- Menghani E, Jain SC, Jain R. Scientific validation of Piper nigrumby HPLC and antioxidative assay markers. Asian J Biotechnol2010;2:133-8.
- Karsha PV, Lakshmi OB. Antibacterial activity of black pepper (Piper nigrumLinn.) with special reference to its mode of action on bacteria.Indian J Nat Prod Resour2010;1:213-5.
- Khushbu C, Roshani S, Patel A, Mayuree MC. Phytochemical and therapeutic potential of Piper longumLinn. A Review. Indian J Nat Prod Resour2011;2:157-61.
- Kumar S, Kamboj J, Suman, Sharma S. Overview for various aspects of the health benefits of Piper longumLinn. J Acupunct Meridian Stud 2001;4:134-42.
- Bajad S, Singla AK, Bedi KL. Liquid chromatographic method for determination of piperine in rat plasma: application to pharmacokinetics. J Chromatogr B 2002;776:245-9.
- Tasleem F, Azhar I, Ali SN, Perveen S, Mahmood ZA. Analgesic and anti-inflammatory activities of Piper nigrumL. Asian Pac J Trop Med2014;7:461-8.
- Heinrich M, Barnes J, Gibbons S, Williamson EM.Fundamentals of Pharmacognosy and Phytotherapy. 2nd ed. Edinburgh: Elsevier; 2012.
- Malaysian Herbal Monograph, Herbal Monograph Committee. Vol. 2. Kuala Lumpur: Forest Research Institute Malaysia; 2009.
- Husain K, Jamal JA, Jalil J. Practical Handbook. Kuala Lumpur: UniversitiKebangsaan Malaysia; 2012.
- Sahu VK, Irchhaiya R, Shashi A, Gurjar H. Phytochemical investigation and chromatographic evaluation of the ethanolic extract of whole plant extract of Dendrophthoefalcata(L.F.) Ettingsh. Int. J Pharm Sci Res2009;1:39-45.
- Kirk R S, Sawyer R. Pearson’s Compositions and Analysis of Foods. 9thed. UK:Longman Group; 1991.
- Association of Official Analytical Chemists, Ash of Spices, Official method 941.12. 17thed. Maryland: Association of Official Analytical Chemists; 2000.
- American Public Health Association, Standard Methods for the Examination of Water and Wastewater, 20thed.Washington D.C: American Public Health Association; 1998.
- European Herbal Infusions Association, Microbiological Status of Untreated Herbal Materials, Hamburg; 2012.
- British Pharmacopoeia. Appendix B and F. London: Her Majesty's Stationery Office, (HMSO); 2012.
- World Health Organization, Quality Control Methods for Medicinal Plant Materials, Geneva, 2011.
- Jantan I, Yasein MSM, Chin CB, Chen LL, Sim NL. Antifungal activity of the nine species of Zingiberaceae species. Pharm Biol2003;41:392-7.
- Rafi IAA, Jantan I, Jalil J. Platelet-activating factor (PAF) receptor binding activity of Curcuma aeruginosaRoxb. Fine chemicals fromnatural resources, Proceedings of the 17th Seminar of the Malaysian Natural Product Society, Shah Alam, Malaysia; 2001,17-8.
- Sonawane LV, Poul BN, Usnale SV, Waghmare PV, Surwase LH. Bioanalytical method validation and its pharmaceutical application-A review. Pharm AnalActa2014;5:288.
- ICH, Q2 (R1), International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedures: Text and Methodology, Geneva, 2005.
- Trivedi MN, Khemani A, Vachhani UD, Shah CP, Santani DD. Pharmacognostic, phytochemical analysis and antimicrobial activity of two Piper species. Int J Comp Pharm 2011;2:1-4.
- Ravindran PN. Black pepper: Piper nigrumseries: Medical and Aromatic Plants - Industrial Profiles, Center for Medicinal Plants Research, Kerala, 2000.
- Rai N, Yadav S, Verma AK, Tiwari L, Sharma RK. Quality specifications on Piper nigrumL.-A spice and herbal drug of Indian commerce. Int J Adv Food SciTechnol 2012;1:1-11.
- European Pharmacopoeia. France: European Directorate for the Quality of Medicines and Health Care, Council of Europe; 2007.
- Robbers JE, Speedie MK, Tyler VE. Pharmacognosy and Pharmacobiotechnology, Pennsylvania: Williams and Wilkins;1996, p. 337.
- Evans WC. Trease and Evans Pharmacognosy, 16thed. London: Saunders Elsevier; 2009.
- Pengelly A. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine, 2nded. Oxon: CABI Publishing; 2004.
- Ministry of Health Malaysia, Drug Registration Guidance Document, National Pharmaceutical Control Bureau, Kuala Lumpur; 2012.
- World Health Organization, Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. World Health Organization, Geneva, 2007.
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J Chromatogr A 2002;976:265-75.
- Menon AN, Padmakumari KP. Studies on essential oil composition of cultivars of black pepper (Piper nigrumL.)-V. J Essent Oil Res 2005;17:153-5.
- United States Pharmacopoeia/National Formulary, Validation of Compendial Procedures, Rockville: United States Pharmacopoeial Convention; 2008.
- Jansz ER, Pathirana IC, Packiyasothy EV. Determination of piperine in pepper (Piper nigrumL). J NatnSciCoun Sri Lanka1983;11:129-38.
- Verzele M, Qureshi S. HPLC determination of piperine in pepper and in pepper extract. Chromatographia 1980;13:241-3.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Alhadrami HAA, Bhandari A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrumLinn. fruits. Asian Pac J Trop Biomed 2015;5:101-7.