- *Corresponding Author:
- R. Sambath Kumar
Department of Pharmaceutics, S.A. Raja Pharmacy College, Tirunelveli 627116, Tamil Nadu, India
E-mail: sambathju2002@yahoo.co.in
| Date of Received | 06 July 2021 |
| Date of Revision | 24 September 2021 |
| Date of Acceptance | 30 September 2022 |
| Indian J Pharm Sci 2022;84(5):1189-1196 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The plasma pharmacokinetic study of abacavir sulphate was investigated after oral administration of free abacavir sulphate solution, freshly prepared niosomal and proniosomal abacavir sulphate dispersions. The abacavir sulphate loaded niosome vesicles were prepared by the thin-film hydration method and proniosomes were prepared by the slurry method using tween 60 as surfactant cholesterol as a membrane stabilizer and dicetyl phosphate as a negative charge inducer. Maltodextrin was used as a carrier in the proniosome formulations. The formulations were evaluated for various evaluation parameters such as drug-polymer interaction, phase identification analysis and in vivo pharmacokinetic study. The comparative pharmacokinetics of free, niosome encapsulated and proniosome encapsulated abacavir sulphate was evaluated in rats at a dose of 8.57 mg/kg of abacavir sulphate. The results of niosome and proniosome encapsulated abacavir sulphate showed a significant increase in bioavailability and prolonged release characteristics. Increased half-life of 3.40 h in the case of niosomes and 4.88 h in the case of proniosomes were observed. Based on the results, it can be concluded that niosomal and proniosomal formulation could be a promising delivery system for abacavir sulphate with improved oral bioavailability and prolonged drug release profiles.
Keywords
Abacavir sulphate, pharmacokinetics, niosomes, proniosomes, bioavailability
Acquired Immune Deficiency Syndrome (AIDS) is the most common problem worldwide because of the rapid increase in the number of victims. World Health Organization recently uses the term ‘Global epidemic’ to describe the Human Immune Deficiency Virus (HIV). In 2019, approximately 38 million people were infected by HIV and 690 000 deaths due to HIV related illnesses in 2019[1]. Antiretroviral therapy has undergone significant changes over the past decades. Antiretroviral drugs are active against the HIV. They help prolong and improve the quality of life and postponing complications of AIDS or AIDS related complex[2]. Although progress in managing people alive with HIV and the significant developments in morbidity and mortality, failure in retroviral therapy still occurs. Along with treatment failure, HIV becomes more resistant to antiretroviral drugs[3,4].
Encapsulation of medicaments in the vesicular organization can be expected to prolong the availability of the medicaments in the systemic circulation and hence augment penetration into objective tissue and diminish toxicity. The constituents of vesicles influence their physicochemical properties, such as their charge, elasticity, size, lamellarity and thermodynamic phase. The vesicular construction can also be altered to afford controlled or sustained drug delivery for extended periods[5]. Vesicular drug transporters such as liposomes, niosomes, virosomes, transferosomes, proteasomes, archaesom, sphingosomes, phamacosomes, ethosomes showing a very hopeful role in drug delivery and avoiding demerits associated with conventional dosage forms because these particles can act as drug reservoirs[6]. Niosomes are unilamellar or multilamellar microscopic vesicles, formed on admixtures of nonionic surfactant and cholesterol with subsequent hydration in the aqueous media can entrap both hydrophilic and lipophilic drugs, either in the aqueous region or in a vesicular membrane made of lipid materials[7,8]. Since the 1980s, niosomes have gained wide attention by researchers for their use as drug targeting agents, drug carriers to have various merits while avoiding demerits associated with the conventional form of drugs[9,10]. Liver act as a depot for many drugs where niosomes containing drugs may be taken up and broken down by lysosomal lipase slowly to release the free drug and re-enter the circulation. Hence niosomes are slowly degraded providing a more sustained effect[11]. However, aqueous suspensions of niosomes may exhibit physical instability problems such as aggregation and fusion of vesicles and leaking or hydrolysis of the encapsulated drug. Proniosomes minimize problems of niosomes and provide additional convenience in transportation, distribution, storage and dosing, which would make dry niosome a promising industrial product[12,13]. By considering the above advantages, proniosomes and niosomes of abacavir sulphate were prepared in this study.
The crucial purpose of pre-clinical pharmacokinetics studies is to validate the tools used to envisage human kinetics. Pharmacokinetic data can be used to predict plasma concentrations, target tissue doses and the fate of the administered dose. The pharmacokinetic profile provides a strong rationale for the development of a prolonged release formulation[14]. To the best of our knowledge, no pharmacokinetic studies have been conducted on the proniosomes and niosomes of abacavir sulphate. In this regard, the present work has been designed to study the pharmacokinetic profile abacavir sulphate of niosomes and proniosomes in rats.
Materials and Methods
Abacavir sulphate was obtained as a gift sample from Cipla Limited, Mumbai. Tween 60, Methanol was bought from Ultra International, Bangalore. Cholesterol and Chloroform were bought from SDFCL-SD Fine Chemicals Limited, Mumbai. Maltodextrin was obtained from Loba Chemie private limited, Mumbai. All other chemicals and solvents were of analytical grade.
Preparation of niosomes:
Niosome containing abacavir sulphate formulations were prepared by thin film hydration method. Tween 60, cholesterol and dicetyl phosphate were accurately weighed and transferred into a long necked 100 ml round-bottom flask and dissolved in 10 ml chloroform. The flask was attached to a rotary evaporator. The organic solvent was slowly evaporated at 60° under reduced pressure at 100-150 rpm such that a thin, dry film of the constituents was formed on the inner wall of the flask. Any excess chloroform was removed by leaving the flask in a desiccator under vacuum overnight. The dried thin film was then hydrated with 10 ml pH 7.4 phosphate buffer saline containing 25 mg abacavir sulphate (Drug Loaded Niosomal Formulation (DNF)) or 10 ml pH 7.4 phosphate buffer saline (Blank Niosomal Formulation (BNF)) by rotating the flask in the same rotary evaporator under normal pressure at 60° to ensure complete hydration of the film. The prepared niosomal preparations were stored at 4° to 8° in a refrigerator for further evaluations[15,16].
Preparation of proniosomes:
Proniosome formulations were prepared by the slurry method. In brief, accurately weighed amounts of lipid mixture comprising of tween 60 and cholesterol, with 5 μM dicetyl phosphate were dissolved in 4 ml chloroform. The drug was dissolved in 6 ml methanol and the resultant solutions were transferred to a 250 ml round bottom flask having a maltodextrin carrier. Additional chloroform:methanol solution was added to form slurry in the case of inferior surfactant loading. The flask was attached to a rotary flash evaporator to evaporate solvent at 100-150 rpm, a temperature of 60° and a reduced pressure of 600 mm of Hg until the mass in the flask had become a dry, free flowing product. After ensuring the complete removal of the solvent, the resultant materials were further dried overnight in a desiccator under vacuum at room temperature. These proniosome granules were stored in a tightly closed container at refrigerator temperature at 4° to 8° until further evaluation. Blank proniosomes were made in the same way without incorporating drug[17,18]. The composition of abacavir sulphate niosomal and proniosomal formulations are represented in Table 1.
| Composition | Niosome | Proniosome | ||
|---|---|---|---|---|
| DNF | BNF | DPF | BPF | |
| Abacavir sulphate | 25.00 mg | - | 25.00 mg | - |
| Cholesterol | 96.66 mg | 96.66 mg | 96.66 mg | 96.66 mg |
| Tween 60 | 312.50 µl | 312.50 µl | 312.50 µl | 312.50 µl |
| Dicetyl phosphate | 2.73 mg | 2.73 mg | 2.73 mg | 2.73 mg |
| Maltodextrin | - | - | 500.00 mg | 500.00 mg |
Note: DNF-Drug loaded niosomal formulation; BNF-Blank niosomal formulation; DPF-Drug loaded proniosomal formulation and BPF-Blank proniosomal formulation.
Table 1: Composition of Abacavir Sulphate Niosomal and Proniosomal Formulations.
Characterization of niosomes and proniosomes:
The niosome and proniosome derived formulations were characterized for vesicle formation by the optical microscopy method[5]. Vesicle size, size distribution and zeta potential of niosomes and niosome vesicles derived from proniosome samples were determined by photon correlation spectroscopy using the Malvern Zetasizer[19]. Free abacavir sulphate was separated from conventional niosomes and proniosome derived niosomes by centrifugation at 15 000 rpm and 4° for 1 h using a cooling centrifuge and the encapsulation efficiency was determined[20]. Abacavir sulphate content in both formulations was estimated at 285 nm by a Ultraviolet (UV) spectrophotometric method[21]. The morphology of abacavir sulphate optimized niosomal formulation was investigated by transmission electron microscopy[18]. In vitro release model of niosomal dispersion was carried out by dialysis bag method[22,23]. 3 ml of abacavir sulphate noisome dispersion was taken in dialysis bag (Hi Media). Dialysis bag was mounted in a beaker containing 100 ml of 0.1N HCl and pH 6.8-phosphate buffer. Magnetic stirrer was used and the temperature was maintained at 37°±1°. Samples were collected periodically up to 24 h. The sink condition was continued throughout the experiment. The withdrawn samples were suitably diluted and analyzed for drug content using UV spectrophotometer at 285 nm keeping phosphate buffer pH 7.4 as blank. All the observations were made in triplicate. The exterior characteristics of the proniosome powder and maltodextrin were examined by scanning electron microscope (JSM 6390LA, Jeol, Tokyo, Japan)[24]. The physical stability study was carried out to investigate the degradation of the drug from niosome and proniosome formulations during storage[25,26].
The molecular state of abacavir sulphate in niosome and proniosome formulations was investigated by performing Differential Scanning Calorimetric (DSC) analysis. The DSC thermograms of pure Abacavir sulphate, the (DNF, Drug Loaded Proniosomal Formulation (DPF)), maltodextrin, tween 60 and cholesterol were obtained by a differential scanning calorimeter. Each formulation was positioned in flat bottomed aluminium pans and then crimped with an aluminum wrap. The samples were heated from 30° to 400° using a platinum crucible and heat flow rate was kept at 10°/min with a nitrogen stream at 20 ml/ min[10,24]. The crystallinity of abacavir sulphate after encapsulation into vesicular and provesicular system was evaluated by X-Ray Diffraction (XRD) recorded for pure abacavir sulphate, formulations (DNF, DPF) and individual formulation excipients. XRD analysis was carried out using an Ultima 3 theta-theta goniometer for pure drug, formulation excipients, lyophilized blank and Abacavir sulphate loaded niosome and proniosome formulations. The samples were measured with a K-beta filter, fixed monochromatic using 40 kV and 32 mA current. The continuous scanning was carried out at the scanning mode of 2θ[25,26].
Pharmacokinetics studies in rats:
Study design: The pharmacokinetic study was carried out with the approval of the Institutional Animal Ethical Committee (JKKN/IAEC/PHD/03/2017), J. K. K. Nattraja College of Pharmacy, Namakkal District, Tamil Nadu, India. The study was aimed to compare the pharmacokinetic profile of abacavir sulpahate-proniosomal formulation and niosomal formulations. The expression of several phase II drug metabolizing enzymes appears to be affected by female sex hormone in rats. So, in this study male Albino Wistar rats were selected for pharmacokinetic evaluation. The selected male Albino Wistar rats (200-250 g) had free access to water and food. Before dosing, the rats were kept for overnight fasting. The rats were divided into four groups containing three in each. The treated animals were set aside in separate cages and upheld under a laboratory environment. The study was planned as a single oral dose. All groups of animals received an equivalent of 8.57 mg of abacavir sulphate/kg body weight of rats. Group I received pure drug solution, Group-II received blank proniosome formulation, Group III received Abacavir sulphate niosomal formulation and Group IV received Abacavir sulphate proniosomal formulation. 500 μl blood samples were collected from the tail vein into heparinized tubes at 0.5, 1, 2, 4, 8, 12 and 24 h after administration.
The plasma was separated by centrifugation process at 10 000 rpm for 10 min in a micro-centrifuge (Eppendorf AG, Germany. 5430R,) and stocked at -20° until drug analysis was done by High-Performance Liquid Chromatography (HPLC) method[20,27].
Chromatographic conditions:
Chromatographic separations were made on Phenomenex C18 column (250×4.60 mm ID, 5 μm particle size) and the injected volume was 10 μl. The column temperature was maintained at 30°. The mobile phase was 0.1 % formic acid in milli-Q-water:0.1 % formic acid in methanol (70:30 % v/v). The absorbance was monitored at 298 nm. The chromatographic analyses were performed using HPLC system consisting of an Agilent 1200 Series components. Detection was achieved by a Diode Array Detector (DAD).
Preparation of standard solution:
Abacavir sulphate equivalent to 10 mg Abacavir was weighed into 10 ml standard volumetric flask, dissolved with methanol and shook well and made up to the mark with methanol. From the 100 µg/ml diluted stock solution, 1, 2, 5, 10, 25 and 50 µg/ml standard solution were prepared using methanol.
Sample preparation and analysis:
For analysis, 0.1 ml of plasma was transferred into the eppendorf vial and added 0.4 ml of methanol. It was shaken well and centrifuged with a cooling centrifuge at 3000 rpm for 10 min until the precipitate was settled completely. Decanted the supernatant and transferred in HPLC injector vial for HPLC-DAD analysis. 10 µl of the methanol aliquot of sample was injected into HPLC.
Screening parameters:
Pharmacokinetic parameters such as half-life (t1/2), time to peak concentration (Tmax), maximum plasma concentration (Cmax), Area Under the Curve (AUC), Area Under the Moment Curve (AUMC) and Mean Residence Time (MRT), were determined by the software program PKsolver using plasma concentration-time profile data for pure drug, optimized niosome formulation and optimized proniosome formulation[20,28].
Statistical analysis:
Data are presented as mean±Standard Deviation (SD). Statistical analysis was performed by Student t-test using GraphPad Prism software. Level of significance defined at p<0.05.
Results and Discussion
The present study was undertaken to formulate abacavir sulphate niosome by thin film hydration method and proniosome by slurry method. Based on the results of preformulation studies, 250:250 μM ratio concentration of tween 60: Cholesterol was fixed for formulations. The proniosome formulation by the slurry method was found to be a convenient method than the conventional niosome formulations prepared by thin film hydration method. The major difficulty found in thin film hydration method in the preparation of conventional niosome is the requirement of additional time for the entire hydration and prevent the loss of surfactant-lipid film still after the hydration period of 1 h at 60°. This problem may be occurred due to the conversion of thin film to viscous and adhere to the surface of the round bottom flask after the initial hydration. The formulation of proniosomes by the slurry method was found to be more convenient and the hydration of proniosome to niosome had taken a short period of time (2 min at 80°) in vortex mixture. The convenience during hydration of proniosome is due to the more surface area of the surfactant-lipid film that occurs over the water soluble carrier particle maltodextrin.
Most optical microscopy images of niosome and proniosome derived niosome vesicles are multilamellar, discrete and spherical with sharp boundaries without much aggregation (fig. 1). The size of blank and drug loaded niosome vesicle was found to be 179.1±7.43 nm and 182.1±16.69 nm respectively. In the case of proniosome formulation the size of blank and drug loaded formulations were found to be 171.4±6.05 nm and 175.0±5.12 nm respectively. The zeta potential values were not shown a statistically significant difference (p<0.05) between niosome and proniosome formulations. Poly dispersity index results had shown uniformity of niosome and proniosome vesicle sizes.
The results of encapsulation efficiency determination indicated that the niosome formulation (DNF: 83.02 %±1.085 %) and the proniosome formulation (DPF: 85.02 % ±1.56 %) had efficient encapsulation efficiency due to the presence of cholesterol, negative charge inducer dicetyl phosphate and hydrophilic surfactant tween 60. Furthermore, the abacavir sulphate proniosome formulations had shown statistically non-significant (p>0.05) higher encapsulation efficiency than niosome formulations. This may be due to the short hydration period of proniosome formulation than the time-consuming hydration period of niosome formulations. Uniformity in abacavir sulphate content of niosomal dispersion and proniosomal formulation was confirmed to ensure uniformity in dosages. The differences in drug content among DNF and DPF were statistically non-significant (p>0.05).
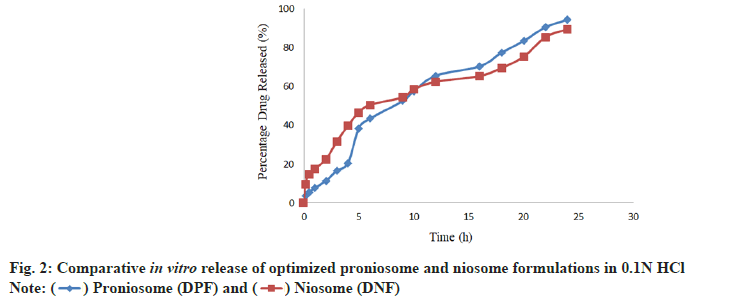
In vitro release was found to be appreciable for proniosome formulations as compared with niosome formulations due to their limited initial release and the gradual improvement in further release. The optimized proniosome formulation had shown the prolonged in vitro release of 94.46 %±1.39 % in 0.1N HCl and optimized niosome formulation had shown the release of 85.59 %±1.31 % in the same pH. Comparative in vitro release profile of optimized proniosome and niosome formulations has shown in the fig. 2. From the stability study results of vesicular size, encapsulation efficiency and drug content of the niosome and proniosome formulations it was concluded that the niosome formulation was stable at refrigeration temperature and proniosome formulation was quite stable at refrigeration temperature and room temperature as well.
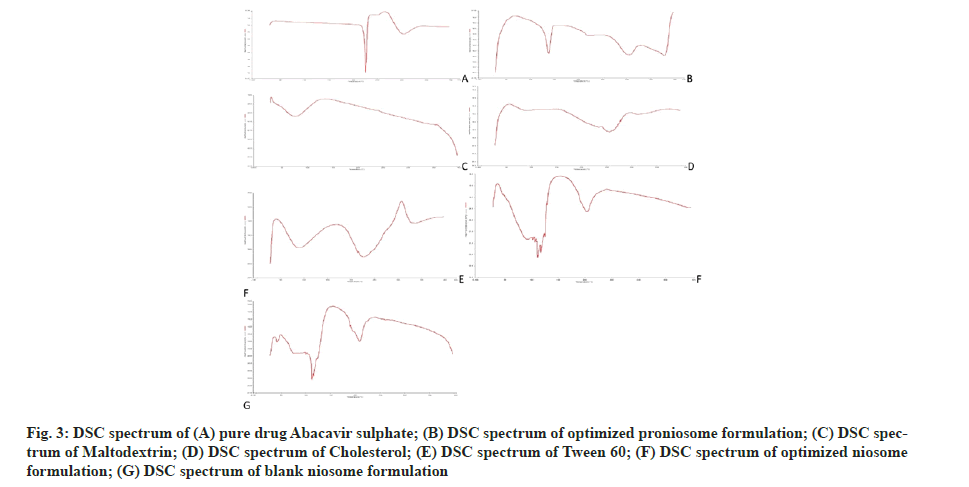
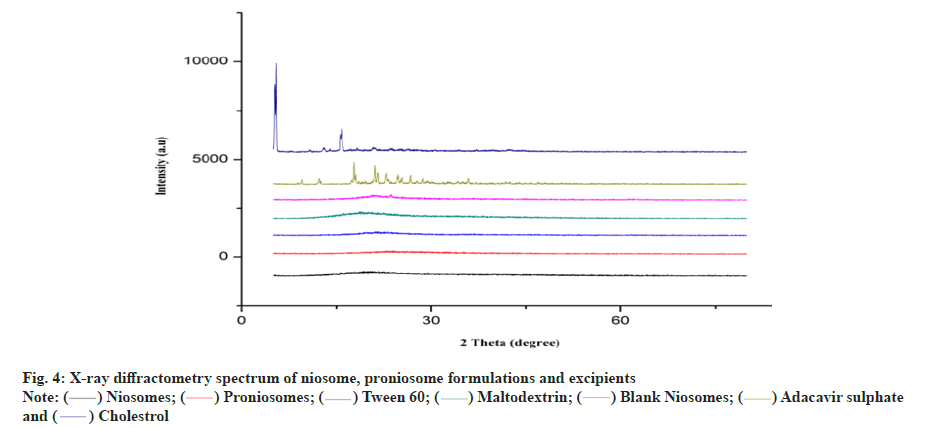
The endothermic peak of pure abacavir sulphate was observed at 228° in DSC spectrum (fig. 3). In the niosome and proniosome formulations, abacavir sulphate’s endothermic peak was disappeared, indicating that the drug is molecularly dispersed in both formulations. XRD diffractograms of pure abacavir sulphate, abacavir sulphate encapsulated niosome, abacavir sulphate encapsulated proniosomes, blank niosomes and proniosome excipients such as tween 60, maltodextrin and cholesterol were presented in fig. 4. The diffractograms point out the loss of crystallinity of abacavir sulphate after encapsulation. The diffraction patterns of abacavir sulphate illustrated many peaks around 2θ of 10°-30°, whereas a high intensity peak at 2θ=21° symbolizes its crystallinity. On the other hand, these peaks have vanished in the Abacavir sulphate encapsulated niosomes and proniosomes. It indicates that abacavir sulphate is molecularly dispersed or encapsulated in formulation and its crystal nature is not there in both niosome and proniosome formulations.
Fig. 3: DSC spectrum of (A) pure drug Abacavir sulphate; (B) DSC spectrum of optimized proniosome formulation; (C) DSC spectrum of Maltodextrin; (D) DSC spectrum of Cholesterol; (E) DSC spectrum of Tween 60; (F) DSC spectrum of optimized niosome formulation; (G) DSC spectrum of blank niosome formulation.
A HPLC method was used to analyze the plasma concentration of abacavir from the collected blood samples. Fig. 5 shows the plasma abacavir concentration profile as a function of time after the oral administration of pure abacavir sulphate solution, niosomal suspension and reconstituted proniosomal suspension. Pharmacokinetic parameters such as Tmax, Cmax, AUC, AUMC, MRT, t1/2, were determined by the software program PKsolver for pure drug, optimized niosome formulation and optimized proniosome formulation. The mean pharmacokinetic parameters of abacavir from the administered formulations are represented by Tmax(h), Cmax (μg/ml), AUC (μg.h.ml-1), AUMC (μg.h2.ml-1), MRT and t1/2 (h) are summarized in Table 2. All values were expressed in the mean±SD. The AUC of Abacavir sulphate was increased from 1.171 μg.h.ml-1 to 8.213 μg.h.ml-1 in niosome formulations and 11.302 μg.h.ml-1 in proniosome formulations (p<0.05). The value of Tmax was shifted from 2.034 h to 3.4 h for niosome formulations and 4.885 h for proniosome formulations (p<0.05).
| Parameters | Pure drug | DNF | DPF |
|---|---|---|---|
| t1/2 (h) | 1.441±0.071 | 2.411±0.124* | 3.442±0.198** |
| Tmax (h) | 2.034±0.359 | 3.401±0.535* | 4.885±0.643** |
| Cmax (μg/ml) | 0.212±0.013 | 0.895±0.054* | 0.889±0.032** |
| AUC0-t ( μg.h.ml-1) | 1.171±0.095 | 8.214±1.232* | 11.302±1.693** |
| AUC0-∞ ( μg.h.ml-1) | 1.171±0.047 | 8.270±0.901* | 11.816±1.211** |
| AUMC ( μg.h2.ml-1) | 4.765±0.756 | 56.263±2.357* | 115.459±2.008** |
| MRT (h) | 4.070±0.6656 | 6.803±1.081* | 9.771±1.147** |
Note: DNF-Drug loaded niosomal formulation; DPF-Drug loaded proniosomal formulation. Each value represents the mean±SD (n=3); *p<0.05 compared to the corresponding parameters of pure drug and **p<0.05 compared to the corresponding parameters of DNF.
Table 2: Mean Pharmacokinetic Parameters of Abacavir Sulphate.
Cmax of the abacavir sulphate was increased from 0.212 μg/ml to 0.895 μg/ml for niosome formulation and 0.889 μg/ml for proniosome formulation (p<0.05). The results demonstrate a significant increase in oral bioavailability of abacavir sulphate. The present study clearly showed that niosomes and proniosome derived niosomes are capable of encapsulating abacavir sulphate and the formulations are suitable for oral administration. The pharmacokinetic data obtained from in vivo study in rats shows better bioavailability of proniosome formulation when compared with pure abacavir sulphate and niosome formulation. It may be due to niosome and proniosome derived niosome vesicles of abacavir sulphate may efficiently take up by macrophages of the Reticulo Endothelial System (RES) in liver and spleen due to their suitable nanoscale sizes. The results reported here indicate that proniosomal carrier system can be successfully used to enhance the bioavailability of abacavir sulphate.
Acknowledgments:
We are grateful to Chairperson, Director, J.K.K. Nattraja College of Pharmacy and Dean, Department of Pharmaceutical Technology, Anna University for facilitating required research sources. Also, we want to express the gratitude to Cipla Ltd. for the gift sample of Abacavir sulphate.
Conflict of Interests:
The authors declared no conflict of interest.
References
- World Health Organization. HIV data and statistics. 2021.
- Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV AIDS 2012;4:117-24.
[Crossref] [Google Scholar] [PubMed]
- Aldous JL, Haubrich RH. Defining treatment failure in resource-rich settings. Curr Opin HIV AIDS 2009;4(6):459.
[Crossref] [Google Scholar] [PubMed]
- Kumar S. Proniosomal gel of flurbiprofen: Formulation and evaluation. J Drug Deliv Ther 2012;2(1):1-5.
- Akhilesh D, Hazel G, Kamath JV. Proniosomes–A propitious provesicular drug carrier. Int J Pharm Pharm Sci Res 2011;1(3):98-103.
- Akhilesh D, Faishal G, Prabhu P, Kamath J. Development and optimization of proniosomes for oral DELIVERY of glipizide. Int J Pharm Pharm Sci 2012;4(3):307-14.
- Nasr M. In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. AAPS PharmSciTech 2010;11(1):85-9.
[Crossref] [Google Scholar] [PubMed]
- Kumar K, Rai AK. Development and evaluation of proniosome-encapsulated curcumin for transdermal administration. Trop J Pharma Res 2011;10(6):697-703.
- Sankar V, Ruckmani K, Durga S, Jailani S. Proniosomes as drug carriers. Pak J Pharm Sci 2010;23(1):103-7.
[Google Scholar] [PubMed]
- Sahoo RK, Biswas N, Guha A, Sahoo N, Kuotsu K. Development and in vitro/in vivo evaluation of controlled release provesicles of a nateglinide–maltodextrin complex. Acta Pharm Sin B. 2014;4(5):408-16.
[Crossref] [Google Scholar] [PubMed]
- Jain NK, editor. Controlled and novel drug delivery. CBS publishers & distributors; 1997.
- Hu C, Rhodes DG. Proniosomes: A novel drug carrier preparation. Int J Pharm 1999;185(1):23-35.
[Crossref] [Google Scholar] [PubMed]
- Sudhamani T, Priyadarisini N, Radhakrishnan M. Proniosomes-a promising drug carriers. Int J PharmTech Res 2010;2(2):1446-54.
- Singla S, HariKumar SL, Aggarwal G. Proniosomes for penetration enhancement in transdermal system. Int J Drug Dev Res 2012;4(2):1-13.
- Hao YM. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm 2011;403(1-2):245-53.
[Crossref] [Google Scholar] [PubMed]
- Akbari V, Abedi D, Pardakhty A, Sadeghi-Aliabadi H. Release studies on ciprofloxacin loaded non-ionic surfactant vesicles. Avicenna J Med Biotechnol 2015;7(2):69-75.
[Google Scholar] [PubMed]
- Blazek–Welsh AI, Rhodes DG. SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharm Res 2001;18(5):656-61.
[Crossref] [Google Scholar] [PubMed]
- Naggar VF, El Gamal SS, Allam AN. Proniosomes as a stable carrier for oral acyclovir: Formulation and physicochemical characterization. J Am Sci 2012;8(9):417-28.
- Khudair N, Agouni A, Elrayess MA, Najlah M, Younes HM, Elhissi A. Letrozole-loaded nonionic surfactant vesicles prepared via a slurry-based proniosome technology: Formulation development and characterization. J Drug Deliv Sci Technol 2020;58:101721.
- Ruckmani K, Sankar V, Sivakumar M. Tissue distribution, pharmacokinetics and stability studies of zidovudine delivered by niosomes and proniosomes. J Biomed Nanotechnol 2010;6(1):43-51.
[Crossref] [Google Scholar] [PubMed]
- Rangasamy M, Ayyasamy B, Raju S, Gummadevelly S, Shaik S. Formulation and in vitro evaluation of niosome encapsulated acyclovir. J Pharm Res 2008;1(2):163-6.
- Shilakari Asthana G, Sharma PK, Asthana A. In vitro and in vivo evaluation of niosomal formulation for controlled delivery of clarithromycin. Scientifica 2016;2016.
[Crossref] [Google Scholar] [PubMed]
- Parthibarajan R, Rubinareichal C, Loganathan S. Formulation and evaluation of methotrexate proniosomal powder. Int J Pharm Pharm Sci 2012;4(11):175-8.
- Gurrapu A, Jukanti R, Bobbala SR, Kanuganti S, Jeevana JB. Improved oral delivery of valsartan from maltodextrin based proniosome powders. Adv Powder Technol 2012;23(5):583-90.
- Devi AS, Pinnika A, Divya P. Formulation and evaluation of candesartan cilexetil transdermal proniosomal gel. J Drug Deliv Ther 2014;4(2):90-8.
- Aburahma MH, Abdelbary GA. Novel diphenyl dimethyl bicarboxylate provesicular powders with enhanced hepatocurative activity: Preparation, optimization, in vitro/in vivo evaluation. Int J Pharm 2012;422(1-2):139-50.
[Crossref] [Google Scholar] [PubMed]
- Agarwal S, Mohamed MS, Raveendran S, Rochani AK, Maekawa T, Kumar DS. Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. RSC Adv 2018;8(57):32621-36.
[Crossref] [Google Scholar] [PubMed]
- Ahmed S, El-Setouhy DA, Badawi AA, El-Nabarawi MA. Provesicular granisetron hydrochloride buccal formulations: In vitro evaluation and preliminary investigation of in vivo performance. Eur J Pharm Sci 2014;60:10-23.
[Crossref] [Google Scholar] [PubMed]

 .
.
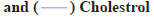 .
.
 .
.