- *Corresponding Author:
- A. Swargiary
Department of Zoology, Bodoland University, Kokrajhar-783 370, India
E-mail: ananbuzoo101@gmail.com
| Date of Submission | 05 July 2016 |
| Date of Revision | 09 December 2016 |
| Date of Acceptance | 02 March 2017 |
| Indian J Pharm Sci 2017;79(2):212-219 |
Abstract
Hodgsonia heteroclita (Roxb) is an important medicinal plant of Northeast India. The fruit pulp of H. heteroclita is traditionally used as antidiabetic medicine. Due to its pharmacological properties, the present study was aimed to investigate the phytochemical, antioxidant and heavy metal contents of the plant. Preliminary phytochemical screening revealed the presence of phytochemicals like phenolics, flavonoids, alkaloids, saponins, and steroids. The heavy metal content when analysed using Analytik Jena AAS vario-6 Graphite furnace spectrometer revealed highest content of iron followed by chromium and copper. Two toxic metals, cadmium and lead were found within the acceptable range. The antioxidant capacities of alcoholic extract of plant was studied by 1,1-diphenyl-2-picryl-hydrazyl, ferric reducing antioxidant power assay, lipid peroxidation scavenging activity assay and phosphomolybdate assay showed significant free radical scavenging potential. Pearson correlation revealed strong relationship between the phytochemical contents and antioxidant capacity of the plant. The present study revealed that the plant extract possessed good antioxidant activity and less quantity of toxic metals, which therefore can be used as a source of natural free radical scavenger. However, further study need to be carried out to know its mode of action.
Keywords
Wild plants, phytochemicals, antioxidant, trace elements, Assam
Wild plants remain to be a major source of traditional medicine in rural areas of North-Eastern (NE) region of India. In this part of India several wild and aromatic plants have traditionally been used as medicine against several health complications such as heart attack, cancer, diabetes, malaria, jaundice, inflammation and wound healing [1-3]. Despite of remarkable progress in the field of medical sciences and synthetic medicines more than 25% of commercial drugs/chemicals come directly or indirectly from plants. According to World Health Organization (WHO) report, more than 80% population of developing countries like India depends on traditional medicine for daily healthcare needs because of its easy accessibility, less preparation costs and absence of any undesirable side effects [4,5]. Over the last few decades several potent chemotherapeutic drugs and molecules have been derived from plants. Out of 20% plant species studied scientifically worldwide, only about 6% are screened for its pharmaceutical potential [6,7]. Screening of various phytochemical constituents and antioxidant properties including heavy metal is a major part of pharmaceutical drug discovery. Although NE India is full of medicinal plants very few studies have been carried out to explore its phytochemical contents and antioxidant properties [8-10]. In addition to antioxidant activities, presence of metallic elements at certain concentration is beneficial to both plants and animals [11]. Trace metals serve either as cofactors or activators of enzymes forming enzymes/ substrate-metal complex and exert catalytic property or regulators of nerve transmission, muscle contraction, osmotic pressure and salt-water balance [12]. It is known that several elements such as cobalt (Co), copper (Cu), chromium (Cr), iron (Fe), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), selenium (Se) and zinc (Zn) are essential compounds required for various biochemical and physiological functions. Inadequate supply of these micro-nutrients results in variety of deficiency diseases [13]. On the other hand, heavy metals like arsenic (As), cadmium (Cd), Cr, lead (Pb) and mercury (Hg) do not have any well-known biological role and are known to be systemic toxicant that induces multiple organ damages and diseases [14].
Hodgsonia heteroclita (Roxb), family Cucurbitaceae, is a perennial, climber plant that reaches up to 30 m in length and grows well in hilly terrain of southern Asia such as Bangladesh, Bhutan, Cambodia, Laos, Myanmar, Thailand, Vietnam and India. It is a deciduous plant having a long life span of up to 70 y [15]. The flower and fruit setting of plant is temperature dependent and flowers open only during the night time. In the NE region of India, the plant is mainly distributed in the hilly areas of Assam, Arunachal Pradesh, Meghalaya, Nagaland and Mizoram. Distributed within the geographical locations of 89° 50/E to 96° 10/E and 24° 30/N to 28° 10/N, Assam is one among the richest biodiversity zones of NE India with diverse ethnicity and rich flora and fauna. Several medicinal and wild edible plants have been studied for its pharmacological properties from this part of India [16,17]. Inhabited with different ethnic groups like Bodos, Rabhas, Mishing and Garo this part of India is rich in traditional knowledge of healthcare systems. H. heteroclita is one such traditionally used medicinal plant, the fruit extract of which is used as antihyperglycemic agent [18]. The plant is also reported to be used against various ailments like nose complain, fever, helminth and bacterial infections [19]. Although the fruit extract of H. heteroclita is used by the local people as antihyperglycemic agent, to the best of our knowledge no scientific report has been published on the antioxidant activity and heavy metal content of this plant. In view of its medicina value, the present study was designed to explore the phytochemical and heavy metal content and antioxidant potential of H. heteroclita.
Materials and Methods
Ascorbic acid (AA), gallic acid, quercetin, aluminum chloride (AlCl3), ferric chloride (FeCl3), Folin- Ciocalteu, bovine serum albumin (BSA), oxalic acid, thiobarbituric acid (TBA), 1,1-diphenyl-2- picryl-hydrazyl (DPPH), trichloroacetic acid (TCA), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), sulphuric acid (H2SO4), hydrochloric acid (HCl), ammonium molybdate, potassium ferricyanide (K3Fe(CN)6), sodium dodecyl sulphate (SDS), chloroform and alcohols were purchased from HiMedia Laboratories, Mumbai and SRL Pvt. Ltd., Mumbai, India. All the chemicals used were of analytical grade.
Collection, identification and preparation of plant extract:
Fresh fruits of H. heteroclita were collected from nearby jungles of Kokrajhar town and were identified in the Department of Botany, Bodoland University. After collection, the fruits were washed with distilled water, pulp extracted and completely dried in hot air oven at 50°. Dried samples were ground to a powder and soaked in 80% methanol. Solution was filtered after 24 h of soaking and fresh solvent was added. The process was repeated four times and the filtrate obtained was evaporated in a rotary evaporator. Dry, semi-solid extracts (crude extract) obtained was kept at 4° for further use.
Heavy metal analysis:
Heavy metal content of plant was analysed following the method reported by Welz and Sperling [20]. Briefly, 1 g of plant powder was digested with concentrated HNO3:HCl (3:1 ratio) at 85° for 3 h. After adding 1 ml of concentrated HClO4, the solution was filtered and diluted to 50 ml of distilled water. An Analytik Jena AAS vario-6 Graphite furnace spectrometer furnished with PC-controlled 6-piece lamp turret and argon gas supply was used for all of the absorption measurements of metal contents of the plant. The elements instrumental conditions are given in Table 1.
| Element | Wavelength (nm) |
Slit width (nm) | Atomisation temperature (°) | Matrix modifiers | Interference wavelength (nm) |
|---|---|---|---|---|---|
| Cr | 357.9 | 0.8 | 2100-2200 | NH4H2PO4 | Fe 358.1, Nb 358.0 |
| Mn | 279.5 | 0.2 | 1600-1650 | Mg(NO3)2+Pd(NO3)2 | Mg 279.5, Fe 279.5, Pb 280.2 |
| Fe | 248.3 | 0.2 | 1850-2050 | Mg(NO3)2 | -- |
| Cu | 324.8 | 0.8 | 1800-1900 | -- | Ni 324.3, Mn 324.9, Pd 324.3, Ag 324.8, Eu 324.8 |
| Zn | 213.9 | 0.8 | 1000-1100 | Pd(NO3)2 | Cu 213.9, Te 214.3, As 214.4, Fe 213.6, Fe 213.9 |
| Cd | 228.8 | 0.8 | 900-1200 | NH4H2PO4+Mg(NO3)2 | As 228.9, Fe 228.8 |
| Pb | 217 | 0.5 | 1200-1350 | Pd(NO3)2+Mg(NO3)2 | Cu 216.5, Fe 216.7, Ni 216.6, Sb 217.6, Pt 216.5 |
Table 1: Instrumental analytical conditions of aas vario-6 graphite furnace elements instrument
Qualitative phytochemical study:
The presence of phytochemicals such as flavonoids, phenolics, reducing sugar, saponins, steroids and tannins in the plant was analysed following standard protocols [21,22]. For anthraquinones, 100 mg of plant extract was boiled with 10 ml of 1% HCl and filtered. Filtrate shaken with 3 ml of benzene and 2 ml of 10% ammonia solution and mixture was filtered. Presence of anthraquinone was confirmed by the presence of pink, violet or red colour in the ammonical phase of the solution. Presence of cardiac glycoside was detected when 5 ml (10 mg/ml methanol) of plant extract mixed with 2 ml glacial acetic acid and few drops of FeCl3 were added. Appearance of a brown ring at the interface of solution after the addition of 1 ml of concentrated H2SO2 established the presence of cardiac glycosides. Presence of flavonoid was confirmed by the appearance of yellow colour in a solution of 1 ml of plant extract and few drops of 1% AlCl3 solution.
Phenolic content of plant was detected when 0.5 g of plant extract dissolved in water, mixed with a few drops of 5% FeCl3 solution, appearance of a dark green colour indicated the presence of phenolic compounds. For phlobatannin detection, 50 mg of extract was boiled in 1% HCl and deposition of a red precipitate indicated its presence. The presence of free reducing sugars was detected by the appearance of a red precipitate in a solution of 2 ml of plant extract (50 mg/ml) when mixed with equal volumes of Fehling’s solution A and B. Saponins were detected by boiling 50 mg extract with 10 ml distilled water, filtered and was mixed with distilled water and shaken vigorously until a stable persistent froth is obtained. The frothing was mixed with 2 to 3 drops of olive oil and shaken vigorously. The formation of emulsion indicated the presence of saponins. Presence of tannins was detected by boiling 50 mg plant extract with 5 ml of distilled H2O, and addition of a few drops of 1% AlCl3 turned the solution into blue-black or blue green colour. Presence of terpenoids was confirmed by mixing 5 ml (1 mg/ml) of extract with 2 ml of chloroform and 3 ml of H2SO4. A reddish brown colour at the interface confirmed the presence of terpenoids.
Quantitative phytochemical study:
The presence of total carbohydrate content in the plant extract was estimated following the anthrone method [23]. Results were expressed as μg sugar/mg crude extract using the calibration curve of glucose (y=0.0017x; R2=0.9996). The protein content of the plant was estimated following Lowry’s method [24]. Results were expressed as μg protein/mg plant extract using the calibration curve of BSA (y=0.0061x; R2=0.9968). The vitamin-C content was estimated titrimetrically [23]. Briefly, 1 ml of 1 mg/ml of AA solution in 4% oxalic acid and the plant extract was taken in separate conical flasks and 10 ml of 4% oxalic acid was added in each flask. The mixture was then titrated against 2,6-dichlorophenol indophenol till the end-point colour pink was observed and the amount of dye consumed was noted. Results expressed as μg ascorbic acid equivalent (AAE)/mg crude extract.
The total phenolic content (TPC) of H. heteroclita is estimated by Swin and Hills with slight modification [25,26]. Briefly, 1 ml of plant extracts (200 μg/ml) was mixed with 3 ml of 10% Folin-Ciocalteu reagent and 0.5 ml of sodium carbonate (10% w/v). The mixture was vortexed for 15 s and incubated at 40° for 30 min for colour development. The absorbance was measured at 765 nm. The amount of TPC was calculated from a calibration curve of gallic acid (y=0.0161x; R2=0.9963) and the results expressed as mg gallic acid equivalent (GAE)/mg crude extract.
The flavonoid content was determined by mixing 1 ml of plant extract (two concentrations 0.25 and 0.5 mg/ml, prepared in 80% ethanol) with 0.5 ml of 2% AlCl3 (prepared in 80% ethanol). The assay mixture was made 3 ml by adding distilled water. The mixture was incubated at room temperature for 30 min and the formation of yellow colour was measured at 430 nm [27]. The total flavonoid content was calculated from the standard curve (y=0.01x; R2=0.9786) of quercetin (concentration 5-25 μg/ml) and the values represented as μg quercetin equivalent (QE)/mg of crude extract.
Total antioxidant activity (phosphomolybdate assay):
The total antioxidant capacity (TAC) of the plant extract was estimated following phosphomolybdate assay [28]. One millilitre of the extract (500 μg/ml) was mixed with 1 ml distilled water and 1 ml reagent solution (600 mM sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The reaction mixture was incubated at 95° for 30 min and absorbance was measured at 695 nm against blank solution. TAC was expressed as μg AAE/mg plant extract.
DPPH radical scavenging activity:
The DPPH scavenging activity of the extract was estimated by mixing 2 ml of DPPH reagent (0.135 mM, prepared in methanol) with 1 ml of AA and 1 ml plant extracts (25-500 μg/ml). After 30 min of incubation at room temperature decrease in absorbance was observed at 517 nm [29]. The scavenging activity of plant extract was calculated using Eqn., DPPH scavenging activity (%) = (Abs control–Abs sample/Abs control)×100, where, Abs control is the absorbance of DPPH and methanol, Abs sample is the absorbance of DPPH and plant extract or AA.
Ferric reducing antioxidant power assay (FRAP assay):
One millilitre of AA (5-100 μg/ml) and plant extract (25-500 μg/ml) was mixed with 2 ml of FRAP reagent, which is a mixture of 10 ml acetate buffer (pH 3.6), 1 ml of 10 mM TPTZ solution in 40 mM HCl and 1 ml of 20 mM FeCl3. After 30 min of incubation at 50° the absorbance was measured at 593 nm. The FRAP activity of plant extracts were compared with that of the standard AA [30].
Lipid peroxidation scavenging activity assay (TBARS assay):
TBARS assay was done to measure the lipid peroxide formation using egg yolk homogenate as lipid-rich media [31]. Lipid peroxidation was induced in 0.1 ml of egg homogenate (10% v/v) by adding 1 ml plant extract/standard (concentration range 0.05-1.0 mg/ ml) and 0.05 ml of 75 mM FeSO4. The mixture was incubated for 30 min at 37°. Then, 1 ml each of 10% TCA and 0.8% (w/v) TBA in 1.1% SDS was added and the resulting mixture vortexed and heated for 1 h at 95°. After cooling, 3 ml of butanol was added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured at 532 nm. Inhibition of lipid peroxidation (%) by the extract was calculated using Eqn., percent inhibition = (Abs control–Abs sample/Abs control)×100, where, Abs control is the absorbance of the reaction mixture without the sample or standard, Abs sample is the absorbance of reaction mixture with sample/standard.
Statistical analysis:
All data are presented as mean ± standard deviation (SD) for at least three replications for each experiment. The results are considered to be significant at P<0.05. All statistical analysis was performed in MS-Excel and the graphs were drawn using OriginPro8 software. Pearson correlation was done using IBM SPSS Statistics 23.
Results and Discussion
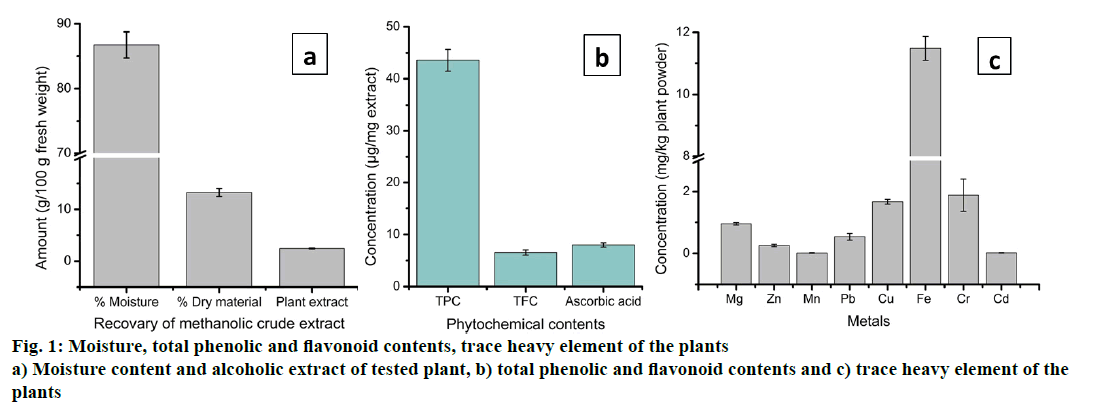
The dry weight of the plant and its moisture content, methanolic crude extract and heavy metal content of H. heteroclita is shown in the Figure 1. The present study showed that the percentage moisture content, dry weight and methanol extract recovered from 100 g of fresh fruit pulp were 86.72%, 13.27 g and 2.44 g, respectively (Figure 1a and b). The semi-solid plant extract recovered was yellow in colour having strong bitter taste and dissolved completely in water. Qualitative study showed the presence of phytochemical contents such as phenolics, flavonoids, alkaloids, saponins, glycosides etc. in the methanol extract of H. heteroclita. The presence of high quantity of phytochemicals including secondary metabolites such as phenolics and flavonoids, might contribute to the pharmacological activity possessed by certain plants [32]. Flavonoids are important secondary metabolites that exhibit medicinal properties such as antioxidant, antiinflammatory, anticancer, antibacterial and antiviral activity [33]. Table 2 showed the phytochemical contents of the tested plant. Qualitative analysis of H. heteroclita revealed the presence of alkaloids, flavonoids, phenol, reducing sugar, saponins, steroids, tannins, terpenoids and cardiac glycosides while anthraquinone and phlobatannins were found to be absent. In addition, the present study revealed high concentrations of carbohydrate, protein, vitamin C, TPC and TFC in the plant (Table 3). Concentration of carbohydrate was found to be highest 445.11 ± 3.09 μg/mg extract followed by protein 73.95 ± 2.52 μg/mg extract. Similarly, the concentrations of TPC, TFC and AA were found to be 43.56 ± 2.09, 6.51 ± 0.51 and 24.46 ± 1.13 μg/ mg extract, respectively (Table 3). Presence of high concentration of carbohydrates and proteins indicate high nutritional value of the plant. Vitamin C or AA is an important biomolecule with free radical scavenging property. Although most mammals can synthesize AA, humans cannot, due to defective L-gulono-1,4- lactone oxidase, the last enzyme in the AA biosynthetic pathway. Therefore, humans need to obtain AA from dietary sources [34]. Various phytochemical studies have revealed the concentration of AA ranging from 8 to 1426 μg/g fresh weight [35].
| Phytochemicals | Reagents/chemicals | Observation | Results |
|---|---|---|---|
| Alkaloids | Wagner's reagent | Brown/red precipitate | + |
| Flavonoids | FeCl3 | Blue green colour | + |
| Phenol | Folin-Ciocalteu | Blue green colour | + |
| Reducing sugar | Fehling’s solution | Orange red precipitate | + |
| Saponins | Distilled water heating | Frothing seen | + |
| Steroids | Liebermann-Burchard test | Bluish green | + |
| Tannins | FeCl3 | Blue green precipitate | + |
| Terpenoids | CHCl3+H2SO4 | Reddish brown ring | + |
| Anthraquinone | C6H6+NH3 | Red, pink or violet colour | - |
| Cardiac glycosides | FeCl3+H2SO4 | Brown ring | + |
| Phlobatannins | HCl+boil | Red precipitate | - |
Table 2: Phytochemical screening of methanol extracts of H. heteroclita
| Phytochemicals contents/IC50 values | H. heteroclita | Standard chemical |
|---|---|---|
| Carbohydrates (µg/mg extract) | 445.11 ± 3.09 | |
| Protein (µg/mg extract) | 73.95 ± 2.52 | |
| Vitamin-C (µg AAE/mg extract) | 8.00 ± 0.04 | |
| TPC (µg GAE/mg extract) | 43.56 ± 2.09 | |
| TFC (µg QE/mg extract) | 6.51 ± 0.51 | |
| TAA (µg AAE/mg extract) | 24.46 ± 1.13 | |
| DPPH, IC50 (µg) | 1284.93 ± 31.20 | 6.31 ± 0.13* |
| TBARS, IC50 (µg) | 431.16 ± 36.37 | 100.17 ± 3.07* |
Table 3: Phytochemical content and ic50 values of free radical scavenging assays of methanol extract of H. heteroclita
The trace element contents in the fruit pulp of H. heteroclita are given in the Figure 1c. The different trace elements such as Mg, Zn, Mn, Pb, Cu, Fe, Cr and Cd estimated in the present study ranged from 0.011 to 11.48 mg/kg plant powder. Trace elements are important molecules for normal functioning of many biological systems. Normal functioning of many proteins, enzymes, metabolic and catabolic activities is regulated by the presence of trace elements. For instance, Fe is an important trace element of biological importance deficiencies of which may lead to vital physiological imbalances in the body. According to WHO estimates, worldwide about 700 million people are suffering from Fe deficiency [36]. In the present study, out of eight trace elements, Fe was found to be highest (11.48 ± 0.386 mg/kg dry plant powder), while Mn showed lowest concentration (0.011 ± 0.001 mg/kg) (Figure 1c). Cu and Cr were present in high concentration compared to other elements and the values are 1.667 ± 0.006 and 1.883 ± 0.523 mg/kg plant powder, respectively. Besides its significant biological importance, there are certain trace elements, which are toxic leading to several diseases and health complications [37]. A large number of researches have investigated the toxicity and side effects of these toxic elements. Among the heavy metals, Pb and Cd were toxic to human even at very low concentrations. According to the United States Pharmacopeia, Limits for Nutritional Supplement, the accepted standard toxicity levels of Pb and Cd for ingested products is 10.0 and 3.0 ppm, respectively. In the present study, the fruit pulp of H. heteroclita was found to contain very little concentration of Pb (0.534 ± 0.107 mg/kg) and Cd (0.015 ± 0.002 mg/kg), which is much less as per the toxicity level.
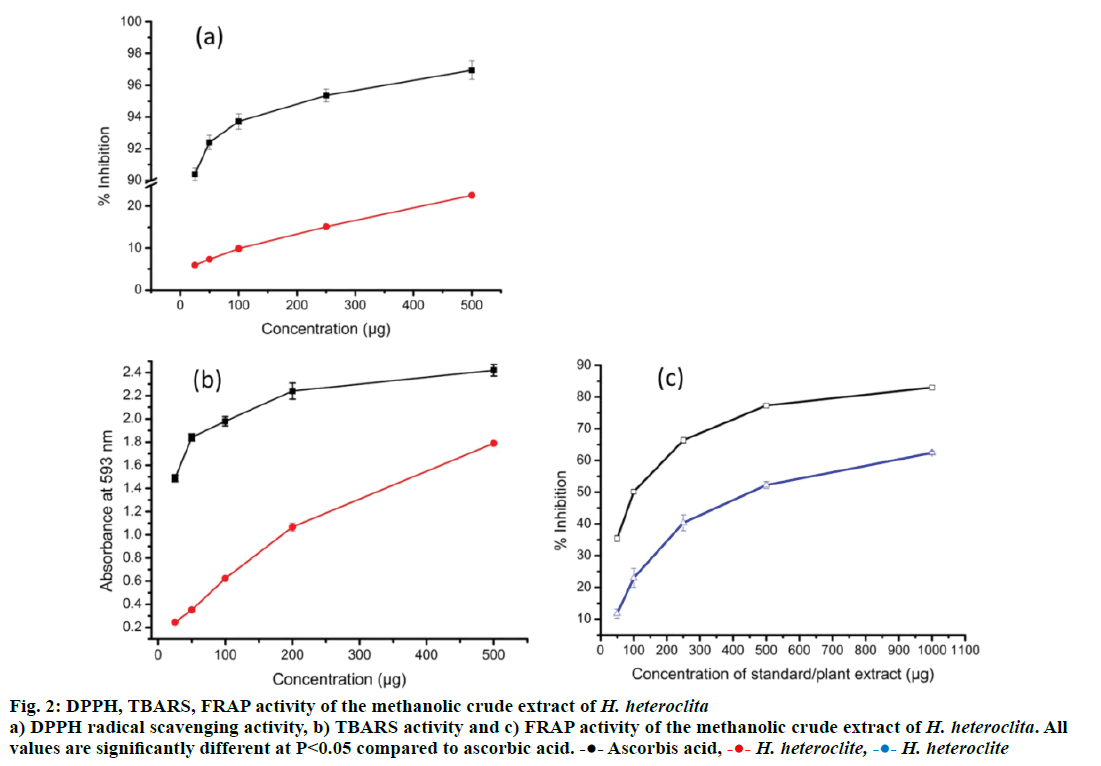
Free radicals, also known as reactive oxygen species (ROS) are atoms or group of atoms with unpaired electrons that are generated in the body during normal physiological conditions. ROS are harmful to the body leading to diseases such as cancer and diabetes [38]. Our body has an innate capacity of neutralizing those harmful ROS called antioxidant capacity or property. However, our innate antioxidant capacity to neutralize ROS is limited to certain concentration of free radicals and beyond that concentration our body fails to neutralize ROS. Plants act as a source of antioxidant molecules and can be used to boost our antioxidant capacity. In the present study, the total antioxidant activity of H. heteroclita was found to be 24.46 ± 1.13 μg AAE/mg extract (Table 3). Similarly, DPPH, TBARS and FRAP assay of antioxidant activity revealed concentrationdependent activity of H. heteroclita (Figure 2). Increase in plant extract showed increased antioxidant activity with R2=0.9909 and R2=0.9947 for DPPH and TBARS, respectively. FRAP result also showed good relation between extract concentration and peroxidation activity (R2=0.8338). The IC50 values of DPPH and TBARS assay is found to be 1284.93 ± 31.20 μg/ml and 431.16 ± 36.37 μg/ml, respectively. Standard AA showed better antioxidant property with IC50 values of 6.31 ± 0.13 and 100.17 ± 3.07 μg/ml for DPPH and TBARS, respectively. The presence of phytochemical contents, mainly TPC, TFC and AA showed strong correlation with the antioxidant activity of the plant (Table 4). All the phytochemical contents viz. TPC, TFC and AA showed good correlation (P<0.05) with antioxidant capacity (TAA, DPPH and TBARS) of the plant extracts. The high content of phytochemicals appeared to have been responsible for the high antioxidant capacity of the plant. In many reports, high lipid peroxidation activity possessed by plant extracts could be correlated with high phenolic content and number of hydroxyl group in the compounds [39]. Similar to the present investigation, a large number of reports also showed increasing trend of reducing power activity with increase of plant concentrations [40]. Therefore, the reducing capacity of the plant extracts may function as an indicator of potential antioxidant capacity of the plant.
| AA | TPC | TFC | TAA | DPPH | TBARS | |
|---|---|---|---|---|---|---|
| AA | 1 | |||||
| TPC | 0.986 | 1 | ||||
| TFC | 0.990 | 1.000 | 1 | |||
| TAA | 0.997 | 0.969 | 0.975 | 1 | ||
| DPPH | 0.984 | 1.000 | 0.999 | 0.966 | 1 | |
| TBARS | 0.898 | 0.959 | 0.952 | 0.859 | 0.962 | 1 |
Table 4: Pearson correlation of the different in vitro antioxidant assays of H. heteroclita
Figure 2: DPPH, TBARS, FRAP activity of the methanolic crude extract of H. heteroclita a) DPPH radical scavenging activity, b) TBARS activity and c) FRAP activity of the methanolic crude extract of H. heteroclita. All
values are significantly different at P< 0.05 compared to ascorbic acid.  Ascorbis acid,
Ascorbis acid,  H. heteroclita,
H. heteroclita, H. heteroclita
H. heteroclita
The presence of phytochemicals such as phenolics, alkaloids, flavonoids, steroids and saponins in H. heteroclita provide a reason why the plant possessed biological activities that are of pharmacological significance. The presence of high phenolic, flavonoid compounds and vitamin C contents could be attributed to its pharmacological activity associated with free radical scavenging activity. Furthermore, evaluation of total antioxidant activity, DPPH, FRAP and TBARS also indicated the high potential of scavenging free radicals by the plant extract. The trace element content of H. heteroclita has also been found within the permissible limits from the studies. The present data would certainly help to ascertain the potency of the tested part of the plant for medicinal use and functional food and nutraceutical applications. Therefore, further investigations are needed for the isolation and identification of the active components of the plant and also to elucidate the mechanism of action responsible for the biological activity and antioxidant activities as well.
Acknowledgments
Authors thank the Head, Department of Zoology, Bodoland University for providing necessary facilities to carry out the work. We are also grateful to the Heads of SAIF, NEHU, Shillong, Department of Biotechnology, Bodoland University and Department of Food Processing and Technology, Central Institute of Technology, Kokrajhar, for providing instrumentation facilities. We also thank Dr. Sanjib Baruah, Department of Botany, Bodoland University for identifying the plant material.
Conflict of interests
Authors declare no conflict of interests.
Financial support and sponsorship
Nil.
References
- Das NJ, Saikia SP, Sarkar S, Devi K. Medicinal plants of North-Kamrup district of Assam used in primary healthcare system. Indian J Tradit Know 2006;5:489-93.
- Saikia AP, Ryakala VK, Sharma P, Goswami P, Bora U. Ethnobotany of medicinal plants used by Assamese people for various skin ailments and cosmetics. J Ethnopharmacol 2006;106:149-57.
- Choudhary MK, Bodakhe SH, Gupta SK. Assessment of the Antiulcer Potential of Moringaoleiferaroot-bark extract in rats. J Acupunct Meridian Stud 2013;6:214-20.
- Nyahangare ET, Mvumi BM, Mutibvu T. Ethnoveterinary plants and practices used for ecto-parasite control in semi-arid smallholder farming areas of Zimbabwe. J EthnobiolEthnomed 2015;11:30.
- http://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/.
- Lahlou M. The success of natural products in drug discovery. Pharmacol Pharm 2013;4:17-31.
- Cragg GM, Newman DJ. Natural products: A continuing source of novel drug leads. BiochimBiophysActa 2013;1830:3670-95.
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 2001;109:69-75.
- Vimal K, Gogoi BJ, Meghvansi MK, Singh L, Srivastava RB, Deka DC. Determining the antioxidant activity of certain medicinal plants of Sonitpur, (Assam), India using DPPH assay. J Phytol 2009;1:49-56.
- Swargiary A, Roy B. In vitroanthelmintic efficacy of Alpinianigraand its bioactive compound, astragalin against Fasciolopsisbuski. Int J Pharm PharmSci 2015;7:30-5.
- Handique JG, Boruah MP, Kalita D. Antioxidant activities and total phenolic and flavonoid contents in three indigenous medicinal vegetables of north-east India. Nat Prod Commun 2012;7:1021-3.
- Wintz H, Fox T, Vulpe C. Functional genomics and gene regulation in biometals research. BiochemSoc Transactions 2002;30:766-8.
- Ozcan MM, Akbulut M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem 2008;106:852-8.
- Prashanth L, Kattapagari KK, Chitturi RT, Baddam VR, Prasad LK. A review on role of essential trace elements in health and disease. J NTR Univ Health Sci 2015;4:75-85.
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metals toxicity and the Environment. EXS 2012;101:133-64.
- https://lilac.uni-hohenheim.de/de/publikationen/2007_Schreiter.pdf.
- Namsa ND, Mandal M, Tangjang S. Antimalarial herbal remedies of northeast India, Assam: An ethnobotanical survey. J Ethnopharmacol 2011;133:565-72.
- Narzary H, Swargiary A, Basumatary S. Proximate and vitamin-C analysis of wild edible plants consumed by Bodos of Assam, India. J MolPathophysiol 2015;4:128-33.
- Swargiary A, Boro H, Brahma BK, Rahman S. Ethno-botanical study of antidiabetic medicinal plants used by the local people of Kokrajhar district of Bodoland Territorial Council, India. J Med Plants Stud 2013;1:51-8.
- Basumatary S, Das AK, Raaman N, Sarma GD, Baalan L, Bora R. Phytochemical screening and antimicrobial activity of leaf and fruit extracts of Hodgsoniaheteroclita(Roxb.) Hook.f. & Thomson. J IntAcad Res Multidisciplinary 2015;3:358-66.
- Welz B, Sperling M. Atomic absorption Spectroscopy. 3rd ed. Weinheim: Wiley-VCH VerlagGmbh; 1999. p. 614-47.
- Trease GE, Evans WC. Pharmacognosy. 11th ed. London: Macmillian Publishers Ltd.; 1989. p. 60-75.
- Sofowara AE. Medicinal plants and traditional medicine in Africa. 2nd ed. Nigeria: Spectrum Books Ltd.; 1993. p. 289.
- Sadasivam S, Manickam A. Biochemical Methods. 3rd ed. New Delhi: New Age International; 2008.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J BiolChem 1951;193:265-75.
- Swain T, Hillis WE. Phenolic constituents of Prunusdomestica. I.—The quantitative analysis of phenolic constituents. J Sci Food Agr 1959;10:63-8.
- Iloki S, Lewis L, Rivera G, Gil A, Acosta A, Meza-cueto CY, Rubio-Pino JL. Effect of maturity and harvest season on antioxidant activity, phenolic compounds and ascorbic acid of MorindacitrifoliaL. (noni) grown in Mexico. Afr J Biotechnol 2013;12:4630-9.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Amer J EnolViticult 1965;16:144-58.
- Huda-Faujan N, Noriham A, Norrakiah AS, Babji AS. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr J Biotechnol 2009;8:484-9.
- Mehrotra MS, Kirar AV, Vats P, Nandi SP, Negi PS. Phytochemical and antimicrobial activities of Himalayan Cordycepssinensis(Berk.) Sacc. Indian J ExpBiol 2015;53:36-43.
- Iloki‑Assanga SB, Lewis‑Luján LM, Lara‑Espinoza CL, Gil‑Salido AA. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucidabuceras L. and Phoradendroncalifornicum. BMC Res Notes 2015;8:396.
- Ohkawa H, Onishi N, Yagi K. Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Patra B, Schluttenhofer C, Wu Y, Pattanaik S, Yuan L. Transcriptional regulation of secondary metabolite biosynthesis in plants. BiochimBiophysActa 2013;1829:1236-47.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: An overview. Scientific World J 2013;2013:162750.
- Gallie DR. Increasing vitamin-C content in plant foods to improve their nutritional value-successes and challenges. Nutrients 2013;5:3424-46.
- http://www.who.int/nutrition/publications/micronutrients/9241546123/en/.
- Liva NDR. Facing the problem of dietary-supplement heavy metal contamination: How to take responsible action. J Integr Med 2007;6:36-8.
- Kumpulainen JT, Salonen JT. Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease. London: Woodhead Publishing; 1999. p. 178-87.
- Weidner SS, Chrzanowski S, Karamać M, Król A, Badowiec A, Mostek A, et al. Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitiscalifornicaseeds submitted to cold stress conditions and recovery after the stress. Int J MolSci 2014;15:16211-25.
- Kibiti CM, Afolayan AJ. Preliminary phytochemical screening and biological activities of Bulbineabyssinicaseed in the folk medicine in the Eastern Cape Province, South Africa. J Evid Based Complementary Altern Med 2015;2015:617607.