- *Corresponding Author:
- S. K. Saha
Department of Zoology, Fish Biology Research Unit, Siksha Bhavana (Institute of Science), Visva-Bharati (A Central University), Santiniketan, West Bengal 731235, India
E-mail: sahaskvb@gmail.com
| Date of Received | 01 January 2021 |
| Date of Revision | 13 September 2021 |
| Date of Acceptance | 26 May 2022 |
| Indian J Pharm Sci 2022;84(3):660-668 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acacia auriculiformis, widely used for afforestation, produces large amount of fruits which has no specific use other than as fuel for cooking by the ethnic people. The aim of our study is to determine chemical constituents with their definite proportion from different solvent extracts of Acacia auriculiformis pericarp at different maturity stages. Qualitative phytochemical analyses of three different solvents extracts viz. methanolic, 80 % ethanolic and aqueous of nine different maturity stages pericarps were screened to identify the presence of secondary metabolites. Quantitative analyses were performed through standard biochemical procedures to obtain the amount of the secondary metabolites. Finally, liquid chromatographyelectrospray ionization-tandem mass spectrometry analysis was performed to identify different fractions of the major phytochemical groups. The biochemical assay of the preparation helped to identify saponin, phenols, tannin, flavonoid, alkaloid, steroid, terpenoids, glycosides, cardiac glycosides and reducing sugar as the major groups. Methanolic extract of the 9th maturity stage pericarp contains the highest amount of saponin and a fairly good amount of phenol, flavonoids, tannin and proanthocynidin. With the help of liquid chromatography-electrospray ionization-tandem mass spectrometry analyses 7 triterpenoid saponins (acaciasides) with their relative percentages, 22 flavonoids, 8 tannins and 1 phenolic rich fractiongalloyl glucose were detected. Among the 7 triterpenoid saponins, acaciasides A and B are worked out as the major components. A detail toxicological study may help to establish it as a substitute of conventionally used piscicides as well for other pharmaceutical purposes.
Keywords
Acacia auriculiformis pericarp, liquid chromatography-electrospray ionization-tandem mass spectrometry peaks, triterpenoid saponin, tannin, flavonoid
Acacia auriculiformis (A. auriculiformis), commonly called Northern black wattle and in Bengali as akashmoni, has been used widely in afforestation for its rapid growth rate. It produces large amount of fruits which have no particular utility other than as fuel by the ethnic people and that too causing coughing to the users. So, there is an availability of the fruit in plenty from a small area at a time. Research on chemical component of the fruit is still in its infancy; only 7 compounds have been identified till date[1-3]. Limited general pharmacological activities of the fruit ingredients have been established[4-8]. However, it is neither used in medicinal purpose at industrial scale nor explored for other usages. Though the whole plant with fruit is famous as one of the folk medicines in its natural habitat Australia, there is a dearth of information about detailed phytochemical analysis of the fruit. It is a general feature that chemical composition and percentage (%) content of the ingredients of a plant part varies with the plant’s habitats, maturity stages and extraction procedures[9-11]. At full maturity, the pods are split, the seeds are dispersed and the pericarps are shed off from the plant. It becomes easier to collect the fallen fruit parts lying underneath the plant. Keeping all these considerations in mind a comprehensive study has been apprehended for phytochemical analyses of Acacia pericarp to explore the possibility of its utilization. The present study is thus aimed to analyse the phytochemical contents of A. auriculiformis pericarp at different maturity stages.
Materials and Methods
Collection of plant material:
A. auriculiformis (A. Cunn. ex Benth.) pericarps of 9 different maturity stages were collected at every 15 d intervals, ranging from small and green to ripen. The samples were collected from Sonajhuri forest (23º41´36´´N 87º40´33´´E), a part of Ballavpur wildlife sanctuary adjacent to Santiniketan, Bolpur, West Bengal, India. The collected pericarps were cleaned, sundried and pulverized separately in powdered form and sieved through uniform mesh no. 40.
Preparation of Acacia pericarp extracts:
Solvent extractions of 9 different stages of pericarp were done with petroleum ether in 60°-80° followed with Chloroform (CHCl3). The aqueous extract were done soaking the pericarp powder in distilled water and subsequently filtered through Whatman filter paper No.1 and evaporated through rotary evaporator. Both alcoholic (methanolic and 80 % ethanolic) extractions were done in soxhlet apparatus at 40°-50° for 4 h and then evaporated through rotary evaporator.
Qualitative analyses of phytochemicals:
All the solvent extracts from 9 different stages of pericarp were screened to identify the presence of secondary metabolites viz. saponin, phenols, tannin, flavonoid, alkaloid, steroid, terpenoids, glycosides, cardiac glycosides, reducing sugar and starch using standard assay methods[12-15] for phytochemicals.
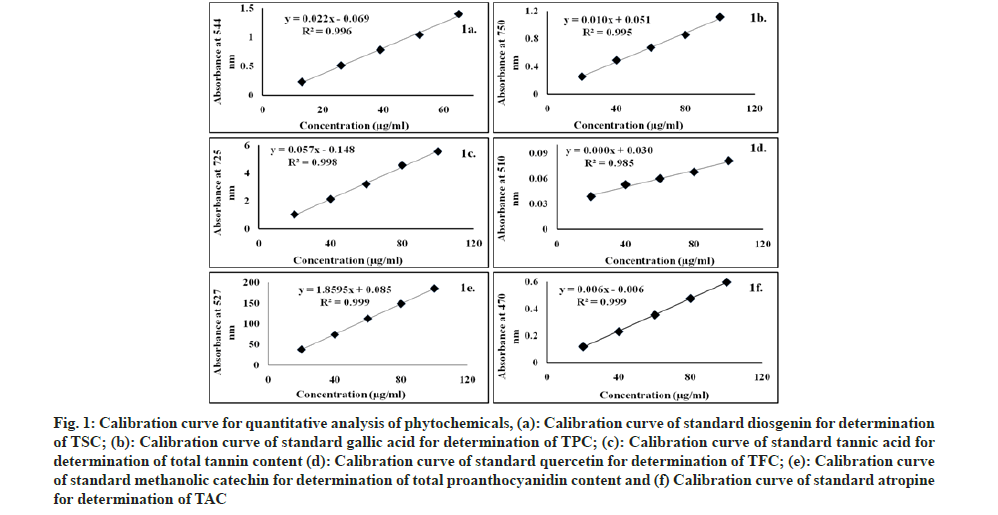
Total Saponin Content (TSC): A volume of 1 ml methanolic extract (1 mg/ml) was added to same volume 8 % vanillin (w/v) and incubated in ice-water bath. After adding 8 ml 77 % (v/v) sulphuric acid, the test tube was placed in hot water bath at 60° for 10 min. After cooled down in ice-water bath for 5 min, absorbance was measured at 544 nm by UVvisible spectrophotometer (Beckman Coulter DU730). Diosgenin standard curve was used. TSC was expressed as mg Diosgenin Equivalents (DE) per gram (g) of extract[16].
Total Phenolic Content (TPC): A volume of 1 ml methanolic extract (1 mg/ml) was taken with 9 ml distilled water. Then 1 ml of Folin and phenol reagent was added and mixed thoroughly. After 5 min incubation, 10 ml 7 % Sodium carbonate (Na2CO3) solution was added and made the volume up to 25 ml with distilled water. After incubation for 90 min absorbance was recorded at 750 nm. TPC were calculated using gallic acid as standard and expressed as mg Gallic Acid Equivalent (GAE) per g of extract[17].
Total Tanin Content (TTC): Total tannin was determined with small modification of Folin-Ciocalteu method. A volume of 0.1 ml methanolic extract (1 mg/ ml) was taken with 7.5 ml distilled water and 0.5 ml of Folin-Ciocalteu phenol reagent. Thereafter, 1 ml Na2CO3 solution (35 %) was added and adjusted the volume to 10 ml with distilled water. After incubation for 30 min, absorbance was measured at 725 nm. TTC were calculated using tannic acid as standard and expressed as Tannic Acid Equivalent (TAE) per g of extract[18,19].
Total Flavonoid Content (TFC): Aluminium chloride colorimetric assay has been used to quantify TFC. A volume of 1 ml methanolic extract (1 mg/ml) was taken with 4 ml distilled water and 0.3 ml 5 % Sodium nitrite (NaNO2). After 5 min incubation, 0.3 ml 10 % aluminium chloride was added. Thereafter, 2 ml of 1 M Sodium hydroxide (NaOH) was added and total volume was made 10 ml with distilled water. Absorbance was taken at 510 nm. TFC was calculated using quercetin as standard and expressed as mg of Quercetin Equivalents (QE) per g of extract[20].
Proanthocynidin Content (PC): Total condensed tannin was evaluated by slightly modified method of Tounsi et al.[21]. In brief, 50 μl of the methanolic solution (1 mg/ml) was mixed with 2 ml of 4 % methanol vanillin solution and 450 μl concentrated sulphuric acid. After 15 min, absorbance was read at 527 nm and results were expressed as mg CE/g of extract using catechin methanol as standard solution.
Total Alkaloid Content (TAC): A volume of 1 ml water extract (1 mg/ml) was mixed with same amount 2 N Hydrochloric acid (HCl) and filtered. Then transferred to separatory funnel and washed three times with 10 ml CHCl3. pH was adjusted to neutral with 0.1 N NaOH. After that 5 ml of Bromocresol Green (BCG) solution and 5 ml of phosphate buffer were added to the solution and shaken. The formed complex was then extracted with 1, 2, 3 and 4 ml CHCl3 by vigorous shaking. Diluted in 10 ml with CHCl3 and absorbance was determined at 470 nm. TAC was expressed as mg of Atropine Equivalent (AE)/g of extract using atropine as standard[22].
Liquid Chromatography–Electrospray Ionization– Tandem Mass Spectrometry (LC-ESI-MS/MS) analysis:
An untargeted LC-ESI-MS/High-Performance Liquid Chromatography (HPLC)-MS analysis was performed to identify the saponin fractions and other chemical components. A comparison of relative % of the saponin fractions was also subsequently done from the MS data. This analysis was performed on Waters ACQUITY® TQD equipped with Sunfire C18 column of 250×4.6 mm, 5 μm. ‘Xevo TQ-S micro’ quadrupole ion trap mass spectrometer fitted with high performance spray dual-orthogonal Atmospheric Pressure Ionization (API) sources. Ion transfer optics with Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) sensitivity was with automated Multiple Reaction Monitoring (MRM) sensitivity (1 pg reserpine S/N 2000:1). The column was held at 95 % solvent A (0.1 % acetic acid in water) and 5 % solvent B (0.1 % acetic acid in acetonitrile). The column temperature was fixed at 35° at a constant flow rate of 0.4 ml/min. The injection volume was 20 μl. The MS analysis was performed using ESI in the negative and positive ion MS and MS/MS modes in the range of 150–2000 m/z with acquisition speed: 10 scan/s, polarity switching (ES+/ES-): 20 ms and ESI mode switching: 20 ms.
Statistical analysis:
Statistical analyses were done through Analysis of Variance (ANOVA) test with repeated measurements (n=5) using Tukey method in Minitab 17 software.
Results and Discussion
A. auriculiformis fruit needs 135 d on an average from formation to become fully matured and dried up in vivo. The physical characteristic of the fruit have been recorded at every 15 d intervals of maturity period as shown in Table 1. Analysis for 11 major chemical groups resulted in confirmative presence of 10 groups viz. saponin, phenols, tannin, flavonoid, alkaloid, steroid, terpenoids, glycosides, cardiac glycosides and reducing sugar in all stages of pericarps as shown in Table 2. Among the solvent extracts the methanol extract has been resulted the highest yield as shown in Table 1. Based on this result only the methanolic extract has been selected for further quantitative analyses. The standard curves for biochemical assays are depicted in the fig. 1. Although the pericarp extracts from all the maturity stages are having similar chemical components, their concentration varied considerably as shown in Table 3. While the chemicals such as phenol, flavonoids, tannin, proanthocynidin and alkaloid were found maximum in 1st stage pericarps, 2nd highest amounts were obtained in the 9th maturity stage. Saponin concentration of the extracts was estimated >39 % in all the maturity stages with slightly higher in the stage nine. The phenolics ranks 2nd highest in content. Although the first maturity stage contains good amount of secondary metabolites, it estimates a less biomass content in comparison to that of the 9th maturity stage pericarps. For this reason the methanolic extract of 9th maturity stage pericarp was selected for LC-ESI-MS analysis.
| Different maturity stages of fruit | Small description of fruit pericarp | Distilled water extract (%) | 80 % Ethanolic extract (%) | Methanolic extract (%) |
|---|---|---|---|---|
| 1st stage | Green, small, leafy, without seed | 16.73 | 18.35 | 23.6 |
| 2nd stage | Green longer pericarp but leafy without seed | 20.27 | 22.88 | 28.6 |
| 3rd stage | Pericarp thicker and longer without seed | 23.32 | 26.33 | 32.9 |
| 4th stage | Long and thick pericarp, very small green colour seed appeared | 21.27 | 24 | 30 |
| 5th stage | Pericarp larger with small mid dilation, with green small seed | 26 | 29.27 | 36.66 |
| 6th stage | Green thick with the matured green seed | 24.57 | 27.66 | 34.66 |
| 7th stage | Reddish green pericarp with green seed, | 21.93 | 24.68 | 30.93 |
| 8th stage | Semi dried dark brown pericarp with brownish seed | 25.06 | 28.2 | 35.33 |
| 9th stage (fully matured) | Fully dried dark brown pericarp with separated pods and exposing seeds attached through funicle with pods | 29.85 | 33.6 | 42.1 |
Table 1: Solvent Extractions of A. auriculiformis Pericarp of Different Maturity Stages.
| Phytochemicals | Methanolic extract of nine stage pericarp | 80 % Ethanolic extract of nine stage pericarp | Distilled water extract of 9th stage pericarp |
|---|---|---|---|
| Saponin | +ve | +ve | +ve |
| Phenolic content | +ve | +ve | +ve |
| Tanin | +ve | +ve | +ve |
| Flavonoid | +ve | +ve | +ve |
| Alkaloid | -ve | +ve | +ve |
| Steroid | +ve | +ve | +ve |
| Terpenoids | +ve | +ve | +ve |
| Glycosides | +ve | +ve | +ve |
| Cardiac glycosides | +ve | +ve | +ve |
| Reducing sugar | +ve | +ve | +ve |
| Starch | -ve | -ve | -ve |
Note: (+ve): Presence of the phytochemical group and (–ve): Absence of the phytochemical group
Table 2: Qualitative Analysis of Phytochemicals in Solvent Extracts of A. auriculiformis Pericarp Preparation.
Fig. 1:Calibration curve for quantitative analysis of phytochemicals, (a): Calibration curve of standard diosgenin for determination of TSC; (b): Calibration curve of standard gallic acid for determination of TPC; (c): Calibration curve of standard tannic acid for determination of total tannin content (d): Calibration curve of standard quercetin for determination of TFC; (e): Calibration curve of standard methanolic catechin for determination of total proanthocyanidin content and (f) Calibration curve of standard atropine for determination of TAC.
| Different maturity stages of fruit | TSC (mg of DE/g methanolic extract) | TPC (mg of GAE/g methanolic extract) | TFC (mg of QE/g methanolic extract) | Total tannin content (mg of TAE/g methanolic extract) | PC (mg of catechin equivalent/g methanolic extract) | TAC (mg of AE/g water extract) |
|---|---|---|---|---|---|---|
| 1st stage | 390.2824±0.029 | 219.179245±0.2331 | 26.98±0.004 | 20.45256±0.051 | 0.414377±0.0002 | 14.078± 0.039 |
| 2nd stage | 390.46759±0.012 | 208.235849±0.196 | 21.82±0.0038 | 19.94021±0.042 | 0.307898±0.00013 | 13.124± 0.021 |
| 3rd stage | 390.60648±0.025 | 200.00943±0.312 | 18.9±0.0290 | 18.90798±0.039 | 0.29768±0.00021 | 12.253± 0.052 |
| 4th stage | 390.3287±0.061 | 197.3113208±0.411 | 16.72±0.0036 | 15.91729±0.057 | 0.139575±0.0003 | 12.0325± 0.084 |
| 5th stage | 380.72685±0.032 | 167.3113208±0.156 | 10.81±0.0047 | 12.95704±0.07 | 0.034709±0.00034 | 11.0685± 0.077 |
| 6th stage | 390.14352±0.033 | 169.00943±0.185 | 10.88±0.0031 | 15.22091±0.028 | 0.042776±0.00051 | 12.71± 0.069 |
| 7th stage | 390.18981±0.046 | 171.198113±0.325 | 11.68±0.0032 | 16.52449±0.045 | 0.150624±0.00049 | 12.971± 0.056 |
| 8th stage | 400.11667±0.035 | 176.179245±0.423 | 13.18±0.00279 | 17.16971±0.033 | 0.230433±0.00047 | 12.66± 0.029 |
| 9th stage (fully matured) | 410.22685±0.065 | 213.933962±0.3521 | 24.92±0.00511 | 19.314±0.059 | 0.399268±0.00035 | 13.72± 0.042 |
Note: Values are mean±standard error of the mean, n=5
Table 3: Quantitative Analysis of Some Secondary Metabolites in Methanolic and Water Extract from Different Maturity Stages Pericarp of A. auriculiformis.
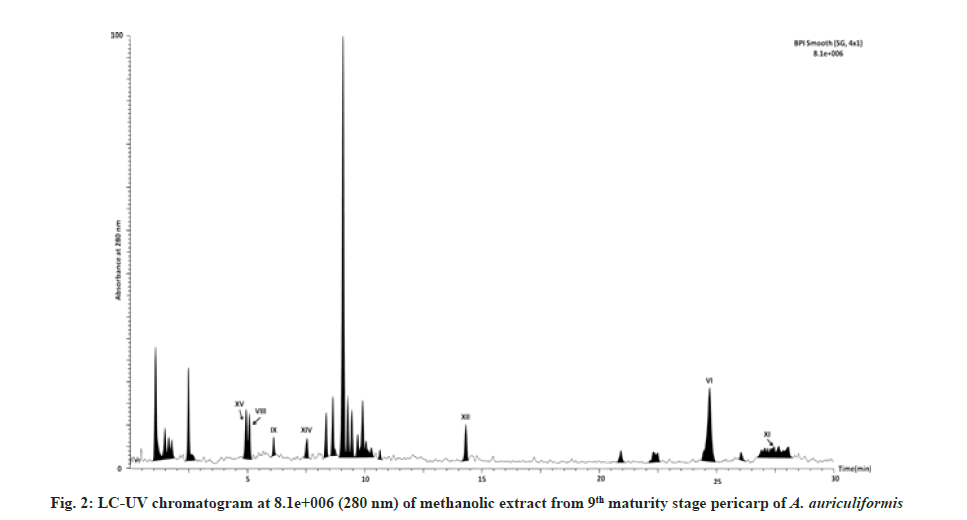
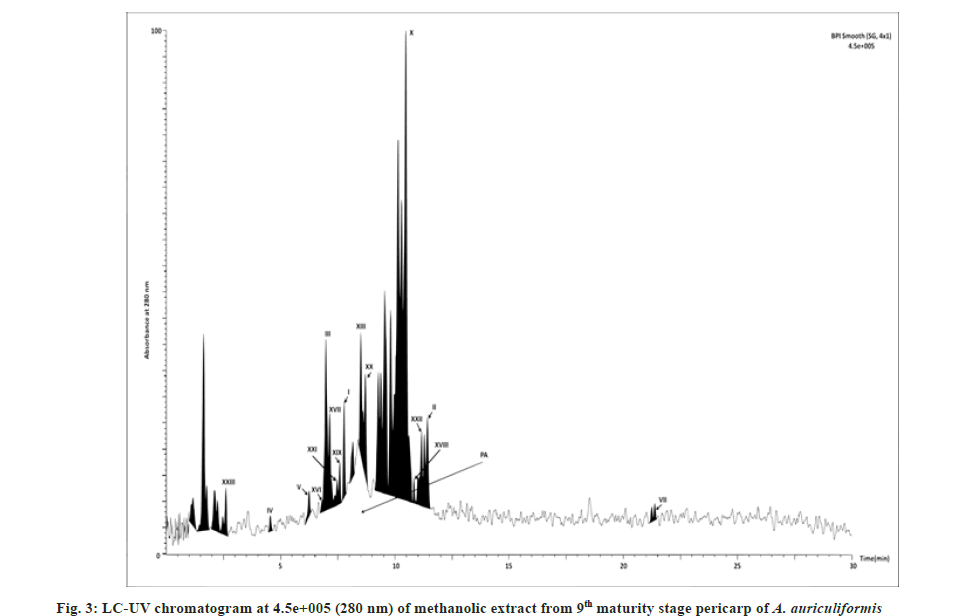
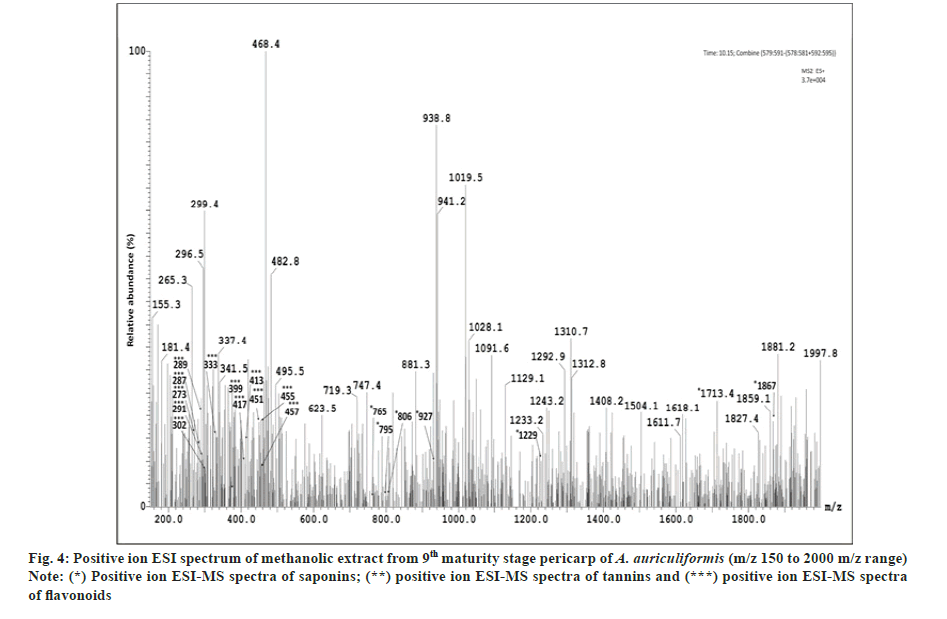
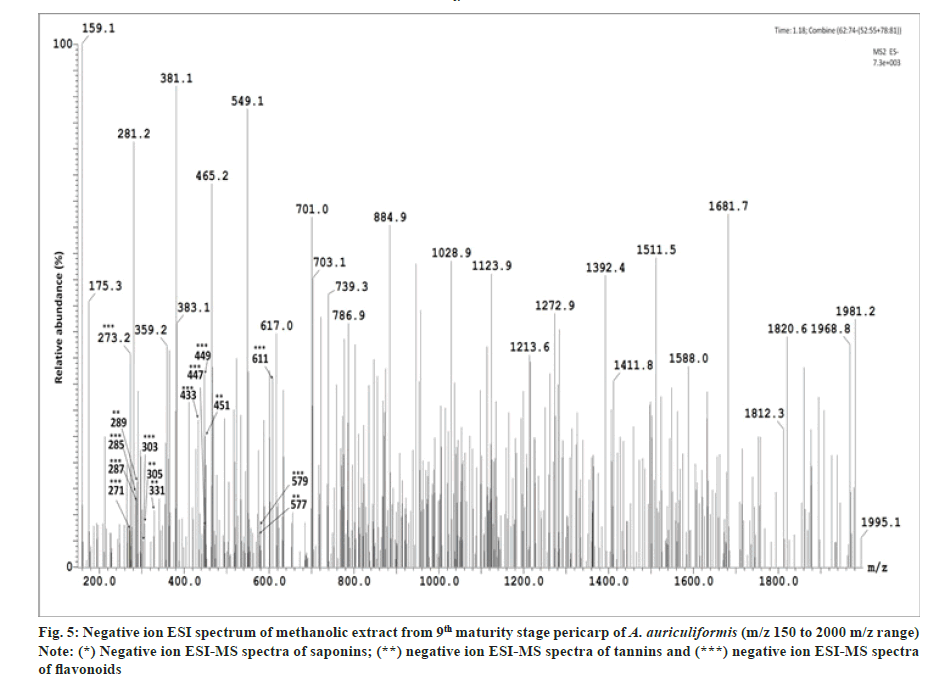
In the previous studies fourteen flavonoids (1-14) have been reported from heartwood[23-25] and another flavonoid quercetin from bark[26] and seedpod[27] of A. auriculiformis. Seven other flavonoids (16-22) have been previously identified from different species of Acacia[25,28] but not in A. auriculiformis. In our study, all those 22 flavonoids have been detected from the pericarp itself of A. auriculiformis through LC-ESIMS analysis (fig. 2, fig. 3, fig. 4, fig. 5 and Table 4). The three flavonoids isoteracacidin I, isoteracacidin II and teracacidin which have been detected in accordance with their retention time have exhibited same peak having same m/z value. Thus these three flavonoids are considered as isomers. In corroboration with the earlier report[25] present study also detected teracacidin and melacacidin (a 3,4-diol like teracacidin) in dimeric forms[2M-H]-ion in negative ESI spectra. The peak XII has been identified as flavonoid but the specific characteristic of this compound is still not known.
| SL. No | Identified compounds from pericarp of A. auriculiformis | Molecular weight (MW) | Peak in LC-UV chromatogram | Ion in ESI-MS spectra | m/z value | References |
|---|---|---|---|---|---|---|
| Saponins | ||||||
| 1 | Acaciaside A | 1712 | [M+H]+ | 1713 | [2] | |
| 2 | Acaciaside B | 1844 | [M+Na]+ | 1867 | [2] | |
| 3 | Acaciaside C | 1206 | [M+Na]+ | 1239 | [1] | |
| 4 | Proacaciaside-I | 794 | [M+H]+ | 795 | [3] | |
| 5 | Proacaciaside-II | 764 | [M+H]+ | 765 | [3] | |
| 6 | Acaciamine | 805 | [M+H]+ | 806 | [3] | |
| 7 | Acaciaside | 926 | [M+H]+ | 927 | [29] | |
| Flavonoids | ||||||
| 1 | 2,3-trans-3,4',7,8-tetrahydroxyflavanone | 288 | I | [M-H]-[M+H]+ | 287 289 | [14] |
| 2 | 4',7,8-trihydroxyflavanone | 272 | II | [M-H]-[M+H]+ | 271 273 | [14] |
| 3 | Teracacidin | 290 | III, IV, V | [2M-H]-[M-H2O+H]+[M+H]+ | 579 | [12] [14] |
| 4 | Isoteracacidin-I | 273 | ||||
| 5 | Isoteracacidin-II | 291 | ||||
| 6 | α-spinasterol (C29H48O) | 412 | VI | [M+H]+ | 413 | [13] |
| 7 | Auriculoside (C22H26O10) | 450 | [M+H]+ | 451 | [13] | |
| 8 | 4,7,8-trihydroxy-2,3-trans-dihydroflavonol (C15H12O6) | 288 | [M+H]+ | 289 | [12] | |
| 9 | 4,7,8-trihydroxyflavonol (C15H10O6) | 286 | [M+H]+ | 287 | [12] | |
| 10 | 4,7,8-trimethoxy-2,3-cis-flavan-3,4-trans-diol (C18H20O6) | 332 | [M+H]+ | 333 | [12] | |
| 11 | 3,4-trans-diacetoxy-4,7,8-trimethoxy-2,3-cis-flavan (C22H24O8) | 416 | [M+H]+ | 417 | [12] | |
| 12 | 3,4,7,8-tetraacetoxy-2,3-trans-dihydroflavonol (C23H20O10) | 456 | [M+H]+ | 457 | [12] | |
| 13 | 4,7,8-triacetoxyflavanone (C21H18O8) | 398 | [M+H]+ | 399 | [12] | |
| 14 | 3,4,7,8-tetraacetoxyflavone (C23H18O10) | 454 | [M+H]+ | 455 | [12] | |
| 15 | Quercetin | 301 | XIII | [M+H]+ | 302 | [15] [16] |
| 16 | Kaempferol | 286 | XIV | [M-H]- | 285 | [17] |
| 17 | 2,3,4,4-tetrahydroxychalcone (C15H12O5) | 272 | VII | [M+H]+ | 273 | [17] |
| 18 | Melacacidin | 306 | VIII | [2M-H]- | 611 | [14] |
| 19 | Taxifolin | 304 | IX | [M-H]- | 303 | [14] [17] |
| 20 | Flavonoid hexoside | 448 | X | [M-H]- | 447 | [17] |
| 21 | Flavonoid glycoside | 434 | XI | [M-H]- | 433 | [17] |
| 22 | Flavonoid | 450 | XII | [M-H]- | 449 | [17] |
| Tannins | ||||||
| 1 | Catechin hexoside | 452 | XV | [M-H]- | 451 | [17] |
| 2 | Epigallocatechin | 306 | XVI, XVII | [M-H]- | 305 | [17] |
| 3 | Galloepicatechin | |||||
| 4 | Procyanidin dimer I | 578 | XVIII, XIX, XX | [M-H]- | 577 | [17] |
| 5 | Procyanidin dimer II | |||||
| 6 | Procyanidin dimer III | |||||
| 7 | Catechin | 290 | XXI, XXII | [M-H]- | 289 | [17] |
| 8 | Epicatechin | |||||
| Phenolic rich fraction | ||||||
| 1 | Gallyl glucose | 332 | XXIII | [M-H]- | 331 | [17] |
Table 4: List of Compounds Detected in Methanolic Extract of 9th Maturity Stage Pericarps from A. auriculiformis through LC-ESI-MS/MS Analysis.
Tannins of eight different components have been detected first time in the pericarp extract through negative ion ESI-MS spectra (fig. 2, fig. 3, fig.5 and Table 4). In Ultraviolet (UV) chromatogram, epigallocatechin has shown earlier retention time than that of galloepicatechin. So these are established as isomers. In LC-UV chromatogram procyanidin dimer- II has an earlier retention time, followed by procyanidin dimer-III and procyanidin dimer-I has longer retention time. These three fractions are also considered as isomers. In UV chromatogram catechin has an earlier retention time than that of epicatechin. These are exhibiting isomeric feature. There is no earlier report of tannins from A. auriculiformis but reported from other species of Acacia[28].
This is for the 1st time, one phenolic rich fraction-gallyl glucose has also been detected in A. auriculiformis (fig. 3 and fig. 5) (Table 4). In other Acacia species however, it had been reported earlier[28].
Proanthocyanin like compounds have been identified as an amalgamation of broad peak on UV chromatogram but no ESI-MS spectra of those has been detected because of their molecular weights beyond the range of 2000 m/z. A small % of proanthocyanidin has been quantified in the methanolic extract (Table 3). To get it in ESI-MS, depolymerization of the compounds is required. Those compounds, not reported earlier from A. auriculiformis, are needed to analyse by Nuclear Magnetic Resonance (NMR) spectroscopy for further detailing.
One triterpenoid saponin (acaciaside) (7) which was previously reported from seed[29] has now been identified from the pericarp of A. auriculiformis (fig. 3) (Table 4). Acaciamine and five other saponins (1-6) have also been detected in pericarp, were previously detected by Fast Atom Bombardment-MS (FAB-MS)[1-3]. However, in the present study relative % of those compounds has been estimated to come to the inference that among the seven triterpenoid saponin, acaciaside A and acaciaside B are the major components as shown in Table 5.
| Triterpenoid saponins | m/z value | Relative concentrations (%) |
|---|---|---|
| Acaciaside A | 1712 | 29.27 |
| Acaciaside B | 1844 | 21.95 |
| Acaciaside | 926 | 12.195 |
| Acaciaside C | 1206 | 12.195 |
| Acaciamine | 805 | 12.195 |
| Proacaciaside-I | 794 | 9.756 |
| Proacaciaside-II | 764 | 2.439 |
Table 5: Relative Concentration of Saponin Fractions According to LC-ESI-MS/MS Chromatogram of Methanolic Extract from 9th Maturity Stage Pericarp of A. auriculiformis.
Other than pharmacological applications[4-8,30], in aquaculture saponin and tannin containing plant products are used as piscicides for prestocking pond management[31]. Since Acacia pericarp contains good amount of saponin and tannin, it it has been recommended very recently[32] as a better substitute of the conventionally used piscicide mohua (Bassia latifolia) oil cake which also contains saponin as the principal ingredient[33].
It can be concluded that highest amount of saponin (41 %) with other phytochemicals are present in the methanolic extract of the 9th stage pericarps. LC-ESIMS/MS analyses revealed 7 triterpenoid saponins, 22 flavonoids, 8 tannins and 1 phenolic rich compoundgallyl glucose in this preparation. Among these components acaciaside A is present in maximum concentration, followed by acaciaside B.
Acknowledgements:
We thankfully acknowledge University Grants Commission, New Delhi for financial assistance in the form of UGC-NET-JRF fellowship, Sl. No. 2061530652. We are also thankful to Central Drug Research Institute, Lucknow for giving access to their instrumental facility.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Uniyal SK, Badoni V, Sati OP. A new triterpenoidal saponin from Acacia auriculiformis. J Nat Prod 1992;55(4):500-2.
[Crossref] [Google Scholar][PubMed]
- Mahato SB, Pal BC, Nandy AK. Structure elucidation of two acylated triterpenoid bisglycosides from Acacia auriculiformis cunn. Tetrahedron 1992;48(32):6717-28.
- Garai S, Mahato SB. Isolation and structure elucidation of three triterpenoid saponins from Acacia auriculiformis. Phytochemistry 1997;44(1):137-40.
- Chakraborty T, Sinhababu SP, Sukul NC. Antifilarial effect of a plant Acacia auriculiformis on canine dirofilariasis. Trop Med1995;37(1):35-7.
- Ghosh NK, Sinhababu SP, Sukul NC. Cestocidal activity of Acacia auriculiformis. J Helminthol1996;70(2):171-2.
- Mandal P, Sinhababu SP, Mandal NC. Antimicrobial activity of saponins from Acaciaauriculiformis. Fitoterapia 2005;76(5):462-5.
[Crossref] [Google Scholar][PubMed]
- Sathya A, Siddhuraju P. Role of phenolics as antioxidants, biomolecule protectors and as anti-diabetic factors-Evaluation on bark and empty pods of Acacia auriculiformis. Asian Pac J Trop Med2012;5(10):757-65.
- Sathya A, Siddhuraju P. Protective effect of bark and empty pod extracts from Acaciaauriculiformis against paracetamol intoxicated liver injury and alloxan induced type II diabetes. Food Chem Toxicol 2013;56:162-70.
- Dhanani T, Shah S, Gajbhiye NA, Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem2017;10:1193-9.
- Harhar H, Gharby S, El Idrissi Y, Pioch D, Matthaus B, Charrouf Z, et al. Effect of maturity stage on the chemical composition of argan fruit pulp. OCL 2019;26:1-8.
- Abd ElGawad AM, Elshamy AI, Al Rowaily SL, El Amier YA. Habitat affects the chemical profile, allelopathy and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019;8(11):1-20.
[Crossref] [Google Scholar][PubMed]
- Harborne JB. Phytochemical methods. 1st ed. London: Chapman and Hall Ltd; 1973.
- Trease GE, Evans WC. Pharmacognosy. 11th ed. London:Bailliere Tindall and Cox; 1989.
- Sofowara A. Medicinal plants and traditional medicine in Africa. Ibadan: Spectrum Books Ltd; 1993.
- Ganesan S, Bhatt RY. Qualitative nature of some traditional crude drugs available in commercial markets of Mumbai, Maharashtra, India. Ethnobot Leaflets2008;2008(1):348-60.
- Gu LW, Gu WY. Spectrophotometric determination of soyasaponins. J Chinese Cereals Oils Assoc 2000;15:38-42.
- Maizura M, Aminah A, WanAida WM. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. Int Food Res J 2011;18(2):529-34.
- Folin O, V. Ciocalteu. On tyrosine and tryptophane determinations in proteins. J Biol Chem 1927;73(2):627-50.
- Afify AE, El Beltagi HS, El-Salam SMA, Omran AA. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β-carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac J Trop Biomed2012;2(3):203-9.
- Kalita P, Barman TK, Pal TK, Kalita R. Estimation of total flavonoids content (TPC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivumLinn. J Drug Deliv Ther2013;3(4):33-7.
- Tounsi MS, Ouerghemmi I, Ksouri R, Wannes WA, Hammrouni I, Marzouk B. Hplc-determination of phenolic composition and antioxidant capacity of cactus prickly pears seeds. Asian J Chem2011;23(3):1006-10.
- A new triterpenoidal saponin from Acacia auriculiformis
- Drewes SE, Roux DG. A new flavan-3, 4-diol from Acacia auriculiformis by paper ionophoresis. Biochem J 1966;98(2):493-500.
- Sahai R, Agarwal SK, Rastogi RP. Auriculoside, a new flavan glycoside from Acacia auriculiformis. Phytochemistry 1980;19(7):1560-2.
- Barry KM, Mihara R, Davies NW, Mitsunaga T, Mohammed CL. Polyphenols in Acaciamangium and Acacia auriculiformis heartwood with reference to heart rot susceptibility. J Wood Sci 2005;51(6):615-21.
- Kaur A, Sohal SK, Arora S, Kaur H. Phenolic rich fractions from the bark of Acaciaauriculiformis. Bull Pure Appl Sci Zool 2014;33(1-2):1-5.
- Chaki S, Ghosh B, Bandyopadyhay S, Mukerjee M, Das S, Dastidar SG. Detection of various phytochemical compounds from seeds of A. auriculiformis for possibilities of obtaining potent antimicrobial agents. Int J Biol Pharma Res 2015;6(2):120-8.
- Subhan N. Phytochemical and pharmacological investigations of Australian Acacia: An ethnomedicine-guided bioprospective approach. Charles Sturt University 2016.
- Mahato SB, Pal BC, Price KR. Structure of acaciaside, a triterpenoid trisaccharide from Acaciaauriculiformis. Phytochemistry 1989;28(1):207-10.
- Prakash D, Upadhyayb G, Pushpangadan P. Antioxidant potential of some under-utilized fruits. Indo Glob J Pharm Sci 2011;1(1):25-32.
- Das SK, Mondal B, Biswas B, Mandal A. Herbal piscicides in inland aquaculture-A review. J Ecol2018;2(3):00133.
- SahaB, SahaSK. Acacia auriculiformispericarp preparation-A substitute of Mohua oil cake as piscicide in pre-stocking pond management. Aquaculture 2022;548(1):737586.
- Ayyappan S, Jena JK. Carp culture in India: A sustainable farming practice. In: Natarajan P, editor. Advances in aquatic biology and fisheries. Trivendrum: University of Kerala; 1998. p. 123-53.