- *Corresponding Author:
- Chanya Chaicharoenpong
Faculty of Science,
Molecular Crop Research Unit,
Chulalongkorn University,
Bangkok 10330,
Thailand
E-mail: chanya.c@chula.ac.th
| Date of Received | 10 November 2020 |
| Date of Revision | 11 April 2021 |
| Date of Acceptance | 11 November 2021 |
| Indian J Pharm Sci 2021;83(6):1144-1154 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Free radicals can cause oxidative damage in biomolecules and lead to pathological diseases. Antioxidants can protect against the oxidation of living cells. The aim of this study was to evaluate the total phenolic and flavonoid contents and the antioxidant and antityrosinase activities of different parts of Manilkara zapota and measure the quantity of (+)-dihydrokaempferol in different plant parts. The bark, flowers, fruit, leaves, roots, seeds and wood of Manilkara zapota were extracted with methanol and water. All the crude extracts were evaluated for biological activities and measured the quantity of (+)-dihydrokaempferol using high performance liquid chromatography. The methanol crude extract of the flowers showed the highest total phenolic (743±29 milligrams of gallic acid equivalent per gram crude extract) and flavonoid contents (133.8±3.1 milligrams of quercetin per gram of crude extract). Moreover, it exhibited the strongest antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (half maximal inhibitory concentration of 22.74±0.67 μg/ml), 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (half maximal inhibitory concentration of 20.89±0.17 μg/ml) and ferric reducing antioxidant power values (790.22±0.81 milligrams of trolox equivalent per gram of crude extract). The methanol crude extract of the bark displayed the strongest monophenolase inhibitory activity (half maximal inhibitory concentration of 85.2±2.1 μg/ml), while the methanol crude extract of the roots exhibited the highest diphenolase inhibitory activity (half maximal inhibitory concentration of 33.52±0.68 μg/ml). (+)-Dihydrokaempferol was found in the bark, flowers, leaves, roots and wood. The highest content of (+)-dihydrokaempferol was detected in the methanol crude extract of the bark. These results suggested that the flowers of Manilkara zapota may be used as a source of natural antioxidants and the bark and roots may be beneficial sources of tyrosinase inhibitors.
Keywords
Manilkara zapota, dihydrokaempferol, antioxidant, antityrosinase activity, high performance liquid chromatography
Reactive oxygen species (ROS) are chemically reactivemoleculesand free radicals containing oxygen. ROS are formed as natural by- products of the normal metabolism of oxygen [1]. ROS have roles in cell signaling, including in apoptosis, gene expression and the activation of cell signaling cascades [2]. The effect of ROS on cellular processes is a function of the strength, duration and context of exposure. High levels of ROS lead to cellular damage, oxidative stress and the damaging of lipids, proteins and nucleic acids [3]. Thus, elevated ROS are linked to myriads of pathologies, such as skin photoaging and photocarcinogenesis leading to rheumatoid arthritis, asthma, cancer, diabetes, inflammation and hypertension, as well as neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases [4,5]. Antioxidants protect the body from oxidative stress and damage caused by free radicals. They either protect from the oxidation of radical chain reactions or inhibit the processing of oxidation. Natural antioxidants, such as phenolic compounds and flavonoids were reported to protect against oxidative stress, act as reducing agents for ROS production and delay skin aging [6]. Moreover, they reduce chelated metal ions and inhibit the increasing of melanin synthesis. The antioxidant activity of phenolic compounds and flavonoids depends on the number of hydroxyl groups in the structure [7]. Furthermore, phenolic compounds protect against free radical processes and reduce the production of browning [8]. Phenolic compounds are secondary metabolites in plants that possess several biological activities, such as anticancer, antidiabetic, antihyperlipidemic, antimicrobial, antioxidant and antityrosinase activities [9,10]. Tyrosinase (EC 1.14.18.1) is acopper-containing enzyme that is distributed in microorganisms, animals and plants [11]. Tyrosinase catalyzes both the hydroxylation of L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) and the oxidation of L-DOPA to (2S)-2- amino-3-(3,4-dioxocyclohexa-1,5-dien-1-yl)propanoic acid (dopaquinone) in the melanin biosynthesis pathway [12]. A serious esthetic problem of melanin products is hyperpigmentation, such as melasma, age spots and freckles. Commercial skin whitening agents such as arbutin and kojic acid inhibit tyrosinase from protecting against skin hyperpigmentation. However, they cause long-term side effects such as allergic reactions, photosensitivity and skin irritation [13]. Thus, effective and safe tyrosinase inhibitors from medicinal plants are still being investigated [13,14]. Manilkara zapota (M. zapota) belongs to the Sapotaceae family. It is a native plant of South America and widely cultivated throughout the tropics. Ripe fruits are edible with a sweet taste [15,16]. Previous reports indicated that the phytochemicals of M. zapota exhibited various biological activities. It was reported that triterpenoids and flavonoids from its bark and seeds exhibited antioxidant, anti-inflammatory, antipyretic, antidiabetic, antiaging, antilipidemic and acaricidal activities [17,18]. The fruit of M. zapota is a rich source of polyphenolic compounds that has significant antioxidant, antihyperglycemic and hypercholesterolemic activities [19,20]. Several triterpenoids were isolated from the bark and fruit of M. zapota and they exhibited antitumor activity [21,22]. Its seeds showed antibacterial activity [18] and the seed coats exhibited tyrosinase inhibitory activity [23]. (+)-Dihydrokaempferol (fig. 1), a flavonoid, was isolated from the bark of M. zapota and it exhibited potent in vitro cytotoxicity in various human tumor cell lines, such as the breast (BT474), colon (SW620), gastric (KATO-III), liver (HepG2) and lung bronchus- (Chago-K1) carcinoma cell lines [24]. Additionally, (+)-dihydrokaempferol exhibited significant antityrosinase and antioxidant activities [24]. Therefore, it may be of use as a marker compound for biologically active compounds present in M. zapota. A standardization of the chemical profiles of plant extracts may be useful for the elucidation of the chemical components and biological properties of plant extracts.
In the present study, total phenolic and flavonoid contents of different parts of M. zapota, including bark, flowers, fruit, leaves, roots, seeds and wood, were evaluated. Antioxidant activity of the crude extracts was determined by in vitro assays, namely, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino- bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging assays, with the Ferric Reducing Antioxidant Power (FRAP) assay. The antityrosinase activity of the crude extracts was also investigated in vitro. Furthermore, the quantification of the (+)-dihydrokaempferol content of the different parts of M. zapota was evaluated using High Performance Liquid Chromatography (HPLC).
Materials and Methods
ABTS, DPPH, L-DOPA, L-tyrosine, 2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ), 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox) and mushroom tyrosinase were purchased from Sigma- Aldrich (St. Louis, MO, USA). Folin–Ciocalteu reagent, absolute ethanol, arbutin, gallic acid, Dimethyl Sulfoxide (DMSO), methanol (MeOH), potassium persulfate, kojic acid, disodium hydrogen phosphate, sodium dihydrogen phosphate, acetic acid and quercetin were purchased from Merck (Darmstadt, Germany). HPLC grade solvents were purchased from Merck (Darmstadt, Germany), including Acetonitrile (ACN), water and methanol. Aluminium chloride, ferric chloride, sodium carbonate, sodium hydroxide, sodium acetate (anhydrous) and sodium nitrite were purchased from Fisher Scientific (Loughborough, UK). (+)-Dihydrokaempferol was isolated in our laboratory from M. zapota bark and used as a reference compound for HPLC analysis.
Crude extracts were evaporated using a vacuum rotary evaporator (Tokyo Rikakikai, Tokyo, Japan). Ultraviolet (UV) absorbance was measured on a multimode plate reader (PerkinElmer, Hamburg, Germany). HPLC analysis was performed on an Agilent Series 1100 liquid chromatograph (Agilent Technologies, Waldbronn, Germany). HPLC equipment consisted of a G1379A model vacuum degasser, a G1311A model quaternary pump and a G1315B model diode array detector (Agilent Technologies, Waldbronn, Germany).
Plant material:
The bark, flowers, fruit, leaves, roots, seeds and wood of M. zapota were collected from Saraburi province, Thailand. A voucher specimen (BKF No. 187749) was deposited at the Forest Herbarium Department of National Parks, Wildlife and Plant Conservation, Bangkok, Thailand.
Plant extraction:
The bark, flowers, fruit, leaves, roots, seeds and wood of M. zapota were finely chopped and dried in a hot air oven at 60. Each 500 g of dried sample was extracted with methanol (2×0.5 l) and water (2×0.5 l) by maceration for 24 h and filtered through Whatman No. 1 filter paper. The filtrate was then evaporated under reduced pressure to obtain the methanol and aqueous crude extracts of the seven different parts of M. zapota.
Total phenolic content:
Total phenolic content was determined using a Folin- Ciocalteu assay [25]. First, 100 μl of sample (1 mg/ml) was added to 6 ml of water. After that, 500 μl of undiluted Folin–Ciocalteu reagent was added to the solution and it was incubated for 1 min at 37°. Then, 1.5 ml of 20 % w/v sodium carbonate was added and the reaction mixture was made up to 10 ml in a volumetric flask with water. Absorbance was measured at 760 nm after 2 h incubation. Total phenolic content was expressed as the milligrams of gallic acid equivalent per gram of crude extract (mg GAE/g crude extract).
Total flavonoid content:
Total flavonoid content was determined by a modified method of Tohidi et al. [26]. First, 125 μl of sample (1 mg/ml) was added to 75 μl of 5 % w/v sodium nitrite. The solution was incubated for 6 min at 37° and 150 μl of 10 % w/v aluminium chloride was then added. After 5 min of incubation, 750 μl of 1 M sodium hydroxide was added and the final volume of the reaction mixture was made up to 2500 μl with water. After incubation for 15 min at 37°, absorbance was measured at 510 nm. Total flavonoid content was expressed as the milligrams of quercetin per gram of crude extract (mg QE/g crude extract) by using a quercetin calibration curve.
Antioxidant activity, DPPH radical scavenging assay:
The DPPH radical scavenging activity was determined using a modification method by Sulaiman et al. [27]. Briefly, the reaction solution contained 50 µl of the sample (100 mg/ml) and 150 µl of the 0.05 M DPPH solution in methanol. The reaction mixture was then vortexed and incubated in the dark for 30 min at 37°. Absorbance of the reaction mixture was recorded at 517 nm. Trolox was used as a standard control. The DPPH scavenging effect was calculated using the following equation 1.
DPPH radical scavenging activity (%)= [1– [(As–Ab)/ Ad]×100 (1)
where, As is the absorbance of the sample mixed with DPPH solution, Ab is the absorbance of the sample without the DPPH solution and Ad is the absorbance of the DPPH solution without sample.
ABTS radical scavenging assay:
ABTS scavenging capacity was determined using a method modified from Wootton-Beard et al. [28]. The stock solution contained 100 ml of 7.0 mM ABTS solution and 100 ml of 2.4 mM potassium persulfate solution. The solution mixture was then left in the dark for 14 h at 37°. Then, 1 ml of ABTS solution was added to the reaction mixture and diluted with 60 ml of absolute ethanol to evaluate an absorbance of 0.700±0.001 absorbance units at 734 nm. After that, 500 μl of the sample (100 mg/ml) was added to 500 μl of the ABTS solution and absorbance was measured at 734 nm after 7 min incubation. The ABTS scavenging capacity of Trolox was assayed for comparison. The percentage of scavenging activity was calculated with the equation 2.
ABTS radical scavenging activity (%)= [(Ac–As)/ Ac]×100 (2)
where, Ac is the absorbance of the control and As is the absorbance of the ABTS radical with the sample.
FRAP assay:
The FRAP assay was determined using a protocol modified from Wootton-Beard et al. [28]. The FRAP reagent was prepared by mixing 25 ml of 0.3 M acetate buffer (pH 3.6), 2.5 ml of 20 mM ferric chloride solution and 2.5 ml of 10 mM TPTZ and total volume was adjusted to 50 ml using 40 mM HCl solution. The FRAP reagent was then put in a water bath for 30 min at 50°. Then, 600 μl of the FRAP reagent was reacted with 25 μl of the sample (100 mg/ml). After 4 min of incubation at 37°, absorbance was measured at 595 nm. Trolox was used as a positive control. Ferric reducing capacity was expressed as the milligrams of trolox equivalent per gram of crude extract (mg TE/g crude extract).
Antityrosinase activity:
The inhibition of tyrosinase activity was determined using a method modified from Dej-adisai et al. [29] and performed by measuring the enzymatic reaction using L-tyrosine (monophenolase) and L-DOPA (diphenolase) substrates. Briefly, the sample (1 mg/ml) was dissolved in water. The solution mixture contained 150 µl of 0.2 M sodium phosphate buffer (pH 6.8), 50 µl of the sample and 50 µl of 500 μM substrate solution. The reaction solution was blended and incubated for 10 min at 37°. Absorbance at 490 nm (t=0 min) was immediately recorded after 50 µl of tyrosinase solution (200 U/ml) was added. Finally, the reaction mixture was incubated for 20 min at 37° and absorbance was again measured at 490 nm (t=20 min). The percentage of inhibition was calculated using the equation 3.
Tyrosinase inhibitory activity (%)= [(A–B)–(C–D)/(A– B)]×100 (3)
where, A is the difference of UV absorbance of the control at t=0 and 20 min, B is the difference of UV absorbance of the blank control at t=0 and 20 min, C is the difference of UV absorbance of the test sample and the positive control at t=0 and 20 min and D is the difference of UV absorbance of the blank of the test sample and the positive control at t=0 and 20 min.
Chromatographic conditions:
Quantitative analysis by HPLC was optimized on an octadecylsilyl (ODS) Thermo Hypersil Keystones column (250×4.6 mm internal diameter (i.d.,) 5 μm, YMC Co., Kyoto, Japan) equipped with a guard column (20×3.0 mm i.d., 3.5 μm, Phenomenex Inc., Torrance,California, USA). The gradient-elution system was carried out with 0.1 % v/v formic acid in water (A) and acetonitrile:methanol (1:4 % v/v, B) using the following gradient: 100 % A at 0-10 min, 80 % A at 11-30 min, 60 % A at 31-60 min, 40 % A at 61-100 min, 20 % A at 101-120 min and 100 % B at 121-160 min. Sample was filtered through a 0.45 μm Polytetrafluoroethylene (PTFE) membrane filter and 10 μl of the sample solution was injected into the HPLC system for analysis. The column was operated at 25°. The flow rate of the mobile phase was 1.0 ml/min. The absorbance was measured at a wavelength of 254 nm.
HPLC method validation:
Quantification of (+)-dihydrokaempferol in the aqueous and methanol crude extracts of the different parts of M. zapota was performed after validation of the HPLC method. Analytical method validation was achieved in terms of linearity, sensitivity, accuracy and precision.
Linearity: The (+)-dihydrokaempferol standard solutions were prepared with methanol to obtain eight concentrations of 100, 200, 400, 600, 800, 1000, 1200 and 1400 ng/ml. Solutions were filtered before analysis through a 0.45 μm PTFE membrane filter. Seven repetitions were conducted in this experiment. The calibration curve was plotted with peak area versus concentration using the method of least squares regression analysis. The values of the slope and y-intercept of the plot were calculated.
Sensitivity: Limit of Detection (LOD) and Limit of Quantification (LOQ) were determined using a linear calibration curve of (+)-dihydrokaempferol standards. (+)-Dihydrokaempferol was prepared at different concentrations of 200, 400, 600, 800, 1000 and 1200 ng/ml. Seven repetitions of each concentration were injected into the chromatographic column. The LOD and LOQ were calculated according to the following equation 4 and equation 5.
LOD = [3.3×Sy]/Am (4)
LOQ = [10×Sy]/Am (5)
where, Sy is the standard deviation of the y-intercept and Am is the average slope.
Accuracy: The percentage recovery was used to evaluate the accuracy of the method. Three different concentrations of (+)-dihydrokaempferol (n=7) were obtained from six different concentrations and analyzed. Briefly, (+)-dihydrokaempferol was prepared at concentrations of 400, 600 and 800 ng/ml in methanol. Then, 1 ml of the methanol crude extract of barks (400 ng/ml) was added to 1 ml of (+)-dihydrokaempferol solution. All data were calculated using the following equation 6,
Recovery (%)= [(C1–C2)/C3]×100 (6)
where, C1 is the sample contents after adding standard solution, C2 is the sample before adding standard solution and C3 is the standard solution.
Precision: The percentage relative standard deviation (% RSD) was used to determine both intra and inter- day precision of the method. Intra-day precision was computed by evaluating with three different concentrations of (+)-dihydrokaempferol (n=7) that were obtained from six different concentrations and analyzed. (+)-Dihydrokaempferol was prepared at concentrations of 400, 600 and 800 ng/ml in methanol. Inter-day precision was measured by investigating the above concentration (n=7) on five consecutive days. All data were computed by the equation 7.
% RSD= [SD/x? ]×100 (7)
where, SD is standard deviation and x? is the mean of the concentration of the standard solution.
Quantitative analysis of (+)-dihydrokaempferol in different parts of M. zapota by HPLC:
The validated HPLC method was used for quantitative analysis of (+)-dihydrokaempferol in the aqueous and methanol crude extracts of different parts of M. zapota. The sample solution and (+)-dihydrokaempferol were dissolved in methanol. The (+)-dihydrokaempferol content of the crude extracts of the different parts of M. zapota were expressed as milligrams of (+)-dihydrokaempferol/gram of crude extract (mg/g crude extract).
Statistical analysis:
Results were expressed as mean±SD. Statistical data analysis was performed with Statistical Package for the Social Sciences (SPSS) version 24 and differences were considered significant at p<0.05.
Results and Discussion
Plant extraction was done and the extraction yield of the aqueous crude extracts of M. zapota was in the range of 5.4–34.0 % and the extraction yield of the methanol crude extracts was in the range of 6.7–39.7 % (Table 1). The fruits gave the maximum yield for both the aqueous and methanol crude extracts.
| Plant part | Extraction yield (% w/w dry weight) | |
|---|---|---|
| Aqueous crude extract | Methanol crude extract | |
| Bark | 12.5 | 14.5 |
| Flowers | 9.4 | 6.7 |
| Fruit | 34.0 | 39.7 |
| Leaves | 20.7 | 20.1 |
| Roots | 11.6 | 11.0 |
| Seeds | 5.4 | 7.3 |
| Wood | 10.6 | 18.8 |
Table 1: Extraction Yield of Different Parts of M. Zapota
Total phenolic and flavonoid contents were determined. In the present study, the methanol crude extracts of the flowers and leaves, and the aqueous crude extracts of the fruit and roots showed high total phenolic contents, which were 743±29, 675±19, 727±53 and 698±46 mg GAE/g crude extract, respectively (Table 2). The wood presented a low content of phenolic compounds in both the aqueous and methanol crude extracts. The high content of flavonoids of the methanol and aqueous crude extracts of flowers and the methanol crude extract of seeds, were determined to be 133.8±3.1, 131.6±1.4 and 127.8±1.7 mg QE/g crude extract, respectively (Table 2). These values were not significantly different (p<0.05). This was followed by the methanol crude extract of bark, which was 116.1±4.2 mg QE/g crude extract. The wood displayed a low content of flavonoids in both the aqueous and methanol crude extracts. Phytochemicals of M. zapota were reported as alkaloids, saponins, sterols, tannins, triterpenoids and phenolic compounds [16,24,30-32]. Previous studies reported that the acetone crude extract of M. zapota leaves, the ethanol crude extracts of M. zapota fruit and pulp, and the methanol crude extracts of Manilkara hexandra (M. hexandra) fruit and seeds contained a high amount of phenolic compounds [32-35]. In contrast, the aqueous crude extracts of M. zapota fruit pulps and peels showed low total phenolic content [36]. In addition, the acetone crude extract of M. zapota leaves and the ethyl acetate crude extract of M. zapota seed coats were previously reported to show a high amount of flavonoids [33,37]. The methanol extracts of M. hexandra fruit and seeds also consisted of flavonoids [34]. The phenolic compounds; methyl-4-O-galloylchlorogenate and 4-O-galloylchlorogenic acid were isolated from M. zapota fruit and 3,4-dihydroxybenzoic acid, (+)-dihydrokaempferoland6-hydroxyflavanone were isolated from M. zapota bark [23,24]. Gallic acid, a phenolic acid, was reported to be a major component of M. hexandra fruit and seeds [34]. The contents of phenolic compounds and flavonoids of the extracts depend on extraction procedures, temperature and solvent polarity in extract preparation [9]. Furthermore, the content of flavonoids is correlated with the content of phenolic compounds [38,39] because flavonoids are one of the major phenolic compounds in Manilkara species.
| Plant part | Solvent extract | Total phenolic content (mg GAE/g crude extract) |
Total flavonoid content (mg QE/g crude extract) |
|---|---|---|---|
| Bark | Methanol | 495±40f | 116.1±4.2e |
| Water | 220±34bc | 64.5±2.8b | |
| Flowers | Methanol | 743±29i | 133.8±3.1g |
| Water | 227±17bc | 131.6±1.4fg | |
| Fruit | Methanol | 633±47g | 76.7±1.8c |
| Water | 727±53hi | 74.7±4.1c | |
| Leaves | Methanol | 675±19gh | 97.8±1.5d |
| Water | 545±36f | 75.6±2.1c | |
| Roots | Methanol | 307.5±6.7d | 74.5±2.5c |
| Water | 698±46hi | 101.2±1.0d | |
| Seeds | Methanol | 398±46e | 127.8±1.7f |
| Water | 249±10c | 65.4±1.3b | |
| Wood | Methanol | 178±8ab | 56.7±4.0a |
| Water | 144±31a | 63.6±5.1b |
Values are mean±SD (n=3). Statistical comparison between values of different extracts was done using Duncan’s multiple range test (p<0.05)
Table 2: Total Phenolic and Flavonoid Contents of Different Parts of M. zapota
The DPPH radical scavenging activity of the aqueous and methanol crude extracts of M. zapota was expressed as the Half Maximal Inhibitory Concentration or 50 % Inhibitory Concentration (IC50) as shown in Table 3. The methanol crude extract of flowers showed the highest DPPH radical scavenging capacity with an IC50 value of 22.74±0.67 μg/ml. The aqueous crude extracts of the fruit and roots also displayed potent DPPH radical scavenging capacity with IC50 values of 33.08±0.26 and 44.24±0.49 μg/ml, respectively, whereas, the wood exhibited weak DPPH radical scavenging activity. Trolox exhibited greater DPPH radical scavenging capacity than that of the crude extracts of M. zapota with an IC50 value of 6.06±0.01 μg/ml (24.21±0.04 μM). The results of ABTS radical scavenging activity of the aqueous and methanol crude extracts of M. zapota are presented in Table 3. The methanol crude extract of the flowers exhibited the highest ABTS radical scavenging similar ABTS radical scavenging activity with IC50 values of 54.59±0.22, 55.28±0.24 and 55.43 ±0.55 μg/ml, respectively. The wood showed low ABTS radical scavenging activity. Trolox exhibited ABTS radical scavenging activity with an IC50 value of 6.04 ±0.01 μg/ml (24.13±0.04 μM). FRAP activity of the aqueous and methanol crude extracts of M. zapota was expressed as statistical trolox equivalents (Table 3). The methanol crude extracts of the flowers and leaves, and the aqueous crude extracts of the fruit and roots, showed high reducing capacity with FRAP values of 790.22±0.81, 718.89±0.77, 728.67±0.75 and 725.33±0.75 mg TE/g crude extract, respectively.
| Plant part/Standard | Solvent extract | IC50 (μg/ml) | FRAP (mg TE/g crude extract) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| Bark | Methanol | 66.42±0.70h | 70.54±0.16h | 438.22±0.52e |
| Water | 77.99±0.53k | 95.9±1.1l | 183.78±0.35bc | |
| Flowers | Methanol | 22.74±0.67b | 20.89±0.17b | 790.22±0.81h |
| Water | 77.84±0.20k | 94.93±0.78k | 257.33±0.35cd | |
| Fruit | Methanol | 58.0±1.5f | 55.43±0.55e | 624.22±0.64g |
| Water | 33.08±0.26c | 35.29±0.58c | 728.67±0.75h | |
| Leaves | Methanol | 55.4±1.2e | 55.28±0.24e | 718.89±0.77h |
| Water | 63.20±0.33g | 67.95±0.63g | 530.22±0.60f | |
| Roots | Methanol | 69.89±0.39j | 54.59±0.22e | 307.56±0.41d |
| Water | 44.24±0.49d | 41.34±0.65d | 725.33±0.75h | |
| Seeds | Methanol | 68.2±1.2i | 64.85±0.53f | 411.78±0.51e |
| Water | 77.10±0.33k | 72.93±0.63i | 276.00±0.34d | |
| Wood | Methanol | 84.61±0.24l | 93.89±0.52j | 164.44±0.29b |
| Water | 94.59±0.52m | 98.34±0.44m | 66.67±0.18a | |
| Trolox | 6.06±0.01a | 6.04±0.01a | ||
Values are mean±SD (n=3). Statistical comparison between values of different extracts was done using Duncan’s multiple range test (p<0.05)
Table 3: Antioxidant Activity of Different Parts of M. zapota
This was followed by the methanol crude extract of the fruit with a FRAP value of 624.22±0.64 mg TE/g crude extract, while the wood showed low FRAP activity. This plant has previously been reported that the acetone crude extract of M. zapota leaves exhibited stronger DPPH radical scavenging activity than that of ascorbic acid [33]. The extract of the fresh pulps of M. zapota exhibited stronger DPPH radical scavenging activity than that of the dry pulp extract [30]. The methanol extracts of M. hexandra fruit and seeds also showed potent antioxidant activity [34], similar to the methanol extract of M. zapota leaves [20]. In contrast, the crude extract of M. zapota fruits showed low DPPH radical scavenging activity and the 60 % ethanol crude extract of M. zapota seeds exhibited no effect on DPPH radical scavenging activity [30,40]. Usually, phenolic compounds act as reducing agents and react with free radicals via the hydroxyl groups in hydrogen atom or single electron transfer mechanisms [41,42]. Phenolic compounds and flavonoids were reported to have significant DPPH scavenging and metal chelating activities [32]. The number and substitution pattern of hydroxyl groups in phenolic compounds and flavonoids are responsible for free radical scavenging potential [43,44]. Regarding DPPH scavenging activity, antioxidants transfer hydrogen atoms to free radicals and ABTS radical scavenging activity revealed an electron transfer system. In terms of FRAP, antioxidants reduced ferric ions to ferrous ions [45-47]. Phenolic compounds and flavonoids are well known in protection against skin aging, cancer, diabetes and neurodegenerative diseases [48,49].
The seven different parts of M. zapota were investigated for tyrosinase inhibitory activity, including both monophenolase and diphenolase inhibitory activities (Table 4). Results indicated that the methanol crude extracts of the bark (IC50 85.2±2.1 μg/ml) and roots (IC50 87.9±1.2 μg/ml) showed stronger monophenolase inhibitory activity than that of other crude extracts. The methanol crude extract of the fruit (IC50 466.3±1.9 μg/ ml) showed the weakest monophenolase inhibitory activity. The aqueous crude extracts of the leaves (IC50128.54±0.42 μg/ml) and roots (IC50107.24 ±0.41 μg/ml), and the methanol crude extracts of the bark (IC50 85.2±2.1 μg/ml), leaves (IC50 108.0±1.1 μg ml) and roots (IC50 87.9±1.2 μg/ml) showed stronger monophenolase inhibitory activity than that of arbutin (IC50 134.23±0.53 μg/ml, 493.0±2.0 μM). However, kojic acid showed the strongest monophenolase inhibitory activity with an IC50 value of 1.19±0.02 μg/ml (8.37±0.14 μM). For diphenolase inhibitory activity, the methanol crude extracts of the roots (IC50 33.52 ±0.68 μg/ml), leaves (IC50 48±10 μg/ml) and flowers (IC50 51.01±0.33 μg/ml) exhibited strong antityrosinase activity. The weakest diphenolase inhibitory activity was found in the aqueous crude extract of the bark (IC50 437.6±1.7 μg/ml). Kojic acid and arbutin displayed potent diphenolase inhibitory activity with IC50 values of 2.48±0.02 μg/ml (17.45±0.14 μM) and 8.62 ±0.11 μg/ml (31.66±0.41 μM), respectively. A previousstudy reported that the ethyl acetate crude extract of M. zapota bark exhibited moderate diphenolase inhibitory activity and the methanol crude extract of M. zapota leaves showed moderate monophenolase inhibitoryactivity [23,24].Moreover,3,4-dihydroxybenzoic acid, (+)-dihydrokaempferol, 6-hydroxyflavanone and myricetin-3-O-α-L-rhamnoside that were isolated from M. zapota showed moderate antityrosinase activity [23,24]. Furthermore, antityrosinase activity is based on the number and position of the substitution of hydroxyl groups of phenolic compounds that bind to active sites of tyrosinase and result in low enzymatic activity [44,50].(+)-Dihydrokaempferol was reported to be a strong antioxidant and tyrosinase inhibitor [24]. It was described that its hydroxyl group may be correlated with both high ABTS and FRAP activities [51]. Thus, quantification of (+)-dihydrokaempferol in the aqueous and methanol crude extracts of the different parts of M. zapota was performed via a validated HPLC method.
| Plant part/Standard | Solvent extract | IC50 (μg/ml) | |
|---|---|---|---|
| Monophenolase inhibitory activity | Diphenolase inhibitory activity | ||
| Bark | Methanol | 85.2±2.1b | 66.18±0.82d |
| Water | 175.90±0.76i | 437.6±1.7l | |
| Flowers | Methanol | 181.7±1.2j | 51.01±0.33c |
| Water | 171.0±3.3h | 203±18i | |
| Fruit | Methanol | 466.3±1.9n | 68.2±5.3d |
| Water | 266.8±1.4m | 262.2±6.9j | |
| Leaves | Methanol | 108.0±1.1d | 48±10c |
| Water | 128.54±0.42e | 337.5±9.0k | |
| Roots | Methanol | 87.9±1.2c | 33.52±0.68b |
| Water | 107.24±0.41d | 83.3±1.6e | |
| Seeds | Methanol | 166.79±0.37g | 133.6±1.7g |
| Water | 236.3±2.6l | 82.7±2.7e | |
| Wood | Methanol | 269.24±0.61m | 174.8±1.9h |
| Water | 218.0±1.8k | 117.64±0.32f | |
| Arbutin | 134.23±0.53f | 8.62±0.11a | |
| Kojic acid | 1.19±0.02a | 2.48±0.02a | |
Values are mean±SD (n=3). Statistical comparison between values of different extracts was done using Duncan’s multiple range test (p<0.05)
Table 4: Antityrosinase Activity of Different Parts of M. zapota
Analytical method validation of (+)-dihydrokaempferol was achieved and showed good linearity, sensitivity, accuracy and precision. The linear relationship between concentration of the (+)-dihydrokaempferol solution and peak area was within the range of 200-1200 ng/ml. As a result, the regression equation was y=0.6339x–40.869, with correlation coefficient (R2) 0.9991. LOD and LOQ were estimated for (+)-dihydrokaempferol at a concentration of 200-1200 ng/ml. LOD and LOQ values were found to be 214.5 and 650.0 ng/ml, respectively. Result of accuracy was calculated and reported in terms of percentage recovery (Table 5). The percentage recovery of (+)-dihydrokaempferol was found to range from 99.80 to 102.35 %. Intra and inter-day assays were used to determine the precision of HPLC method (Table 6). The intra and inter-day % RSD were found to be in the range of 0.04-0.07 % and 0.05-0.06 %, respectively.
| Concentration (ng/ml) | Recovery (%) | RSD (%) |
|---|---|---|
| 400 | 99.80 | 0.22 |
| 600 | 101.55 | 0.13 |
| 800 | 102.35 | 0.08 |
Table 5: Accuracy Results of (+)-DIHYDROKAEMPFEROL
| Concentration (ng/ml) | Intra-day | Inter-day | ||
|---|---|---|---|---|
| Mean±SD (ng/ml) | RSD (%) | Mean±SD (ng/ml) | RSD (%) | |
| 400 | 398.97±0.26 | 0.07 | 398.96±0.24 | 0.06 |
| 600 | 602.07±0.31 | 0.05 | 602.06±0.31 | 0.05 |
| 800 | 798.97±0.35 | 0.04 | 798.96±0.36 | 0.05 |
Values are mean±SD (n=7)
Table 6: Precision Results of (+)-DIHYDROKAEMPFEROL
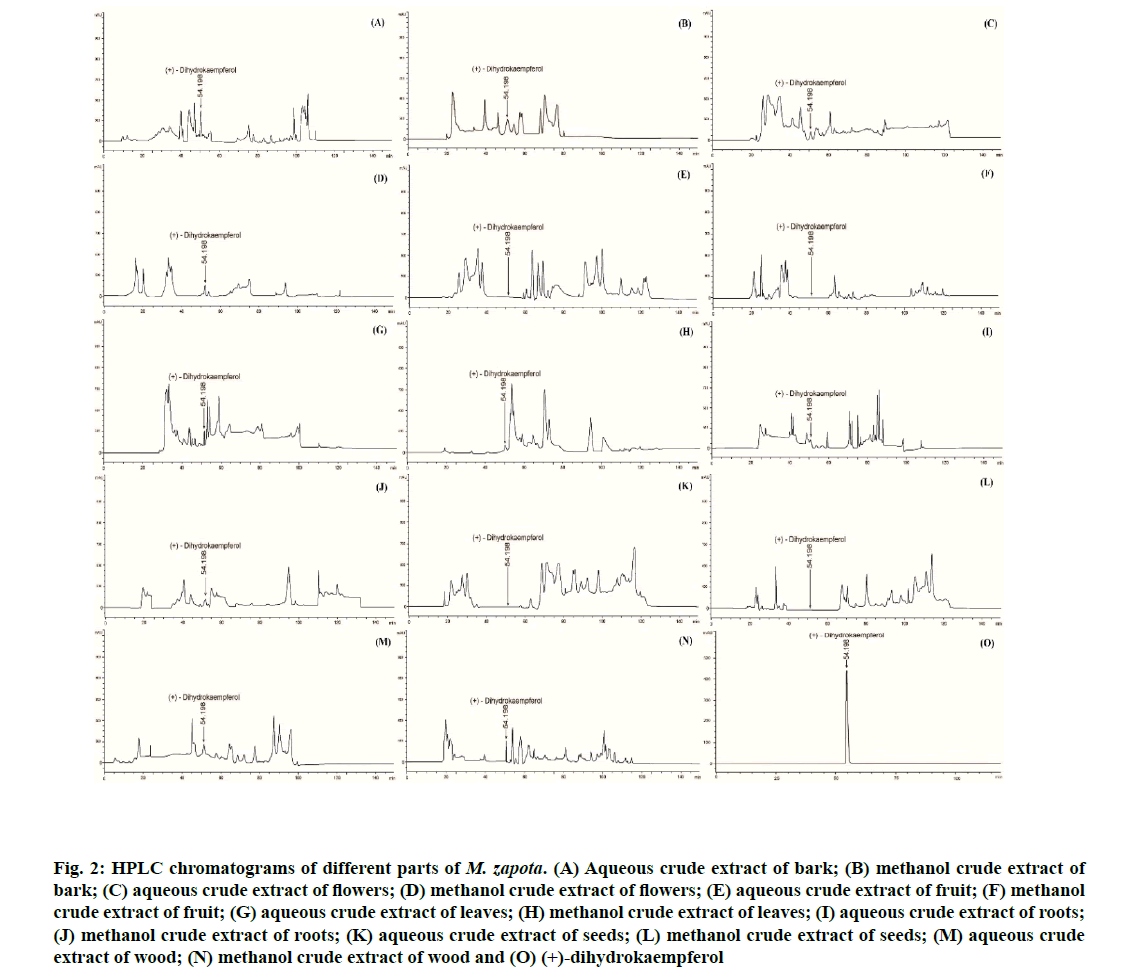
Quantitativeanalysisof (+)-dihydrokaempferol in different parts of M. zapota by HPLC is shown here. Chromatograms of the different parts of M. zapota are presented in fig. 2 and quantification of (+)-dihydrokaempferol is listed in Table 7. (+)-Dihydrokaempferol showed retention time of 54.198 min (fig. 2O) and it was detected in decreasing order of concentration, in: bark>roots>wood>flowers>leaves. The methanol crude extract of the bark (33.62±0.01 mg/g of crude extract) showed the highest content of (+)-dihydrokaempferol, followed by the aqueous crude extract of the bark (27.94±0.01 mg/g of crude extract), the methanol crude extract of the roots (23.20±0.01 mg/g of crude extract) and the methanol crude extract of the wood (22.96±0.01 mg/g of crude extract). However, (+)-dihydrokaempferol was not detected in the fruit or seeds. In the present study, the flowers contained more phenolic compounds and flavonoids than the bark. Moreover, the methanol crude extract of the flowers exhibited strong antioxidant and moderate diphenolase inhibitory activities. Therefore, a high content of (+)-dihydrokaempferol leads to strong antityrosinase activity. The presence of bioactive compounds, such as phenolic compounds and flavonoids, in the extracts of M. zapota relates to their antioxidant and antityrosinase activities.
Figure 2: HPLC chromatograms of different parts of M. zapota. (A) Aqueous crude extract of bark; (B) methanol crude extract of bark; (C) aqueous crude extract of flowers; (D) methanol crude extract of flowers; (E) aqueous crude extract of fruit; (F) methanol crude extract of fruit; (G) aqueous crude extract of leaves; (H) methanol crude extract of leaves; (I) aqueous crude extract of roots; (J) methanol crude extract of roots; (K) aqueous crude extract of seeds; (L) methanol crude extract of seeds; (M) aqueous crude extract of wood; (N) methanol crude extract of wood and (O) (+)-dihydrokaempferol
| Plant part | Solvent extract | Content of (+)-dihydrokaempferol (mg/g of crude extract) |
|---|---|---|
| Bark | Methanol | 33.62±0.01i |
| Water | 27.94±0.01h | |
| Flowers | Methanol | 11.30±0.01e |
| Water | 9.12±0.02c | |
| Fruit | Methanol | ND |
| Water | ND | |
| Leaves | Methanol | 8.46±0.01b |
| Water | 5.70±0.01a | |
| Roots | Methanol | 23.20±0.01g |
| Water | 12.44±0.01f | |
| Seeds | Methanol | ND |
| Water | ND | |
| Wood | Methanol | 22.96±0.01g |
| Water | 10.10±0.01d |
Values are mean±SD (n=3); ND: Value not detected
Table 7: Quatification of (+)-DIHYDROKAEMPFEROL in Different Parts of M. zapota
In conclusion, the analysis of seven different parts of M. zapota indicated that the flowers contained the highest amounts of phenolic compounds and flavonoids and exhibited the strongest antioxidant activity, and showed moderate diphenolase inhibitory activity. The bark presented the strongest monophenolase inhibitory activity and the roots exhibited the strongest diphenolase inhibitory activity. Quantification of (+)-dihydrokaempferol using HPLC analysis showed that this compound was presented in the bark, flowers, leaves, roots and wood, but was absent in the fruit and seeds. The methanol crude extract of the bark presented the highest amount of (+)-dihydrokaempferol. Therefore, the flowers of M. zapota may be used as a natural antioxidant source and the bark and roots may be beneficial for sources of tyrosinase inhibitors.
Acknowledgements:
This research was supported by the Graduate School of Chulalongkorn University (thesis grant), Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (GRU 6203023003-1) and the National Research Council of Thailand (GRB_ BSS_101_59_61_08). The authors would like to thank the Faculty of Science and Technology, Phranakhon Rajabhat University for providing access to the HPLC facility.
Conflicts of interest:
The authors declare that they have no conflicts of interests.
References
- Manda G, Nechifor MT, Neagu TM. Reactive oxygen species, cancer and anti-cancer therapies. Curr Chem Biol 2009;3(1):22-46.
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans 2001;29(2):345-9.
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012;2012:37-63.
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008;4(2):89-96.
- Rahman T, Hosen I, Islam MT, Shekhar HU. Oxidative stress and human health. Adv Biosci Biotechnol 2012;3:997-1019.
- Saxena M, Saxena J, Pradhan A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int J Pharm Sci Rev Res 2012;16(2):130-4.
- Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J Med Plants Res 2011;5(31):6697-703.
- Nirmal NP, Benjakul S, Ahmad M, Arfat YA, Panichayupakaranant P. Undesirable enzymatic browning in crustaceans: Causative effects and its inhibition by phenolic compounds. Crit Rev Food Sci Nutr 2015;55(14):1992-2003.
- Tanase C, Co?arc? S, Muntean DL. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019;24(6):1182.
- Vicente O, Boscaiu M. Flavonoids: Antioxidant compounds for plant defence and for a healthy human diet. Not Bot Horti Agrobot Cluj Napoca 2018;46(1):14-21.
- Sánchez-Ferrer Á, Rodríguez-López JN, García-Cánovas F, García-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1995;1247(1):1-11.
- Prota G. An introduction to melanin research. In: Prota G, editor. Melanins and melanogenesis, California: Academic press; 1992. p. 1-9.
- Burger P, Landreau A, Azoulay S, Michel T, Fernandez X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016;3(4):36-60.
- Rendon MI, Gaviria JI. Review of skin?lightening agents. Dermatol Surg 2005;31:886-90.
- Chantaranothai P. Sapotaceae. In Santisuk T, Balslev H, editors. Flora of Thailand. Bangkok: Department of National Parks, Wildlife and Plant Conservative; 2014. p. 610-55.
- Milind P, Preeti M. Chickoo: a wonderful gift from nature. Int J Res Ayurveda Pharm 2015;6(4):544-50.
- Barbalho SM, Bueno PC, Delazari DS, Guiguer EL, Coqueiro DP, Araújo AC, de Souza MD, et al. Antidiabetic and antilipidemic effects of Manilkara zapota. J Med Food 2015;18(3):385-91.
- Fomani M, Nouga AB, Toze FA, Ndom JC, Waffo AF, Wansi JD. Bioactive Phenylethanoids from the Seeds of Manilkara zapota. J Pharm Res Int 2015;8:1-5.
- Ganguly A, Al Mahmud Z, Uddin MM, Rahman SA. In vivo anti-inflammatory and anti-pyretic activities of Manilkara zapota leaves in albino Wistar rats. Asian Pac J Trop Dis 2013;3(4):301-7.
- Ma J, Luo XD, Protiva P, Yang H, Ma C, Basile MJ, et al. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J Nat Prod 2003;66(7):983-6.
- Fayek NM, Monem AR, Mossa MY, Meselhy MR. New triterpenoid acyl derivatives and biological study of Manilkara zapota (L.) Van Royen fruits. Pharmacognosy Res 2013;5(2):55-9.
- Toze FA, Fomani M, Nouga AB, Chouna JR, Waffo AF, Wansi JD. Taraxastane and Lupane Triterpenoids from the Bark of Manilkara zapota. Int Res J Pure Appl Chem 20157(4):157-64.
- Venkateswara RG, Sahoo MR, Madhavi MS, Mukhopadhyay T. Phytoconstituents from the leaves and seeds of Manilkara zapota Linn. Der Pharm. Lett 2014;6:69-73.
- Chunhakant S, Chaicharoenpong C. Antityrosinase, antioxidant, and cytotoxic activities of phytochemical constituents from Manilkara zapota L. Bark. Molecules 2019;24(15):2798-825.
- Dorman HD, Ko?ar M, Kahlos K, Holm Y, Hiltunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties and cultivars. J Agric Food Chem 2003;51(16):4563-9.
- Tohidi B, Rahimmalek M, Arzani A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem 2017;220:153-61.
- Sulaiman SF, Yusoff NA, Eldeen IM, Seow EM, Sajak AA, Ooi KL. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J Food Compost Anal 2011;24(1):1-10.
- Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int 2011;44(1):217-24.
- Dej-Adisai S, Meechai I, Puripattanavong J, Kummee S. Antityrosinase and antimicrobial activities from Thai medicinal plants. Arch Pharm Res 2014;37(4):473-83.
- Tansirikongkol A. Effects of sapota part and extracting solvent on in vitro anti-aging properties of Manilkara zapota extract. Thai J Pharm Sci 2016;40:100-3.
- Islam MR, Parvin MS, Banu MR, Jahan N, Das N, Islam ME. Antibacterial and phytochemical screening of ethanol extracts of Manilkara zapota leaves and bark. Int J Pharma Sci 2013;3:394-97.
- Pientaweeratch S, Panapisal V, Tansirikongkol A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm Biol 2016;54(9):1865-72.
- Kaneria M, Chanda S. Evaluation of antioxidant and antimicrobial properties of Manilkara zapota L. (chiku) leaves by sequential soxhlet extraction method. Asian Pac J Trop Biomed 2012;2(3):S1526-33.
- Parikh B, Patel VH. Quantification of phenolic compounds and antioxidant capacity of an underutilized Indian fruit: Rayan [Manilkara hexandra (Roxb.) Dubard]. Food Sci Hum Wellness 2017;6(1):10-9.
- Shafii ZA, Basri M, Malek EA, Ismail M. Phytochemical and antioxidant properties of Manilkara zapota (L.) P roen fruit extracts and its formulations for cosmceuetical application. Asian JPlant Sci Res 2017;7(3):29-41.
- Woo PF, Yim HS, Khoo HE, Sia CM, Ang YK. Effects of extraction conditions on antioxidant properties of sapodilla fruit (Manilkara zapota). Int Food Res J 2013;20(5):2065-72.
- Kanlayavattanakul M, Lourith N. Sapodilla seed coat as a multifunctional ingredient for cosmetic applications. Process Biochem 2011;46(11):2215-8.
- Khorasani Esmaeili A, Mat Taha R, Mohajer S, Banisalam B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). Biomed Res Int 2015;2015:285-96.
- Stankovic MS. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J Sci 2011;33(2011):63-72.
- Tansirikongkol A. Comparative in vitro anti-aging activities of Phyllanthus emblica L. extract, Manilkara sapota L. extract and its combination. Thai J Pharm Sci 2016;40:108-11.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2(5):270-8.
- Stagos D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2019;9(1):19-26.
- Gutiérrez-Grijalva EP, Ambriz-Pére DL, Leyva-López N, Castillo-López RI, Heredia JB. Dietary phenolic compounds, health benefits and bioaccessibility. Arch Latinoam Nutr 2016;66(2):87-100.
- Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J Pharm Sci 2018;13(1):12-23.
- Pisoschi AM, Pop A, Cimpeanu C, Predoi G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid Med Cell Longev 2016;2016:76-112.
- Deng YT, Liang G, Shi Y, Li HL, Zhang J, Mao XM, et al. Condensed tannins from Ficus altissima leaves: structural, antioxidant, and antityrosinase properties. Process Biochem 2016;51(8):1092-9.
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 2011;48(4):412-22.
- Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin?lightening agents–existing and new approaches. Int J Cosmet Sci 2011;33(3):210-21.
- Choi MH, Shin HJ. Anti-melanogenesis effect of quercetin. Cosmetics 2016;3(2):18-34.
- Sarkhail P, Sarkheil P, Khalighi-Sigaroodi F, Shafiee A, Ostad N. Tyrosinase inhibitor and radical scavenger fractions and isolated compounds from aerial parts of Peucedanum knappii Bornm. Nat Prod Res 2013;27(10):896-9.
- Csepregi K, Neugart S, Schreiner M, Hideg É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016;21(2):208-25.