- Corresponding Author:
- Purkayastha
Amity Institute of Biotechnology, Amity University, Sector 125, Noida-201 303, India
E‑mail: sp18nov.aib@gmail.com
Date of Submission |
25 August 2011 |
Date of Revision |
25 October 2012 |
Date of Acceptance |
28 October 2012 |
| Indian J Pharm Sci, 2012, 74 (5): 443-450 |
Abstract
The in vitro antibacterial activity of various solvents and water extracts of aloe vera, neem, bryophyllum, lemongrass, tulsi, oregano, rosemary and thyme was assessed on 10 multi-drug resistant clinical isolates from both Gram-positive and Gram-negative bacteria and two standard strains including Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922. The zone of inhibition as determined by agar well diffusion method varied with the plant extract, the solvent used for extraction, and the organism tested. Klebsiella pneumoniae 2, Escherichia coli 3 and Staphylococcus aureus 3 were resistant to the plant extracts tested. Moreover, water extracts did not restrain the growth of any tested bacteria. Ethanol and methanol extracts were found to be more potent being capable of exerting significant inhibitory activities against majority of the bacteria investigated. Staphylococcus aureus 1 was the most inhibited bacterial isolate with 24 extracts (60%) inhibiting its growth whereas Escherichia coli 2 exhibited strong resistance being inhibited by only 11 extracts (28%). The results obtained in the agar diffusion plates were in fair correlation with that obtained in the minimum inhibitory concentration tests. The minimum inhibitory concentration of tulsi, oregano, rosemary and aloe vera extracts was found in the range of 1.56-6.25 mg/ml for the multi-drug resistant Staphylococcus aureus isolates tested whereas higher values (6.25-25 mg/ml) were obtained against the multi-drug resistant isolates Klebsiella pneumoniae 1 and Escherichia coli 1 and 2. Qualitative phytochemical analysis demonstrated the presence of tannins and saponins in all plants tested. Thin layer chromatography and bioautography agar overlay assay of ethanol extracts of neem, tulsi and aloe vera indicated flavonoids and tannins as major active compounds against methicillin-resistant Staphylococcus aureus.
Keywords
Agar well diffusion, bioautography assay, minimum inhibitory concentration, multi?drug resistant, phytochemical analysis
Antibiotics have saved the lives of millions of people and have contributed to the major gains in life expectancy over the last century. However, the clinical efficacy of many existing antibiotics is being threatened by the emergence of multi?drug resistant (MDR) pathogens [1] the recent appearance of strains with reduced susceptibility as well as, undesirable side effects of certain antibiotics [2]. Infectious diseases caused by resistant microorganisms are associated with prolonged hospitalizations, increased cost, and greater risk for morbidity and mortality. Resistance is an especially vexing problem for people with impaired immune systems, such as AIDS, cancer patients and recipients of organ transplants. The promiscuous use of antibiotics accounts for a major part of the community burden of antibiotic use and contributes dramatically to the rising prevalence of resistance among major human pathogens. Vancomycin?resistant enterococci (VRE), methicillin?resistant Staphylococcus aureus (MRSA), MDR Mycobacterium tuberculosis and MDR Gram?negative bacteria are recognised as the most difficult healthcare?associated infections to control and treat. The development of extended?spectrum β?lactamases (ESBLs) and carbapenemases that target Gram?negative bacteria has resulted in infections that can be extremely difficult to treat leading to substantial increased illnesses and death rate. The effect is pronounced in third world as the costly replacement drugs for treating the highly resistant infectious diseases are unaffordable [3]. The resistance problem demands that a renewed effort be made to screen various medicinal plants for their potential antimicrobial traits, which are due to compounds synthesized in the secondary metabolism of the plant. The most important of these bioactive compounds of plants are alkaloids, flavonoids, tannins, phenolic compounds, steroids, resins, fatty acids and gums which are capable of producing definite physiological action on body. Another driving factor that encouraged scientists to search for new antimicrobial substances from various sources including medicinal plants has been the rapid rate of plant species extinction. Medicinal plants are relied upon by 80% of the world’s population and in India there is a rich tradition of using herbal medicine for the treatment of various infectious diseases, inflammations, injuries and other diseases. Many of the plant materials used in traditional medicine are generally proved more effective and relatively cheaper than modern medicine [4] against certain ailments while simultaneously mitigating many of the side effects that are often associated with synthetic antimicrobials [5].
Most of the studies are directed to see the activity of plant extracts against a variety of test bacteria including both pathogenic and nonpathogenic strains. Several workers have made targeted screening against MDR bacteria such as MRSA, VRE, M. tuberculosis, enteric bacteria and others [6?9]. It was documented that acetone and ethanol extracts obtained from fifteen plants used in folk medicine by tribals of Mandla region exhibited significant activity against urinary tract infection (UTI) causing pathogens [10]. Aqil et al. [11] reported significant inhibitory effect of ethanol extracts of various Indian medicinal plants on both clinical isolates of β?lactamase producing MRSA and methicillin?sensitive S. aureus (MSSA). In another study, oregano oil exhibited antibacterial activity against methicillin?sensitive and methicillin?resistant bacteria [12]. Ayachi et al. [13] detected the antibacterial activity of methanol, dichloromethane and ether extracts of Thymus vulgaris against MDR Salmonella typhimurium.
Despite abundant literature on the antimicrobial properties of plant extracts, none of the plant derived chemicals have successfully been exploited for clinical use as antibiotics. A significant part of the chemical diversity produced by plants is thought to protect plants against microbial pathogen. Hence, they have been proven to have antimicrobial importance both in vivo and in vitro [14]. This research was designed to study the antimicrobial potentiality of eight medicinal plants viz. Aloe barbadensis (aloe vera), Azadirachta indica (neem), Bryophyllum pinnatum (bryophyllum), Cymbopogon citratus (lemongrass), Ocimum sanctum (tulsi), Origanum vulgare (oregano), Rosmarinus officinalis (rosemary) and Thymus vulgaris (thyme) against a series of MDR bacteria of clinical relevance. Phytochemical screening was carried out to identify major biologically active phytoconstituents. Moreover, we investigated the biological activity of the potent crude extracts against S. aureus MRSA using agar overlay bioautography assay. It is hoped that these active constituents will provide useful information for discovering new compounds with better activity against MDR bacteria than agents currently available.
Materials and Methods
Fresh leaves of lemongrass, oregano, rosemary and thyme were purchased from INA local market, New Delhi, India. Additionally, leaves of neem, tulsi, aloe vera and bryophyllum free from diseases were obtained from Noida localities, Uttar Pradesh, India. The collected plants were identified taxonomically and authenticated.
Preparation of plant extracts
The leaf samples were washed thoroughly 2?3 times with running tap water and once with sterile water, air?dried, powdered and used for extraction. Fifty grams of each of the air?dried and coarsely powdered plant material was extracted successively with 200 ml each of hexane, chloroform, methanol, ethanol and water in the increasing order of their polarity using a soxhlet evaporator for 48 h [15]. After complete solvent evaporation, extracts were dissolved in 10% dimethyl sulphoxide (DMSO) (Merck (India) Ltd., Mumbai, India) to a final concentration of 50 mg/ml and stored at 5° in labelled sterile screw?capped bottles for further use.
Bacterial cultures and growth conditions
MDR clinical isolates of S. aureus (3 isolates), S. aureus (MRSA), Escherichia coli (3 isolates), Klebsiella pneumoniae (2 isolates) and Proteus mirabilis with their antibiotic resistance profiles were obtained from the Department of Microbiology, Rajiv Gandhi Cancer Research Institute, Delhi, India (Table 1). Standard strains S. aureus ATCC 25923 and E. coli ATCC 25922 were used for quality control. All the test strains were maintained on nutrient agar slants (Hi?Media Laboratories Pvt. Limited, Mumbai, India) at 4° and subcultured on to nutrient broth for 24 h prior to testing. These bacteria served as test pathogens for antibacterial activity assay.
| Antibiotics | Test bacteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kp1 | Kp2 | Ec1 | Ec2 | Ec3 | Pm | Sa1 | Sa2 | Sa MRSA | Sa3 | |
| AK | S | R | S | S | R | S | S | S | S | R |
| AC | R | R | R | R | R | R | S | S | R | R |
| CFX | R | R | R | R | R | R | R | R | R | R |
| CS | R | R | S | R | R | S | S | S | S | R |
| CE | R | R | R | R | R | R | R | S | R | R |
| CI | R | R | R | S | R | S | R | R | R | R |
| CF | R | R | R | R | R | S | S | R | R | R |
| GF | S | R | S | R | R | S | S | S | S | R |
| G | S | R | R | S | R | R | S | S | R | R |
| I | S | S | S | S | S | S | S | S | S | R |
| LE | S | R | R | R | R | S | S | S | S | R |
| MR | S | R | S | S | S | S | S | S | R | R |
| OF | R | R | R | R | R | S | S | R | R | R |
| PT | S | R | S | S | R | R | S | S | R | R |
| VA | - | - | - | - | - | - | S | S | S | S |
| LZ | - | - | - | - | - | - | S | S | S | S |
AK=Amikacin, AC=Amoxycillin/Clavulanic acid, CFX=Cefixime, CS=Cefoperazone+Sulbactum, CE=Cefotaxime, CI=Ceftriaxone, CF=Ciprofloxacin, GF=Gatifloxacin, G=Gentamicin, I=Imipenem, LE=Levofloxacin, MR=Meropenem, OF=Ofloxacin, PT=Piperacillin/tazobactam, VA=Vancomycin, LZ=Linezolid, R=Resistant, S=Sensitive, Kp=Klebsiella pneumoniae, Ec=Escherichia coli, Sa=Staphylococcus aureus
Table 1: Antibiotic Resistance Profile Of Bacterial Isolates Used
Antibacterial activity assay
Antibacterial activity of aqueous and solvent extracts was determined by agar well diffusion method according to National Committee for Clinical Laboratory Standards (NCCLS) [16]. Inoculum containing 106 cfu/ml of each bacterial culture to be tested was spread on nutrient agar plates with a sterile swab moistened with the bacterial suspension. Subsequently, wells of 8 mm diameter were punched into the agar medium and filled with 100 μl (25 mg/ml) of plant extract and allowed to diffuse at room temperature for 2 h. The plates were then incubated in the upright position at 37° for 24 h. Wells containing the same volume of DMSO (10%), hexane, chloroform, methanol, ethanol and distilled water served as negative controls while standard antibiotic discs of imepenem (10 μg) and vancomycin (30 μg) were used as the positive controls. After incubation, the diameters of the growth inhibition zones were measured in mm. Three replicates were carried out for each extract against each of the test organism. Data were expressed as mean±standard deviation.
Minimum inhibitory concentration
Based on the preliminary screening, ethanol and methanol extracts that revealed potent antimicrobial activity were further tested to determine the minimum inhibitory concentration (MIC) for each bacterial sample (Table 2). The MIC of these extracts was determined by broth dilution technique where the stocks of 50 mg/ml of the extracts were resuspended in 10% DMSO to produce two?fold dilutions in the range of 0.39?25 mg/ml. Each dilution was seeded with bacterial suspension (1×106 cfu/ml) and incubated for 24 h at 37°. After incubation the growth of the bacterial isolates in the test tubes were observed as turbidity using spectrophotometer at 600 nm. The least concentration where no turbidity was observed was determined and noted as the MIC value. All samples were tested in triplicates.
| Plant | Ex | Test bacteria (zone of inhibition in mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sa ATCC 25923 | Sa 1 | Sa 2 | Sa MRSA | EcATCC 25922 | Pm | Kp1 | Ec1 | Ec2 | ||
| Ne | H | 17.76±0.75 | - | 7.93±0.05 | - | 11.06±0.20 | 10.00±0.20 | - | - | - |
| C | 16.00±0.65 | - | 6.90±0.05 | - | 8.00±0.35 | 8.00±0.30 | - | - | - | |
| M | 20.10±0.47 | 19.90±0.45 12.00±0.15 | - | 15.00±0.98 | 11.00±0.11 | 11.00±0.10 | 8.00±0.11 | - | ||
| E | 25.00±0.88 | 16.40±0.79 | 14.00±0.20 | 16.96±0.10 | 16.00±0.02 | 15.00±0.15 | 6.00±0.10 | 8.00±0.10 | - | |
| Tu | H | 12.00±0.91 | - | 10.06±0.15 | 12.06±0.05 | 10.00±0.90 | 6.00±0.15 | - | - | - |
| C | 11.93±0.90 | - | - | - | - | 4.00±0.05 | - | - | - | |
| M | 16.23±0.87 | 11.90±0.25 8.03±0.15 | 11.06±0.10 | 14.00±0.30 | - | 6.00±0.10 | 7.00±0.15 | 5.00±0.30 | ||
| E | 20.03±0.90 | 19.00±0.75 12.06±0.15 | 18.10±0.10 | 17.00±0.80 | 12.00±0.17 | 9.00±0.15 | 9.00±0.15 | 8.00±0.30 | ||
| Og | H | 10.00±0.04 | 9.90±0.55 14.00±0.17 | - | - | 4.00±0.10 | 6.00±0.15 | - | - | |
| C | 8.31±0.61 | 10.00±0.20 6.90±0.10 | - | 13.00±0.98 | - | - | 6.00±0.05 | - | ||
| M | 16.06±0.30 | 12.00±0.03 15.06±0.05 | 12.03±0.10 | 17.00±0.40 | 10.00±0.15 | 6.00±0.26 | 14.00±0.11 | 10.00±0.17 | ||
| E | 18.00±0.30 | 11.00±0.80 | 13.00±0.20 | 14.90±0.05 | 15.00±0.55 | 8.00±0.23 | 7.00±0.10 | 12.00±0.20 | 13.00±0.11 | |
| Rm | H | 12.10±0.26 | - | - | - | - | - | - | - | - |
| C | 11.00±0.03 | - | 6.00±0.15 | - | - | 4.00±0.43 | - | - | - | |
| M | 16.10±0.36 | 15.00±0.30 12.03±0.05 | 14.00±0.70 | 14.00±0.30 | 5.00±0.05 | 6.00±0.11 | 10.00±0.05 | 11.00±0.15 | ||
| E | 18.03±0.15 | 14.00±0.80 | 14.93±0.05 | 14.00±0.90 | 10.00±0.10 | 12.00±0.11 | 10.00±0.11 | 9.00±0.05 | 10.00±0.10 | |
| Lg | H | - | - | - | - | - | - | - | - | - |
| C | - | - | - | - | - | - | - | - | - | |
| M | 10.20±0.20 | 6.93±0.60 | - | 10.96±0.20 | 5.00±0.70 | - | 7.00±0.05 | 5.00±0.20 | - | |
| E | 13.90±0.20 | 4.00±0.80 | - | 13.00±0.60 | 10.00±0.70 | - | 14.00±0.25 | 14.00±0.30 | 14.00±0.11 | |
| Av | H | 10.30±0.15 | 10.03±0.70 | - | - | 4.00±0.20 | - | - | - | - |
| C | 8.00±0.10 | 5.60±0.35 14.90±0.10 | - | - | 4.00±0.11 | - | - | - | ||
| M | 13.90±0.17 | 12.00±0.10 16.13±0.11 | 13.96±0.20 | 10.00±0.41 | 10.00±0.05 | 8.00±0.35 | 6.00±0.11 | - | ||
| E | 21.10±0.10 | 15.00±0.10 | - | 17.90±0.35 | 17.00±0.52 | 12.00±0.17 | 14.00±0.10 | 7.00±0.05 | - | |
| Bp | H | 10.96±0.05 | 8.96±0.15 7.06±0.05 | - | 4.00±0.61 | 11.00±0.20 | - | - | - | |
| C | - | 7.03±0.15 5.01±0.07 | - | - | - | - | - | - | ||
| M | 21.90±0.11 | 15.00±0.20 11.03±0.50 | - | 11.00±0.30 | 14.00±0.11 | 10.00±0.05 | 8.00±0.11 | 8.00±0.20 | ||
| E | 15.16±0.15 | 11.10±0.20 9.77±0.58 | - | 10.00±0.50 | 12.00±0.11 | 6.00±0.15 | 7.00±0.20 | 6.00±0.20 | ||
| Th | H | 9.96±0.15 | 12.00±0.20 | - | - | 8.00±0.30 | 6.00±0.11 | 7.00±0.10 | 5.00±0.40 | - |
| C | 7.03±0.15 | 8.20±0.40 | - | - | 6.00±0.45 | - | - | - | - | |
| M | 14.06±0.20 | 12.00±0.15 | - | 11.83±0.10 | 10.00±0.60 | 7.00±0.15 | 7.00±0.05 | - | 7.00±0.11 | |
| E | 21.00±0.10 | 17.00±0.20 7.03±0.05 | 13.76±0.20 | 13.00±0.52 | 11.00±0.10 | 7.00±0.15 | 8.00±0.11 | 4.00±0.20 | ||
| I | NT | NT | NT | NT | 28.00±0.45 | 19.00±0.15 | 17.00±0.35 | 18.50±0.50 | 17.20±0.44 | |
| V | 21.60±0.37 | 18.40±0.15 | 16.60±0.11 | 18.72±0.10 | NT | NT | NT | NT | NT | |
Ne=neem, Tu=tulsi, Og=Oregano, Rm=Rosemary, Lg=Lemongrass, Av=Aloevera, Bp=Bryophyllum, Th=Thyme, Ex=Extract, M=methanol, E=ethanol, hexane, chloroform, Sa=Staphylococcus aureus, Ec=Escherichia coli, Pm=Proteus mirabilis, Kp=Klebsiella pneumoniae,?=No inhibition, NT=Not tested, I=Imepenem, V=Vancomycin. Values expressed as mean±standard deviation of three replicates
Table 2: Antibacterial Activity Of Medicinal Plants Determined By Agar Well Diffusion Method
Phytochemical analysis
Hexane, chloroform, methanol, ethanol and water extracts were subjected to phytochemical analysis to ascertain the presence of metabolites such as reducing sugars, alkaloids, anthraquinones, glycosides, flavonoids, tannins, steroids, saponins, triterpenoids and phlobatanins [17].
Thin layer chromatography
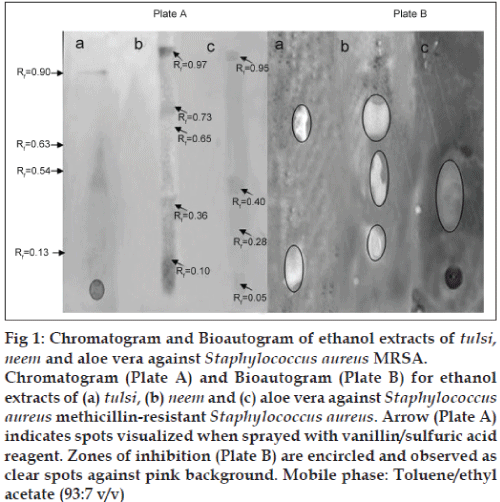
The phytocompounds of the ethanol extracts of neem, tulsi and aloe vera showing significant antimicrobial activity with MIC value of 1.6 mg/ml against S. aureus MRSA (Table 3) were analysed using thin layer chromatography (TLC). About 10 μl of each extract was applied on precoated aluminium silica gel G 25 plates. Developing solvent system used was toluene and ethyl acetate (93:7 v/v). The TLC plates were run in triplicate. Plate A, the reference chromatogram was used to determine the spots as visualised by UV light to see if the separated spots were UV active after which it was sprayed with vanillin sulphuric acid (2%) spray reagent, plate B was used for bioautography and the TLC plates which were used to identify spots with the various TLC reagents to detect the presence of flavonoids, tannins and saponins as described by Johann et al. [18] was denoted as plate C. Individual Rf for each spot was measured.
| Plant Extract | Test bacteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sa ATCC 25923 |
Sa 1 | Sa 2 | Sa MRSA |
Ec ATCC 25922 |
Ec 1 | Ec 2 | Pm | Kp1 | ||
| Ne | E | 0.39 | 1.56 | 1.56 | 1.56 | 1.56 | 12.5 | NT | 6.25 | 12.5 |
| M | 0.78 | 1.56 | 3.12 | NT | 3.12 | 12.5 | NT | 6.25 | 25 | |
| Tu | E | 0.39 | 1.56 | 3.12 | 1.56 | 3.12 | 12.5 | 12.5 | 12.5 | 12.5 |
| M | 0.78 | 3.12 | 6.25 | 6.25 | 1.56 | NT | 12.5 | NT | 25 | |
| Og | E | 1.56 | 6.25 | 3.12 | 3.12 | 1.56 | 6.25 | 6.25 | 12.5 | 12.5 |
| M | 1.56 | 6.25 | 3.12 | 6.25 | 3.12 | 6.25 | 12.5 | 6.25 | 25 | |
| Rm | E | 1.56 | 6.25 | 3.12 | 3.12 | 1.56 | 12.5 | 12.5 | 12.5 | 12.5 |
| M | 1.56 | 3.12 | 3.12 | 3.12 | 1.56 | 12.5 | 12.5 | 25 | 25 | |
| Lg | E | 1.56 | 12.5 | NT | 6.25 | 6.25 | 6.25 | 6.25 | NT | 6.25 |
| M | 1.56 | 12.5 | NT | 12.5 | 12.5 | 12.5 | NT | NT | 12.5 | |
| Av | E | 0.78 | 1.56 | 3.12 | 1.56 | 6.25 | NT | NT | 6.25 | 6.25 |
| M | 1.56 | 3.12 | 3.12 | 3.12 | 12.5 | NT | NT | 25 | 12.5 | |
| Bp | E | 0.78 | 1.56 | 3.12 | NT | 6.25 | 12.5 | 12.5 | 3.12 | 12.5 |
| M | 1.56 | 1.56 | 3.12 | NT | 3.12 | 12.5 | 25 | 3.12 | 12.5 | |
| Th | E | 0.78 | 1.56 | 6.25 | 3.12 | 6.25 | 12.5 | 12.5 | 6.25 | 12.5 |
| M | 1.56 | 3.12 | NT | 6.25 | 6.25 | 25 | 12.5 | 6.25 | 12.5 | |
Ne=neem, Tu=tulsi, Og=Oregano, Rm=Rosemary, Lg=Lemongrass, Av=Aloevera, Bp=Bryophyllum, Th=Thyme, M?Methanol, E=Ethanol, Sa=Staphylococcus aureus, Ec=Escherichia coli, Pm=Proteus mirabilis, Kp=Klebsiella pneumoniae, NT=Not tested
Table 3: Minimum Inhibitory Concentration (Mg/Ml) Of Ethanol And Methanol Extracts Of Medicinal Plants Against Test Bacteria
Bioautography agar overlay
The developed TLC plates were thinly overlaid with molten nutrient agar inoculated with an overnight culture of S. aureus MRSA. The plates were incubated in a dark and humid chamber at overnight at 37°. Subsequently, the bioautogram was sprayed with an aqueous solution of 2,3,5?triphenyl tetrazolium chloride and further incubated for at 37° for 4 h. Microbial growth inhibition appeared as clear zones against a pink background. The Rf values of the spots showing inhibition were determined. The Rf of the inhibition zones on plate B was compared with the Rf of reference chromatogram (plate A) as well as Rf of the spots on plate C. The experiment was repeated twice.
India has profound traditional knowledge which is the most valuable gift to self and to the humanity in general. However, it was not being utilized in a systematic manner as most of us are unaware of our tradition due to this hi-tech life style [1,2]. One of the earliest medical systems,Ayurveda was developed in India. According to Ayurveda, both ahar (edibles) and vihar (behavioural lifestyle) correction is necessary for treating mental disorders. Ramayana, Mahabharata and Bhagavatam, Vedas etc are not just shlokas written on pages, but they have an impact on our attitude and behavior. The techniques/ methods/treatments mentioned in those books are the intellectual properties of our elders and it is our responsibility to understand them thoroughly and prove them scientifically to develop many therapies which are adoptable and affordable to the common man.
Results and Discussion
Out of 40 extracts (hexane, chloroform, methanol, ethanol and aqueous) of eight plants, screened for potential antibacterial activity against MDR bacteria, ethanol and methanol provided more consistent and prominent antimicrobial activity as compared to hexane and chloroform extracts (Table 2). The chloroform extracts exhibited the least antimicrobial activity as compared to other three solvent extracts. The reasons for minimal antibacterial activity in chloroform extracts could be a low concentration of antibacterial compounds in these extracts. None of the aqueous extracts were found effective against any of the assayed bacteria. Water extract may contain a low concentration of antibacterial compounds or may not extract antibacterial compound(s) or all of the identified components from plants active against microorganisms, aromatic or saturated organic compounds are most often obtained through initial ethanol or methanol extraction [19]. In addition, hexane and chloroform extracts of lemongrass showed inactivity against the tested bacteria.
Results (Table 2) showed that the most susceptible organism was S. aureus ATCC 25923 which was sensitive to 29 plant extracts in various solvents except for hexane extract of lemongrass and chloroform extracts of both lemongrass and bryophyllum. S. aureus 1 was sensitive to 24 extracts while S. aureus 2 was sensitive to 21 extracts. The susceptibility of this bacterium to different plant extracts has been documented in literature [20,21]. It was interesting to note that the methanol and ethanol extracts from tulsi, oregano, rosemary, lemongrass, aloe vera and thyme presented antimicrobial activity to S. aureus MRSA. Previous reports also revealed the antibacterial efficacy of the investigated plant extracts and essential oils against S. aureus MRSA [11,22?24]. Although the extracts from bryophyllum did not restrain the growth of S. aureus MRSA, the other standard and MDR bacterial isolates responded differently to the bryophyllum extracts. Methanol extracts of bryophyllum recorded pronounced antibacterial activity against the test pathogens with zones of inhibition varying between 8 mm against MDR E. coli isolates to 22 mm in S. aureus ATCC 25923. This is in fair correlation with Aibinu et al. [25] who reported good antibacterial activity in bryophyllum against some Gram?positive and Gram?negative bacteria using methanol, local gin and aqueous extracts. Maximum inhibition was observed with ethanol extract of neem against S. aureus ATCC 25923 (25±0.88 mm) while minimum activity against K. pneumoniae 1 (6±0.10 mm). Present investigation, is in contrast to Chowdhury et al. [26], who reported strong antibacterial potential against K. pneumoniae as compared to S. aureus.
Amongst the clinical isolates of Gram?negative bacteria, P. mirabilis was found to be the most sensitive, while E. coli 2 was the most resistant. The inhibition zone against E. coli 2 were produced by the methanol and ethanol extracts of five plants, i.e., oregano, rosemary, tulsi, bryophyllum and thyme in which the first and second ones (with inhibition zone of 10?13 mm) appeared to be highly active (Table 2). There is also a significant zone of inhibition of 14 mm given by the ethanol extract of lemongrass against clinical isolates of E. coli and K. pneumoniae. Findings in this study supported the observations of some other researchers about lemongrass which exhibited antibacterial activity against E. coli and K. pneumoniae [27,28]. On the other hand, the extracts of lemongrass did not show any activity on P. mirabilis. The remaining plant extracts (in 3?4 solvents) demonstrated considerable activity with inhibition zones (4?15 mm) against P. mirabilis. This has clearly indicated that antibiotic resistance does not interfere with the antimicrobial action of plant extracts and these extracts might have different modes of action on test organisms. All extracts were virtually inactive against S. aureus 3, E. coli 3 and K. pneumoniae 2 which were found to be resistant to majority of antibiotics (Table 1). The control plate representing DMSO, hexane, chloroform, methanol, ethanol and distilled water did not exhibit inhibition on the tested bacteria. The zones of inhibition produced by one or the other plant extracts observed in the Gram?positive organisms were almost the same with the control antibiotic vancomycin used.
In the present investigation the ethanol extracts of tulsi, thyme, oregano and rosemary showed the most promising broad spectrum antibacterial properties against the reference as well as MDR bacteria in which the diameter of zone of growth inhibition varied between 6 and 20 mm (in tulsi), 4 and 18 mm (in oregano), 4 and 18 mm (in rosemary) and 4 and 21 mm (in thyme). Our results substantiate the findings of Ghaly et al. [27] who demonstrated the antibacterial activity of thyme and rosemary against highly resistant bacterial isolates E. coli, P. mirabilis, K. pneumoniae, P. aeruginosa implicated in UTI. Baydar et al. [29] revealed the inhibitory activity of oregano oil against a series of Gram?positive and Gram?negative bacteria including Bacillus amyloliquefaciens, B. brevis, B. subtilis, S. aureus, Coryenebacterium xerosis, E. coli, K. pneumoniae, P. vulgaris, Mycobacterium smegmatis. Investigators in the past had also clearly shown that ethanol extracts were more effective than water extracts [30,31]. From the present study, water could not have been the best plant solvent, since the entire test isolates were completely resistant to water extracts. Water extract may contain a low concentration of antibacterial compounds or may not extract antibacterial compound(s) or all of the identified components from plants active against microorganisms, aromatic or saturated organic compounds are most often obtained through initial ethanol or methanol extraction [19].
Additionally, the ethanol extract of aloe vera showed maximum inhibitory zone against standard S. aureus isolate but could not suppress the growth of S. aureus 2 and E. coli 2. On the other hand, the extract indicted pronounced antibacterial activity against S. aureus MRSA and K. pneumonia (Table 2). Earlier studies have reported antibacterial properties of ethanol extracts of aloe vera against the pathogens selected in the current study [20,23,32]. According to the antibacterial assay done for screening purpose all extracts in general are more effective on Gram?positive bacteria than on Gram?negative bacteria. The results agree with observations of previous researchers and could be explained by the different cell wall structures of these bacteria. Gram?negative outer membrane comprising of phospholipids and lipopolysaccharides act as a barrier to the entrance and reaction of most antibiotics and/or antimicrobial agents through cell envelope [33,34].
The data obtained through MIC revealed variability in the inhibitory concentrations of each extract for given bacteria. The MIC values of different plant extract against the standard and MDR S. aureus isolates were found in the range of 0.39?12.5 mg/ml (Table 3). The most active plant extract against S. aureus ATCC 25923, S. aureus 1 and S. aureus 2 was neem ethanol extract (MIC values 0.39?1.56 mg/ml) followed by methanol extract of bryophyllum (MIC values 0.39?3.12 mg/ml). However, lemongrass extracts demonstrated the highest MIC value of 12.5 mg/ml against S. aureus 1 (Table 3). Ethanol extracts of neem, tulsi and aloe vera were most effective with MIC values ≤3.12 mg/ml for S. aureus MRSA. Mahfuzul Hoque et al. [35] documented similar MIC values when testing different concentrations of neem extracts on S. aureus isolates. For MDR E. coli and K. pneumoniae isolates, most of the extracts could inhibit the growth only at 12.5 mg/ml, however, the tested extracts could effectively inhibit E. coli ATCC 25923 even at concentrations as low as 1.56?6.25 mg/ml. It was interesting to note that P. mirabilis was the most sensitive species to bryophyllum extracts with MIC of 3.12 mg/ml. However, the MIC of lemongrass (ethanol extract) showed that MDR K. pneumoniae and E. coli isolates was inhibited at 6.25 mg/ml while MIC value of 12.5 mg/ml was found against S. aureus 1. Such results were different from the data reported by Fagbemi et al. [36] who concluded that S. aureus and E. coli were sensitive to ethanol extract of lemongrass at 512 mg/ml while Kleibsiella appeared to be resistant even at MIC value of 512 mg/ml (highest MIC). Variations in the sensitivity of the bacterial species tested on the extracts might be because of differences in the strains employed for the research and perhaps local environmental factors that affect the potency of medicinal plants, such as temperature, rainfall, day length and soil characteristics may have differed between the plant samples used for each study.
Results of phytochemical analysis (Table 4) revealed the presence of saponins and tannins either in both methanol and ethanol extracts or in any of them. Reducing sugars and terpenoids were detected in both methanol and ethanol extracts of six plants. It was observed that flavonoids was positive in both extracts of four plants while found to occur in only methanol extracts of aloe vera and tulsi. It is well known that these phytoconstituents have already exhibited antimicrobial activity [19]. None of the plant extracts tested had shown the presence of phlobatannins. Anthraquinone is observed only in aloe vera extracts.
| Phytochemicals | Plant extracts | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bp | Lg | Av | Tu | Th | Og | Ne | Rm | ||||||||||||||||
| (E) | (M) | (E) | (M) | (E) | (M) | (E) | (M) | (E) | (M) | (E) | (M) | (E) | (M) | (E) | (M) | ||||||||
| Reducing sugar | + | + | − | − | + | + | − | − | + | + | + | + | ++ | + | + | + | |||||||
| Tannins | + | + | + | − | + | − | + | + | + | + | + | + | + | + | − | + | |||||||
| Anthraquinone | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | |||||||
| Glycoside | + | − | − | + | + | + | + | − | + | + | ++ | + | + | + | − | − | |||||||
| Saponins | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | ++ | |||||||
| Flavonoids | + | + | + | + |
+ |
− | + | − | − | − | − | − | + | + | + | + | |||||||
| Phlobatanins | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||
| Steroids | + | − | − | − | − | − | + | + | − | − | − | − | + | + | + | − | |||||||
| Terpenoids | + | + | + | + | − | − | + | + | + | + | − | − | + | + | + | + | |||||||
Ne=neem, Tu=tulsi, Og=Oregano, Rm=Rosemary, Lg=Lemongrass, Av=Aloevera, Bp=Bryophyllum, Th=Thyme, E=Ethanol, M=Methanol, +=Positive, −=Negative
Table 4: Phytochemical Screening Of Ethanol And Methanol Extracts Of Medicinal Plants
TLC analysis revealed the presence of flavonoids, saponins and tannins in all the three extracts tested (data not shown). The assay for bioautography demonstrated strong inhibition zones of tulsi, neem and aloe vera ethanol extracts against the growth of S. aureus MRSA (fig. 1). The clear zones were located in separate places on the TLC plate, suggesting that more than one compound possessed antimicrobial effect. The Rf values of the inhibiting components were 0.13 and 0.63 for tulsi extract, 0.10, 0.36 and 0.65 for neem extract on plate B. Spots with Rf values of 0.10?0.13 and 0.63?0.65 corresponds to the spots representing flavonoids on spraying with on plate C. Furthermore, the ethanol extract from aloe vera showed one large inhibition zone with Rf value ranging from 0.28 to 0.40 which was also positive with 10% FeCl3 spray reagent. This result suggests that the antiMRSA activity present in aloe vera extracts may be due to the presence of tannins. These findings corroborated with the observations of Mattana et al. [37] and Hatano et al. [38] who reported the antibacterial efficacy of flavonoids and tannins against S. aureus MRSA. It is possible that the observed inhibition was likely due to one or more active compounds which overlap possibly due to the solvent system used for screening. In addition to the components with antimicrobial activity several compounds on the reference chromatogram were visible in UV light at 235 nm (data not shown) and others that were visible by using vanillin/sulphuric acid reagent, many of these compounds did not coincide with the antimicrobial components. No inhibition zone was observed corresponding to the spots with the Rf value of 0.54 and 0.05 in tulsi and aloe vera extracts respectively on reference chromatogram (plate A). Likewise, antibacterial activity was not found in the assayed extracts for the spots with Rf values above 0.73 (fig. 1). This could be attributed to evaporation of the active components, photooxidation or insufficient amount of the active component [39]. Synergism might play a major role in extracts that were active when the MIC of the mixture was determined, while the separated components showed no antimicrobial activity. Further investigation need to focus on the isolation and elucidation of active compounds detected by TLC bioautography by employing various developing solvent systems and using different MRSA isolates.
Fig 1: Chromatogram and Bioautogram of ethanol extracts of tulsi, neem and aloe vera against Staphylococcus aureus MRSA.
Chromatogram (Plate A) and Bioautogram (Plate B) for ethanol extracts of (a) tulsi, (b) neem and (c) aloe vera against Staphylococcus aureus methicillin?resistant Staphylococcus aureus. Arrow (Plate A) indicates spots visualized when sprayed with vanillin/sulfuric acid reagent. Zones of inhibition (Plate B) are encircled and observed as clear spots against pink background. Mobile phase: Toluene/ethyl acetate (93:7 v/v)
From the above study, it can be concluded that the selected medicinal plants have great potential as antimicrobial agents against MDR clinical isolates. Furthermore, in a few cases these plant extracts were active against MDR bacteria under very low concentrations thus minimizing the possible toxic effects. Hence, this study would lead to the development of some stable, biologically active compounds which can be employed in the formulation of antimicrobial agents.
Acknowledgements
Authors thank Prof. P. D. Sharma, Retd. Botanist, Delhi University, Delhi, India, for plant authentication.
References
- Bandow JE, Brötz H, Leichert LI, Labischinski H, Hecker M. Proteomic approach to understanding antibiotic action. Antimicrob Agents Chemother 2003;47:948-55.
- Cunha BA. Antibiotic side effects. Med Clin North Am 2001;85:149-85.
- World Health Organization (WHO). Antimicrobial Resistance Fact Sheet No. 194, 2002.
- Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K, Oladosu P, et al. Evaluation of in vitroantimycobacterial activity of Nigerian plants used for treatment of respiratory diseases. Afr J Biotechnol 2008;7:1630-6.
- Iwu MW, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on New Crops and New Uses. Alexandria, VA: ASHS Press; 1999. p. 457-62.
- Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 2001;74:113-23.
- Aqil F, Ahmad I. Antibacterial properties of traditionally used Indian medicinal plants. Methods Find ExpClinPharmacol 2007;29:79-92.
- Nostro A, Bisignano G, Angela Cannatelli M, Crisafi G, Paola Germanò M, Alonzo V. Effects of Helichrysumitalicum extract on growth and enzymatic activity of Staphylococcus aureus. Int J Antimicrob Agents 2001;17:517-20.
- Ríos JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol 2005;100:80-4.
- Sharma A, Verma R, Ramteke P. Antibacterial activity of some medicinal plants used by tribals against UTI causing pathogens. World ApplSci J 2009;7:332-9.
- Aqil F, Khan MS, Owais M, Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of beta-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol 2005;45:106-14.
- Naim A, Tariq P. Evaluation of antibacterial activity of decoction, infusion and essential oil of Origanumvulgare on methicillin resistant and methicillin sensitive Staphylococcus aureus.Int J BiolBiotechnol 2006;3:121-5.
- Ayachi A, Alloui N, Bennoune O, Yakhlef G, DaasAmiour S, Bouzid W, et al. Antibacterial activity of some fruits; berries and medicinal herb extracts against poultry strains of Salmonella. Am-Eurasian J Agric Environ Sci 2009;6:12-5.
- Gibbons S. Anti-staphylococcal plant natural products. Nat Prod Rep 2004;21:263-77.
- Bobbarala VV, Katikala PK, Naidu KC, Penumajji S. Antifungal activity of selected plant extracts against phytopathogenic fungi AspergillusnigerF2723. Indian J SciTechnol 2009;2:6839-46.
- NCCLS. Performance Standards for Antimicrobial Disc Suspectibility Tests. Approved Standard NCCLS Publication M2-A5, Villanova, PA, USA, 1993.
- Kuklinski C. Farmacognosia. España: Omega SA; 2000.
- Johann S, Pizzolatti M, Donnici CL, Resende MA. Antifungal properties of plants used in Brazilian traditional medicine against clinically relevant fungal pathogens. Braz J Microbiol 2007;38:117-20.
- Cowan MM. Plant products as antimicrobial agents. ClinMicrobiol Rev 1999;12:564-82.
- Agarry OO, Olaleye MT, Bello-Michael CO. Comparative antimicrobial activities of aloe vera gel and leaf. Afr J Biotechnol 2005;4:1413-4.
- Aslam F, Rehman KU, Asghar M, Sarwar M. Antibacterial activity of various phytoconstituents of neem. Pak J AgricSci 2009;46:209-13.
- Chao S, Young G, Oberg C, Nakaoka K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. FlavourFragr J 2008;23:444-9.
- Mehrotra S, Srivastava AK, Nandi SP. Comparative antimicrobial activities of neem, amla, aloe, Assam tea and clove extracts against Vibriocholerae, Staphylococcus aureusand Pseudomonas aeruginosa. J Med Plants Res 2010;4:2393-8.
- Oskay M, Oskay D, Kalyoncu F. Activity of some plant extracts against multi-drug resistant human pathogens. Iran J Pharma Res 2009;8:293-300.
- Aibinu I, Adenipekun T, Adelowotan T, Ogunsanya T, Odugbemi T. Evaluation of the antimicrobial properties of different parts of Citrusaurantifolia(lime fruit) as used locally. Afr J Tradit Complement AlternMed 2006;4:185-90.
- Chowdhury N, Ghosh A, Chandra G. Mosquito larvicidal activities of Solanumvillosum berry extract against the dengue vector Stegomyiaaegypti. BMC Complement Altern Med 2008;8:10.
- Ghaly MF, Shalaby MA, Shash SM, Snehata MN, Ayad AA. Synergistic effect of antibiotics and plant extract to control lyses or clinical bacterial isolates implicated in urinary tract death. J ApplSci Res 2009;5:1298-306.
- Nanasombat S, Lohasupthawee P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against Salmonella and other Enterobacteria. KMITL Sci Tech J 2005;5:135-41.
- Baydar H, Osman S, Ozkan G, Karadoan T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Saturejaspecies with commercial importance in Turkey. Food Control2004;15:169-72.
- Joshi B, Lekhak S, Sharma A. Antimicrobial property of different medicinal plants: Ocimumsantum, Cinnamomumzeylanicum, XanthorylumarmatumandOriganummajorana. Kathmandu Univ JSciEngTechnol 2009;5:143-50.
- Saimary IE, Bakr SS, Bassam Y, Khudaier Y, Abass YK. Efficiency of antibacterial agents extracted from Thymus vulgaris (lamiaceae). Internet J Nutri Wellness 2007;4:1015-21.
- Sami R, Al-Zubaydi, Maetham A, Raesan SJ. Antibacterial effect of some medicinal plant extracts against some pathogenic bacterial strains. The 2nd Kurdistan Conference on Biological Sciences. J DuhokUniv 2009;12:244-9.
- Baron EJ, Peterson LR, Finegold SM. ???. Baily and Scott?s Diagnostic Microbiology. 9th ed. St. Louis: Mosby-Year Book Inc.; 1994.
- Lennette EH, Balows A, Hausler WJ, Shadomy HJ. ???. Manual of Clinical Microbiology. 4th ed. Washington: American Society of Microbiologists; 1985.
- MahfuzulHoque MD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S. Antibacterial activity of guava ( Psidiumguajava L.) and neem (Azadirachtaindica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathog Dis 2007;4:481-8.
- Fagbemi JF, Ugoji E, Adenipekun T, Adelowotan O. Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), lemon grass (Cymbopogoncitratus S.) and turmeric (Curcuma longa L.) on pathogens. Afr J Biotechnol 2009;8:1176-82.
- Mattana CM, Satorres SE, Sosa A, Fusco M, Alcaraz LE. Antibacterial activity of extracts of Acacia aroma against methicillin-resistant and methicillin-sensitive Staphylococcus.Braz J Microbiol 2010;41:581-7.
- Hatano T, Kusuda M, Inada K, Ogawa TO, Shiota S, Tsuchiya T, et al. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005;66:2047-55.
- Masoko P, Eloff JN. The diversity of antifungal compounds of six South African Terminaliaecies (Combretaceae) determined by bioautography. Afr J Biotechnol 2005;4:1425-31.