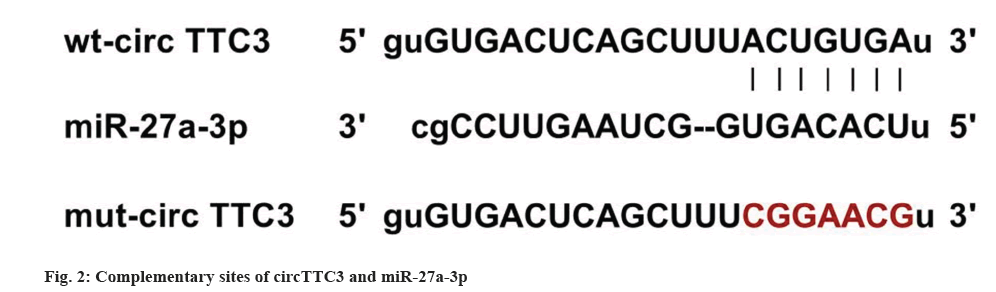- *Corresponding Author:
- Yixue Yu
Department of Pediatrics, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang Province 314001, China
E-mail: yuyixue93@163.com
| Date of Received | 14 January 2023 |
| Date of Revision | 09 September 2023 |
| Date of Acceptance | 21 March 2024 |
| Indian J Pharm Sci 2024;86(2):580-586 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This work was performed to analyze the effect of polysaccharides from semen plantaginis on myocardial cell hypoxia/reoxygenation injury as well as its molecular mechanism. Rat myocardial cells H9C2 were exposed to various concentrations of plantaginis and then subjected to transfection and/or plantaginis treatment, dividing into control, hypoxia/reoxygenation, hypoxia/reoxygenation+L-plantaginis, hypoxia/reoxygenation+M-plantaginis, hypoxia/reoxygenation+H-plantaginis, hypoxia/reoxygenation+si-circTTC3, hypoxia/reoxygenation+si-negative control, and hypoxia/reoxygenation+plantaginis+plasmid cloning deoxyribonucleic acid-circTTC3 groups. Cell viability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay. Flow cytometry, Western blotting as well as enzyme-linked immunosorbent assays were used to analyze cell apoptosis or oxidative stress. CircTTC3 and microRNA-27a-3p levels were detected by real-time quantitative polymerase chain reaction. The targeting relationship between circTTC3 and microRNA-27a-3p was examined by dual-luciferase reporter assay. Treatment with different concentrations of plantaginis increased hypoxia/reoxygenation induced H9C2 cell viability and superoxide dismutase activity but decreased apoptosis rate, cleaved caspase-3 expression levels, malondialdehyde content, and lactate dehydrogenase activity in a concentration-dependent manner, accompanied by a decrease of circTTC3 expression and increase of microRNA-27a-3p expression (p<0.05). CircTTC3 targeted microRNA-27a-3p, and inhibition of circTTC3 expression increased myocardial cell viability and superoxide dismutase activity and decreased apoptosis rate, cleaved caspase-3 expression levels, malondialdehyde content and lactate dehydrogenase activity (p<0.05). Overexpression of circTTC3 reversed the effect of plantaginis on hypoxia/reoxygenation caused myocardial cell damage. Plantaginis regulates circTTC3/microRNA-27a-3p axis to attenuate myocardial cell hypoxia/reoxygenation injury.
Keywords
Polysaccharides, semen plantaginis, circTTC3, microRNA-27a-3p, myocardial cells, hypoxia
Ischemic reperfusion is a commonly used method for treating myocardial ischemia, however, myocardial ischemia-reperfusion damage can cause a series of diseases, seriously affecting the health and prognosis of patients[1,2]. Research has found that traditional Chinese medicine protects myocardial cells from Hypoxia/Reoxygenation (H/R) injury[3]. Semen plantaginis is one of the traditional Chinese medicine materials, which is rich in polysaccharides, flavonoids, volatile oils, and alkaloids[4]. Studies have shown that Polysaccharides from Semen Plantaginis (PSP) have antioxidant effects and can scavenge oxygen free radicals, inhibit lipid peroxidation in liver mitochondria, and activate antioxidant enzyme activity[5,6]. In addition, PSP also have a protective effect on lipopolysaccharide induced liver injury[7]. However, the effect and mechanism of PSP on myocardial cell H/R injury are not yet clear.
Circular RNAs (circRNAs), characterized by the absence 5’-3’ sense translation, are circRNAs molecules generated by trans-splicing of exons, widely expressed in mammalian cells[8,9]. The circRNA/ microRNA (miRNA)/messenger RNA (mRNA) regulatory axis has vital biological regulatory roles. In this regulatory axis, circRNA interacts with miRNA, thereby reducing the regulatory impact of miRNA-mediated gene expression[10,11]. Research has found that circ_0010729 knockdown can alleviate hypoxia induced damage to myocardial cells AC16[12]. Bioinformatics software prediction shows that miR-27a-3p potentially binds to circTTC3. Studies have shown that circTTC3 is elevated in ischemic myocardium as well as hypoxic injured myocardial cells, regulating myocardial cell apoptosis following myocardial infarction via sponging miR-15b[13]. CircTTC3 could protect HaCaT cells against hypoxic damage via downregulating miR-449a[14]. However, the relationship between circTTC3 and miR-27a-3p and its effect on myocardial cell H/R injury are not yet clear. This experiment investigated the effect and mechanism of circTTC3 on myocardial cell H/R injury and explored whether it was related to circTTC3 and miR-27a-3p.
Materials and Methods
Experimental materials:
Rat myocardial cells H9C2 (Shanghai Yaji Biotechnology Co., Ltd.,); Dulbecco’s Modified Eagle Medium (DMEM) (Jiangsu Chi Scientific); PSP (Beijing Yita Biotechnology Co., Ltd.,); 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay kit (Shanghai Bangjing Industrial Co., Ltd.,); apoptosis detection kit (Shanghai Xige Biotechnology Co., Ltd.,); Radioimmunoprecipitation Assay (RIPA) protein lysis buffer (Shanghai Qincheng Biotechnology Co., Ltd.); Malondialdehyde (MDA) content, Lactate Dehydrogenase (LDH) and Superoxide Dismutase (SOD) activity detection kits (Beijing Bio-bridge Technology Co., Ltd.,); Trizol reagent (Hangzhou Xinjing Biochemical Reagent Development Co., Ltd.,); fluorescence quantification kit (Shanghai Yanhui Biotechnology Co., Ltd.,) and dual-luciferase reporter gene detection kit (Chengdu Ansen Shengyuan Technology Co., Ltd.).
PSP pretreatment:
H9C2 cells were cultured in DMEM medium. The cells cultured under normal conditions were used as the control group. The H9C2 cells were placed in sugar-free, serum-free, and hypoxic DMEM medium for 3 h in a hypoxic incubator containing 95 % Nitrogen (N2) and 5 % Carbon dioxide (CO2), and then the normal culture medium was replaced for 3 h in an incubator containing 95 % Oxygen (O2) and 5 % CO2, serving as H/R group. H9C2 cells were pretreated with PSP at concentrations of 100, 200, and 400 μg/ml, followed by H/R treatment, referred to as H/R+L-PSP group, H/R+M-PSP group, and H/R+H-PSP group. H9C2 cells were transfected with circTTC3 small interfering RNA (siRNA) and Negative Control (NC) for 6 h before H/R treatment, referred to as H/R+si-circTTC3 group and H/R+si-NC group. H9C2 cells were transfected with circTTC3 overexpression vector for 6 h, pretreated with 400 μg/ml PSP, and then subjected to H/R treatment, referred to as H/R+PSP+plasmid cloning Deoxyribonucleic Acid (pcDNA)-circTTC3 group.
MTT assay:
After 48 h of culture, cells were exposed to MTT and the incubation was performed for 4 h, and then exposed to dimethyl sulfoxide. After washing cells using PBS, cell viability was analyzed using a microplate reader.
Flow cytometry:
Cells from each group were collected and washed twice with chilled phosphate buffer solution prior to resuspending in binding buffer. Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) were added and incubated in the dark for 10 min. Flow cytometry was utilized for analysis of cell apoptosis.
Western blotting:
Total proteins were extracted from the cells and gel electrophoresis was performed using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) prior to transferring protein bands onto membranes. The membranes were incubated with cleaved caspase-3 antibody (1:500). The protein bands were analyzed by image capture and the grayscale values were analyzed.
Enzyme-Linked Immunosorbent Assay (ELISA):
Cells and supernatants were harvested from each group after 48 h of culture, and indicator detection was performed according to the instructions of each kit.
Real-time fluorescence quantitative Polymerase Chain Reaction (PCR):
TRIzol reagent was mixed with 1×107 cells for Ribonucleic Acid (RNA) isolation. The isolated RNA was dissolved in RNase-free water and universal reverse transcription kit was utilized to synthesize complementary DNA (cDNA). Real time PCR MasterMix and FTC2000 thermocycler were utilized to quantify gene expression. Relative expression level was calculated using the 2-△△Ct method. Primer sequences are circTTC3 5’-CCTGTGTAGAAGCCATCCGT-3’ and 5’-ATCATCAGTGGTAAAGTCAGGAGTA-3’; Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) 5’-AACGGATTTGGTCGTATTGGG-3’ and 5’-CCTGGAAGATGGTGATGGGAT-3’; miR-27a-3p 5’-GCGCATTCACAGTGGCTAAG-3’ and 5’-GTCGTATCCAGTGCAGGGTCC-3'; U6 5’-CTCGCTTCGGCAGCACA-3’ and 5’-AACGCTTCACGAATTTGCGT-3’. The primers were synthesized by Shanghai Shengong Biotechnology Co., Ltd.
Dual-luciferase reporter assay:
CircTTC3 Wild-Type (WT) and mutant luciferase vectors were co-transfected into cardiomyocytes with miR-NC or miR-27a-3p, and then luciferase activity was detected according to the instructions.
Statistical analysis:
Data were analyzed using Statistical Package for the Social Sciences (SPSS) 20.0 software and expressed as mean±standard deviation (x±s). Comparisons between two groups were performed using t-tests, and multiple comparisons was conducted using one-way Analysis of Variance (ANOVA). p<0.05 indicated a significant difference.
Results and Discussion
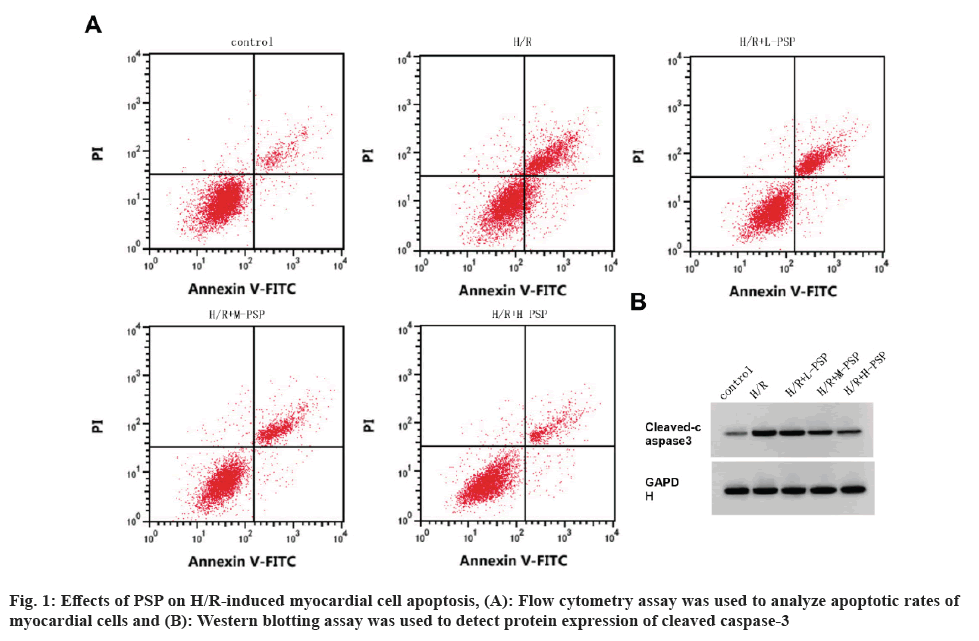
In comparison with control group, myocardial cell viability was decreased, and apoptosis rate and cleaved caspase-3 expression level were increased in the H/R group (fig. 1A, fig. 1B and Table 1). In comparison with the H/R group, myocardial cell viability in the H/R+L-PSP, H/R+M-PSP, and H/R+H-PSP groups was increased, and the apoptosis rate and cleaved caspase-3 expression level were decreased (fig. 1A and fig. 1B, Table 1).
| Group | OD value | Apoptotic rate (%) | Cleaved caspase-3 |
|---|---|---|---|
| Control | 1.17±0.05 | 7.28±0.27 | 0.12±0.01 |
| H/R | 0.42±0.021) | 24.35±0.841) | 0.68±0.041) |
| H/R+L-PSP | 0.58±0.022) | 20.68±0.642) | 0.51±0.042) |
| H/R+M-PSP | 0.82±0.042)3) | 16.71±0.592)3) | 0.35±0.022)3) |
| H/R+H-PSP | 1.03±0.042)3)4) | 12.13±0.432)3)4) | 0.23±0.022)3)4) |
| F | 220.915 | 398.466 | 180.988 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with control group, 1)p<0.05; compared with H/R group, 2)p<0.05; compared with H/R+L-PSP group, 3)p<0.05 and compared with H/R+M-PSP, 4)p<0.05
Table 1: Detection of PSP on H/R cardiomyocyte damage.
As shown in Table 2, in comparison with the control group, MDA content and LDH activity in myocardial cells of the H/R group were increased, and SOD activity was decreased. Moreover, in comparison with the H/R group, MDA content and LDH activity in myocardial cells of the H/R+PSP treatment group were decreased, and SOD activity was increased.
| Group | MDA (nmol/ml) | LDH (U/l) | SOD (U/l) |
|---|---|---|---|
| Control | 97.54±3.55 | 57.16±4.19 | 195.69±4.91 |
| H/R | 393.18±18.291) | 269.95±17.071) | 48.81±2.521) |
| H/R+L-PSP | 315.26±9.972) | 213.60±8.782) | 69.92±3.522) |
| H/R+M-PSP | 223.16±12.002)3) | 158.84±4.572)3) | 98.80±2.812)3) |
| H/R+H-PSP | 155.00±10.102)3)4) | 116.39±8.162)3)4) | 145.01±6.592)3)4) |
| F | 308.005 | 216.918 | 558.718 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with control group, 1)p<0.05; compared with H/R group, 2)p<0.05; compared with H/R+L-PSP group, 3)p<0.05 and compared with H/R+M-PSP, 4)p<0.05
Table 2: The detection of PSP on the expression of MDA, LDH and SOD in H/R cardiomyocytes.
CircTTC3 expression in myocardial cells was increased, and miR-27a-3p was decreased after H/R treatment (Table 3). In comparison with the H/R group, circTTC3 expression in myocardial cells of the H/R+PSP treatment group was decreased, and miR-27a-3p was increased in a concentration-dependent manner (Table 3).
| Group | CircTTC3 | miR-27a-3p |
|---|---|---|
| Control | 1.00±0.00 | 1.00±0.00 |
| H/R | 4.47±0.071) | 0.18±0.011) |
| H/R+L-PSP | 4.04±0.062) | 0.25±0.022) |
| H/R+M-PSP | 3.24±0.042)3) | 0.51±0.032)3) |
| H/R+H-PSP | 1.87±0.092)3)4) | 0.76±0.042)3)4) |
| F | 1761.29 | 593.25 |
| p | 0.000 | 0.000 |
Note: Compared with control group, 1)p<0.05; compared with H/R group, 2)p<0.05; compared with H/R+L-PSP group, 3)p<0.05 and compared with H/R+M-PSP, 4)p<0.05
Table 3: Effects of PSP on CircTTC3 and miR-27A-3p expression in H/R cardiomyocytes.
As shown in fig. 2, there are complementary sequences between circTTC3 and miR-27a-3p. The luciferase activity of cells co-transfected with wt-circTTC3 and miR-27a-3p was significantly decreased (Table 4).
| Group | WT-circTTC3 | Mut-circTTC3 |
|---|---|---|
| miR-NC | 0.96±0.08 | 1.01±0.09 |
| miR-27a-3p | 0.19±0.021) | 0.97±0.06 |
| t | 16.173 | 0.641 |
| p | 0.000 | 0.557 |
Note: Compared with miR-NC group, 1)p<0.05
Table 4: Analysis of luciferase activity.
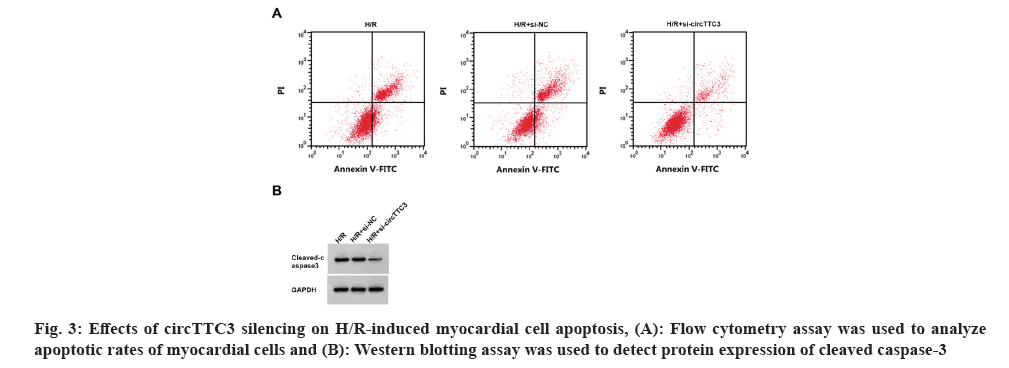
As shown in fig. 3 and Table 5, compared with control groups, circTTC3 expression, cell apoptotic rate, and cleaved caspase-3 protein expression were decreased, whereas miR-27a-3p expression and myocardial cell viability were increased in the H/R+si-circTTC3 group, accompanied by decrease in MDA content and LDH activity, and an increase in SOD activity (p<0.05).
| Group | CircTTC3 | miR-27a-3p | OD values | Apoptotic rate (%) | Cleaved caspase-3 | MDA (nmol/ml) | LDH (U/l) | SOD (U/l) |
|---|---|---|---|---|---|---|---|---|
| H/R | 1.00±0.00 | 1.00±0.00 | 0.43±0.02 | 24.34±0.78 | 0.69±0.06 | 391.46±21.66 | 277.09±23.56 | 50.22±3.56 |
| H/R+si-NC | 0.99±0.03 | 1.02±0.03 | 0.43±0.02 | 24.41±0.92 | 0.68±0.05 | 391.11±26.83 | 273.96±12.47 | 50.82±1.46 |
| H/R+si-circTTC3 | 0.28±0.021)2) | 5.55±0.111)2) | 1.10±0.051)2) | 9.35±0.301)2) | 0.15±0.011)2) | 128.27±7.371)2) | 78.31±3.321)2) | 169.72±6.661)2) |
| F | 1179.920 | 4756.590 | 408.091 | 438.415 | 138.532 | 166.917 | 161.729 | 720.523 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with H/R group, 1)p<0.05 and compared with H/R+si-NC group, 2)p<0.05
Table 5: Effect of CircTTC3 silencing on H/R cardiomyocyte damage and the expression of MDA, LDH and SOD.
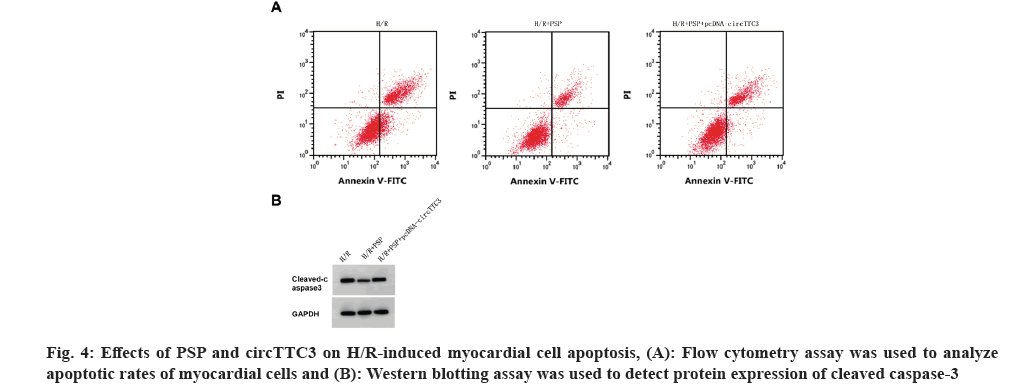
As shown in fig. 4 and Table 6, compared with the H/R+PSP group, circTTC3 expression, cleaved caspase-3 expression, cell apoptotic rate, MDA content, and LDH activity were increased, whereas miR-27a-3p expression, myocardial cell viability and SOD activity were decreased in H/R+PSP+pcDNA-circTTC3 group (p<0.05).
| Group | CircTTC3 | miR-27a-3p | OD values | Apoptotic rate (%) | Cleaved caspase-3 | MDA (nmol/ml) | LDH (U/l) | SOD (U/l) |
|---|---|---|---|---|---|---|---|---|
| H/R | 1.00±0.00 | 1.00±0.00 | 0.42±0.01 | 24.46±0.90 | 0.69±0.05 | 393.21±19.34 | 273.92±16.45 | 48.94±2.57 |
| H/R+PSP | 0.42±0.031) | 4.23±0.071) | 1.03±0.061) | 12.16±0.611) | 0.23±0.011) | 154.85±8.931) | 116.06±12.451) | 148.08±5.791) |
| H/R+PSP+pcDNA-circTTC3 | 0.89±0.062) | 1.45±0.092) | 0.51±0.032) | 21.78±0.792) | 0.57±0.032) | 336.40±10.512) | 231.90±8.322) | 56.73±3.122) |
| F | 189.8 | 2118.9 | 212.152 | 208.463 | 146.4 | 247.244 | 121.571 | 548.526 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with H/R group, 1)p<0.05 and compared with H/R+PSP group, 2)p<0.05
Table 6: The effect of overexpression of CircTTC3 on the H/R cardiomyocyte damage and the expression of MDA, LDH and SOD treated with PSP.
Myocardial ischemia-reperfusion injury severely affects the therapeutic efficacy of ischemic heart disease. Oxidative stress as well as myocardial cell apoptosis are important pathological mechanisms of its injury. Finding methods to inhibit myocardial ischemia-reperfusion damage is an urgent task. Studies have shown that traditional Chinese medicine has certain advantages in treating myocardial reperfusion injury[15,16]. PSP has been reported to alleviate nonylphenol-induced intestinal barrier injury via inhibiting LDH release and increasing tight junction protein expression[17]. PSP can prevent kidney injury by improving renal inflammation in gouty nephropathy rats[18]. PSP can reduce the damage caused by endogenous free radicals produced by lipid peroxidation in rats after exercise[19]. In this experiment, H/R treatment was used to establish an ischemia-reperfusion injury model in cardiac cells. H9C2 cells were pretreated with PSP under H/R conditions, and cell biology methods were used to analyze the cells. We found H9C2 cell viability was increased, the apoptosis rate and cleaved caspase-3 expression level were decreased, the content of MDA and LDH activity were decreased, and the activity of SOD was increased in a concentration-dependent manner, indicating that PSP can inhibit H/R caused myocardial cell damage.
Research has reported that downregulation of circTTC3 reduces cell apoptosis and LDH levels in oxygen and glucose deprivation-induced astrocytes, and the underlying mechanism involved its regulation to miR-372-3p/Toll-Like Receptor 4 (TLR4) pathway[20]. CircTTC3 overexpression could improve acute kidney injury rats by regulating the miR-148a/Rcan2 axis[21]. miR-27a-3p targeted FOXO1 to inhibit brain ischemia-reperfusion injury[22]. miR-27a-3p also exacerbated hypoxia-induced H9C2 cell damage by suppressing BNIP3 production[23]. Our experimental results showed that H/R-induced H9C2 cells had increased circTTC3 and decreased miR-27a-3p content. Inhibition of circTTC3 expression reduced cell apoptosis rate, MDA level, and LDH activity, while increasing SOD activity. Additionally, circTTC3 targeted miR-27a-3p, this suggests that circTTC3 may inhibit H/R-induced cardiomyocyte apoptosis and oxidative stress via modulating miR-27a-3p. Furthermore, PSP decreased circTTC3 expression and increased miR-27a-3p expression, while overexpression of circTTC3 reversed polysaccharides from semen PSP induced influence on H/R-induced cardiomyocyte damage.
In summary, PSP regulated the circTTC3/miR-27a-3p axis to attenuate cardiomyocyte H/R injury. This may imply that modulation of circTTC3/miR-27a-3p axis expression or function can alleviate or prevent the occurrence and development of heart diseases. This is of clinical significance as it could provide new therapeutic targets or strategies for heart diseases. However, further research is needed to validate the specific role and application value of this molecular mechanism.
Conflict of interests:
The authors declared no conflict of interests.
References
- Arroyo-Campuzano M, Gil-Hernández A, Silva-Palacios A. Cardiosome-mediated protection in myocardial ischemia. Clin Chim Acta 2023;545:117374.
- Silva TQ, Pezel T, Jerosch-Herold M, Coelho-Filho OR. The role and advantages of cardiac magnetic resonance in the diagnosis of myocardial ischemia. J Thorac Imaging 2023.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Mao C, Zhang C, Zhang M, Gong J, Wang X. Salvianolic acid B inhibits ferroptosis and apoptosis during myocardial ischemia/reperfusion injury via decreasing the ubiquitin-proteasome degradation of GPX4 and the ROS-JNK/MAPK pathways. Molecules 2023;28(10):4117.
[Crossref] [Google Scholar] [PubMed]
- Peng DH, Kuang HX, Wang QH. Research progress on polysaccharide from Plantago Seed (PS). J Guangdong Pharm Univ 2019;35(5):702-6.
- Zhang R, Yuan CY, Feng N. Effects of polysaccharide from Plantago seed on oxidative stress in diabetic mice. Tianjin Med J 2011;39(3):253-5.
- Yuan CY, Zhang R, Che WW. Effects of polysaccharide from Plantago seed on mitochondrial free radical defense function in rats. Chin J Geriatr Med Surg 2011;31(4):618-20.
- Li F, Huang D, Nie S, Xie M. Polysaccharide from the seeds of Plantago asiatica L. protect against lipopolysaccharide induced liver injury. J Med Food 2019;22(10):1058-66.
[Crossref] [Google Scholar] [PubMed]
- Li H. CircRNA: A promising all-around star in the future. Epigenomics 2023;15(12):677-85.
[Crossref] [Google Scholar] [PubMed]
- Li Q, Ren X, Wang Y, Xin X. CircRNA: A rising star in leukemia. PeerJ 2023;11:e15577.
[Crossref] [Google Scholar] [PubMed]
- Nishita-Hiresha V, Varsha R, Jayasuriya R, Ramkumar KM. The role of circRNA-miRNA-mRNA interaction network in endothelial dysfunction. Gene 2023;851:146950.
[Crossref] [Google Scholar] [PubMed]
- Dakal TC, Kumar A, Maurya PK. CircRNA-miRNA-mRNA interactome analysis in endometrial cancer. J Biomol Struct Dyn 2023;12:1-2.
[Crossref] [Google Scholar] [PubMed]
- Lei D, Wang Y, Zhang L, Wang Z. Circ_0010729 regulates hypoxia induced cardiomyocyte injuries by activating TRAF5 via sponging miR-27a-3p. Life Sci 2020;262:118511.
[Crossref] [Google Scholar] [PubMed]
- Cai L, Qi B, Wu X, Peng S, Zhou G, Wei Y, et al. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J Mol Cell Cardiol 2019;130:10-22.
[Crossref] [Google Scholar] [PubMed]
- Yu L, Wang Q, Liu N, Zhao J, Yu J, Tao S. Circular RNA circ-TTC3 protects HaCaT cells from hypoxic injury by downregulation of miR-449a. IUBMB Life 2020;72(3):505-14.
[Crossref] [Google Scholar] [PubMed]
- Su H, Zhang ZM, Yong WX. Research progress on the influence of traditional Chinese medicine on apoptosis of ischemia-reperfusion injury myocardial cells. World's Latest Med Inform Digest 2020;20(54):31-2.
- Song Z, Yang Z, Tian L, Liu Y, Guo Z, Zhang Q, et al. Targeting mitochondrial circadian rhythms: The potential intervention strategies of traditional Chinese medicine for myocardial ischaemia‒reperfusion injury. Biomed Pharmacother 2023;166:115432.
[Crossref] [Google Scholar] [PubMed]
- Li F, Du P, Yang W, Huang D, Nie S, Xie M. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int J Biol Macromol 2020;164:2134-40.
[Crossref] [Google Scholar] [PubMed]
- Zhao H, Xu J, Wang R, Tang W, Kong L, Wang W, et al. Plantaginis semen polysaccharides ameliorate renal damage through regulating NLRP3 inflammasome in gouty nephropathy rats. Food Funct 2021;12(6):2543-53.
- Li SH. Effects of polysaccharide from Plantago herb on antioxidant and immune capacity in exercise rats. Sichuan Sports Sci Technol 2016;35(2):35-7.
- Yang B, Zang LE, Cui J, Wei L. Circular RNA TTC3 regulates cerebral ischemia-reperfusion injury and neural stem cells by miR-372-3p/TLR4 axis in cerebral infarction. Stem Cell Res Ther 2021;12(1):125.
[Crossref] [Google Scholar] [PubMed]
- Ma X, Zhu G, Jiao T, Shao F. Effects of circular RNA Ttc3/miR-148a/Rcan2 axis on inflammation and oxidative stress in rats with acute kidney injury induced by sepsis. Life Sci 2021;272:119233.
[Crossref] [Google Scholar] [PubMed]
- Li W, Zhu Q, Xu X, Hu X. miR-27a-3p suppresses cerebral ischemia-reperfusion injury by targeting FOXO1. Aging 2021;13(8):11727.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Ren S, Xia J, Wei Y, Xi Y. EIF4A3-induced circ-BNIP3 aggravated hypoxia-induced injury of H9c2 cells by targeting miR-27a-3p/BNIP3. Mol Ther Nucl Acids 2020;19:533-45.
[Crossref] [Google Scholar] [PubMed]