- *Corresponding Author:
- M. Askaripour
Physiology Research Center and Department of Physiology, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran
E-mail: askaripour_m@yahoo.com
| Date of Submission | 05 May 2014 |
| Date of Revision | 15 January 2015 |
| Date of Acceptance | 13 November 2015 |
| Indian J Pharm Sci 2015; 77(6):681-686 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Despite increasing studies on silver nanoparticles, their mechanism of action is not so clear, especially their probable toxicity on reproduction procedure, developmental process and offspring behavior. Therefore in the present study the effect of silver nanoparticles exposure during gestational period on offspring's depression behavior was assessed. Thirty virgin female mice were divided into three groups (n=10 for each group) including: one control and two experimental groups, which received an equal volume (0.2 ml) of suspension containing 0, 0.2 and 2 mg/kg of silver nanoparticles, respectively. After mating, the suspension was injected and repeated every 3 days till accouchement. Depression behaviors were assessed by tail suspension test and forced swimming test, in 45-day-old male and female progenies (6 groups, n=10). In males, both dose of silver nanoparticles (0.2 and 2 mg/kg) decreased mobility and increased immobility time in forced swimming test (P<0.05), but in female no effects were observed in mobility and immobility time. In tail suspension test, 2 mg/kg of silver nanoparticles lead to decrease of mobility time (P<0.05) and increase of immobility time (P<0.05) in female offspring but in males no significant effect was observed on mobility and immobility time.We may concluded that the prenatal exposure to silver nanoparticles probably cause gender-specific depression like behaviors in offspring, possibly through neurotoxic effect during neuronal development.
Keywords
Silver nanoparticles, prenatal exposure, depression behavior, forced swimming test, tail suspension test
Nanomaterials are characterized as nanoobjects or nanostructured materials because their size is between 1-100 nanometers (nm) [1,2]. Silver nanoparticles (Ag-NPs) have a lot of applications in many aspects from medicine [3], biotechnology and drug [4] to industry [5]. Increasing use of Ag-NPs in different materials leads to the increase of exposure to Ag-NPs. Nanomaterials cause some neurotoxicities like short-term memory reduction and decrease of learning ability [6]. Also, it was reported that the prenatal exposure to nanoparticles can effect on neurobehavioral development [7] via ROS accumulation in hippocampus [8]. In particular, females are more prone to nanoparticle diverse effects as their toxicity may lead to impairment of fertility and fetal development [9]. Also, blood brain barrier (BBB) allows the passage of Ag-NPs so; they can accumulate in the brain [10]. On the other hand, it was shown that even in low concentration, silver has long maintenance time in brain than the other tissues [11]. Furthermore, Ag-NPs retention time in mouse body was more than 4 months [12].
These factors provide enough time for Ag-NPs to influence on neural cells natural physiology and development. Also placenta, as a vital barrier, allows the passage of Ag-NPs, which may cause to neurotoxicity in the fetus [13,14]. Some studies reported that Ag-NPs are accumulated in fetus tissues by transporting across placenta [15]. But still little information is available associated to the relation between prenatal Ag-NPs exposure and following behavioral performance.
Widespread applications of Ag-NPs in medicine, passage from the placenta and BBB, long maintaining and long retention time raise this question whether exposure to Ag-NPs during pregnancy can cause damage to neuronal development leading to neurodisorders of the progeny.
Depression is a state of low mood and avoiding activity or reduction in biological function. Hence evaluation of behavioral activities became a useful approach for diagnosis of depression. It is one of the major causes of morbidity in the world with increasing growth in both males and females [16]. Depression and anxiety are stress-related psychiatric disorders. There are several animal models of anxiety and depression that all of them are based on variations in emotionality. They are including open field behavior, light–dark box and elevated plus maze (EPM) [17]. Also sucrose preference test is an indicator of anhedonia, which is related to depression [18]. Among the animal models of depression, mouse forced swimming test (FST) is one of the most used tools for screening depressive mood, which has predictive and reliability validity [19]. Tail suspension test (TST) is another most widely used model for evaluation of antidepressant-like activity in mouse [20]. In both of these methods, dropping in water and suspending by tail are considered as stress, which stimulate animals for more struggling. Therefore, in the present study the toxic effect of the prenatal exposure of Ag-NPs was assessed by FST and TST to detect the probable impairment of neurobehavioral development in mice offspring and possible depression.
Materials and Methods
The used protocol and animals’ ethics were approved by the Research Council of Veterinary Medicine Faculty in Shahid Chamran University of Ahvaz.
Nanoparticle
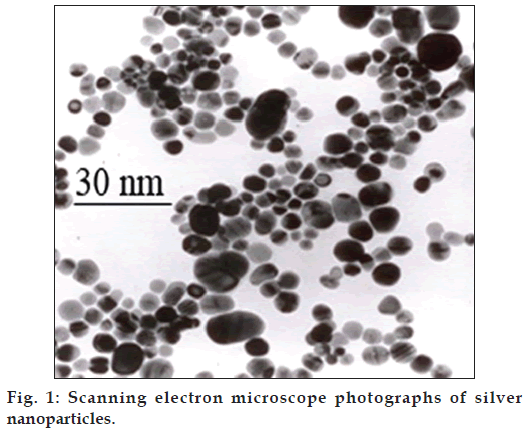
Ag-NPs (size: 10 nm, shape: spherical, purity: 99.9%, density: 1000 ppm) were purchased from Neutrino Corporation (Neutrino Nanovation, Iran). They were prepared by sol-gel method in which silver ions were reduced by sodium borohydride in the presence of citrate (fig. 1).
Animals grouping and Ag-NPs administration: The NMRI outbred mice are the research animal model provided by Harlan Laboratories, which are used in basic research. Forty 5-6 weeks old NMRI (US Naval Medical Research Institute) mice (30 virgin females and 10 males) were purchased from the Laboratory Animal Facility of Jondi Shapoor University of Ahvaz, Iran. Males and females maintained separately in standard plexiglass cages under controlled room temperature (23±2º). The lightness program was planned automatically according to 12:12 h light/dark cycle. The animals were fed ad lib with normal pellet diet (Pars Animal Feed, Iran) and tap water. Following 1 week of acclimatization, the female mice were divided to three groups (n=10 for each group) as follows: control, NP 0.2 and NP 2 groups. They were injected subcutaneously (SC) equal volume of 0.2 ml of suspension, which contained zero, 0.2 and 2 mg/kg of Ag-NP, respectively. Previously, It was reported that silver NPs over dose can results to embryo-malformation [21], so we consider the minimum dose to be sure that Ag-NPs were transferred to the most maternal and embryos organs and extraembryonic tissues without causing any anatomical defects. Therefore neurobehavioral disorders after birth could be due to Ag-NPs, as teratogen, also these disorders were not related to anatomical dysmorphology [22]. So, at first in a preliminary experiment, the probable morphological abnormalities, created with our chosen dose of Ag-NPs were checked. Then, Ag-NPs administration was done only in female mice, immediately following mating and repeated once every 3 days until delivery. Fourteen days later, the males were separated from the females. This time was selected to ensure successful mating. To provide enough space, 17 days after mating, the females were transferred to individual cages (26×47×20 cm3) for comfortable parturition. From 45 days old mice, ten males and ten females were randomly chosen from different colonies for behavioral tests.
Behavioral tests
All experiments were performed between 13:00 h to 17:00 h and behavior parameters recorded as duration (s). The standard behavioral mice model was used for the evaluation of depression behaviors for each FST and TST test, separately. For all tests a video record was done and an observer blind to treatment scored the videotapes.
Forced swim test
In brief, each mouse individually was forced to swim for 6 min. An open glass chamber (25×15×25 cm) was used, which contained fresh water (25±1º) up to the height of 15 cm. At the end of each session (after each 6 min), mice were taken out of water and allowed to dry. Previously, it was shown that used water can alter the animal behavior; so water was changed after each session. This test was done for each mouse, twice. During the first time of FST, the mice were highly active at the beginning, so that they tried to climb the chamber wall or dived to the bottom and strongly had a circle swimming. After 24 h, they again were forced to swim in the similar environment for another 6 min. When the mice stop struggling, they became floated in water. This was considered as immobility. During the final 4 min of the total 6 min, the immobility time was recorded [23,24].
Tail suspension test
This test is usually applied as a behavioral model in mice for detecting depressant-like activity. A box with 25×25×30 cm sizes was provided and to avoid any disturbances, the tests were done in a darkened room with minimum noise. Mice were suspended by tail (1 cm from the end of the tail) with a clamp, so that the head distance from the bottom was 5 cm. The animals were isolated visually and acoustically from each other. Mice were suspended for a total of 6 min, and the duration of immobility was observed and measured during the final 4 min interval of the test. The duration of immobility was considered only when the mouse was completely motionless [25].
Statistical analysis
Statistical analyses were performed by comparing the treatment groups with the control group using SPSS16 (Chicago, USA). All values were expressed as mean±SEM. Statistical comparisons were performed by One Way Analysis of Variance (ANOVA). Post-hoc comparisons between individual groups were carried out by means of Tukey test. P value of <0.05 was accepted as statistically significant.
Results and Discussion
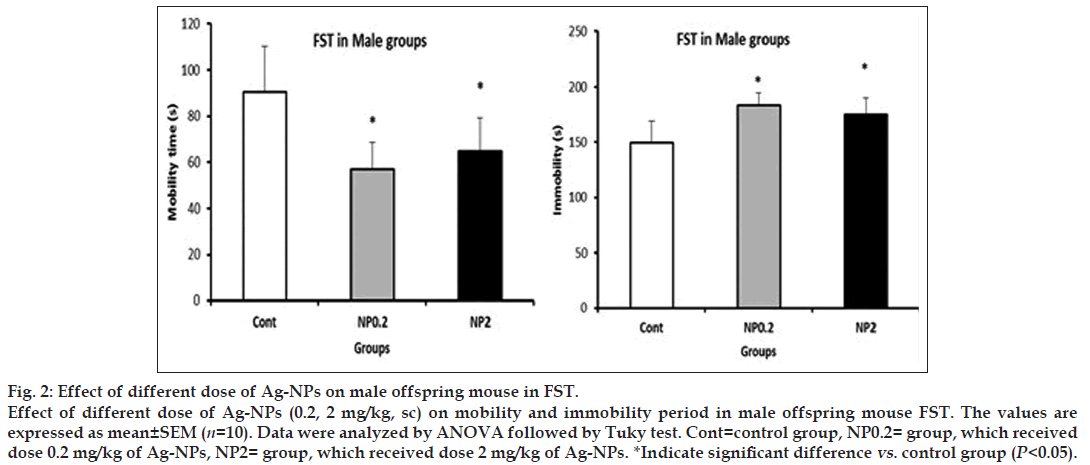
All the results are presented in Table 1. In male progenies, the prenatal exposure of both dose of 0.2 and 2 mg/kg resulted in decreasing of mobility, compared to control groups (control: 90.6±19.5 s, NP 0.2 mg/kg: 56.8±11.67 s, NP 2 mg/kg: 64.7±14.5 s, P<0.05) and increasing in immobility (control: 149.4±19.5 s, NP 0.2 mg/kg: 183.2±11.67 s, NP2 mg/kg: 175.3±14.5 s, P<0.05, fig. 2). But in females, no alteration in mobility and immobility periods in FST test was observed (P>0.05).
| Genders | FST (0.2 and 2 mg/kg) | TST (0.2 and 2 mg/kg) | |||
|---|---|---|---|---|---|
| Mobility | Immobility | Mobility | Immobility | ||
| Male | Decrease | Increase | - | - | |
| Female | - | - | Decrease | Increase | |
Table 1: Summary of Results
Figure 2: Effect of different dose of Ag-NPs on male offspring mouse in FST.
Effect of different dose of Ag-NPs (0.2, 2 mg/kg, sc) on mobility and immobility period in male offspring mouse FST. The values are expressed as mean ± SEM (n=10). Data were analyzed by ANOVA followed by Tuky test. Cont=control group, NP0.2= group, which received dose 0.2 mg/kg of Ag-NPs, NP2= group, which received dose 2 mg/kg of Ag-NPs. *Indicate significant difference vs. control group (P<0.05).
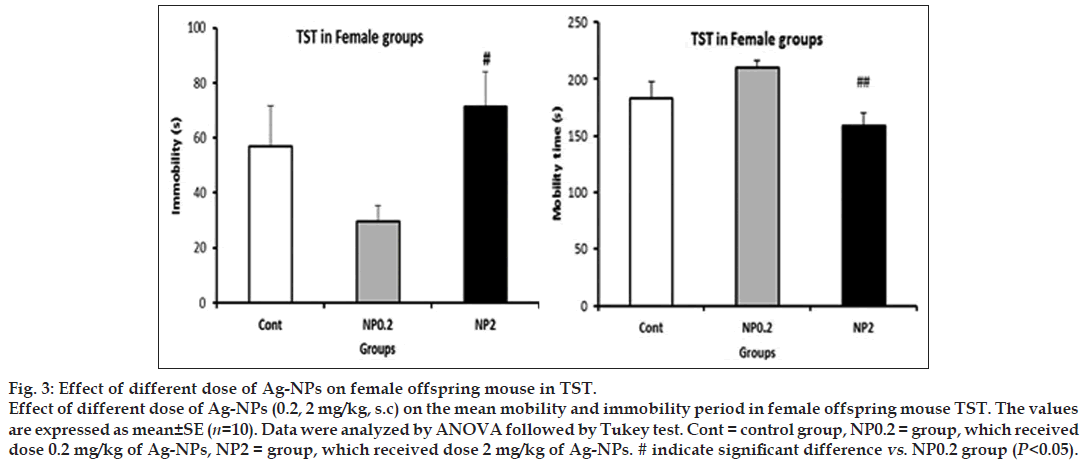
In both dose of 0.2 and 2 mg/kg of Ag-NPs, in tail swimming test, the mobility and immobility periods in male offspring did not alter, but in females, NP 2 dose compared to NP 0.2 resulted to decrease of mobility (NP 0.2 mg/kg: 210.5±6 s, NP 2 mg/kg: 159±11.4 s, P<0.05) and increase in immobility periods (NP 0.2 mg/kg: 29.5±6 s, NP 2 mg/kg: 71.1±12 s, P<0.05, fig. 3).
Figure 3: Effect of different dose of Ag-NPs on female offspring mouse in TST.
Effect of different dose of Ag-NPs (0.2, 2 mg/kg, s.c) on the mean mobility and immobility period in female offspring mouse TST. The values are expressed as mean±SE (n=10). Data were analyzed by ANOVA followed by Tukey test. Cont = control group, NP0.2 = group, which received dose 0.2 mg/kg of Ag-NPs, NP2 = group, which received dose 2 mg/kg of Ag-NPs. # indicate significant difference vs. NP0.2 group (P<0.05).
In our study, FST resulted to in a reduction of mobility and enhancement of immobility in males while TST lead to the same results in females. It is likely that gender-specific depression-like behaviours were observed. An interesting finding of the present study was the dose-dependent increase of immobility and decrease of mobility in FST in the male offspring, which might be related to depression-like behaviors. Depression is an emotional disorder, resulted from the physiological, neurobiological and behavioral interactions [26]. On the basis of FST, the depressed mice after floating in water, abandon swimming and remain immobile. The immobility time is considered as the depression level [27]. Also in TST, the immobility time is considered as a depression state [28]. The antidepressant factors reduced this time because they increase the level of monoaminergic transmitters in CNS [29]. Deficiencies of some monoaminergic transmitters like noradrenalin, serotonin (5-HT) and dopamine (DA) or dysregulation of their receptors in the brain are the major reasons of mental depression [30]. It has been shown that Ag-NPs (15 nm) reduce the DA and its metabolite (dihydroxyphenylacetic acid). They impair neuronal cell differentiation so, they influence on cells capacity for producing of DA [31]. Also, they decrease the expression level of genes, related to the dopaminergic system [32].
Small-sized Ag-NPs transport across the BBB [33]. Ag-NPs with 50–100 nm size can change the action potential of hippocampal CA1 neurons [21], but with 15 nm size they reduce DA and dihydroxyphenylacetic acid [34]. It was reported that silver nanoparticles with 20 nm size were more toxic than larger NPs. Also Ag-NPs have more toxicity than Ag ions [35]. Ag-NPs toxicity is dependent to their ability to penetrate to cell membrane, cell type and NPs size [35].
In the present study, Ag-NPs with size of 10 nm were used. This size of NPs allows the NPs to cross biological barriers. Ag-NPs can accumulate in an in vitro BBB model composed of vascular endothelial cells of rat brain [34]. We administered Ag-NPs subcutaneously because previously it has been reported that SC administration of Ag-NPs can traverse through the BBB and induce pathological changes, necrosis and neuronal degeneration [36]. Also, oral administration in dams led to accumulation in brain [13]. Passage through BBB, enterring fetus blood circulation, accumulation in brain and long maintaining time provide enough chance for Ag- NPs to exert effects on brain. There are several mechanisms attributed to Ag-NPs-induced brain damage and these were, activating the ROS system, impacting on immune system, dysfunction of ion channels, impaired neurotransmission in the brain and changing the action of glutamate, the major excitatory neurotransmitter in the brain.
Oxidative stress and ROS system can generate a wide spectrum of cellular pathological procedures including DNA damage, cell stress, tissue inflammation and apoptosis [37,38]. Besides, there is a direct relationship between depressive disorder and oxidative stress produced by ROS [14,30]. As Ag-NPs induce ROS generation in mother body and can pass BBB, they can enter the fetus blood circulation, activate the ROS system and lead to fetal cell dysfunction [39].
There is a relationship between depression and inflammatory factors, so IL-6 and IL-1 are increased in depressive patients [40]. Therefore their level in serum can change the results of FST and TST [40-42].
Previously it was demonstrated that nanoparticles cause depressive disorders in both genders, by TST and FST evaluation [43]. Also Ivani et al., by FST assessment reported that multiwall carbon nanotubes can lead to depressive disorders via activating the inflammatory cascade [44]. Therefore, parallel to oxidative damage, Ag-NPs impact the immune system leading to production of some inflammatory factors such as cytokines and interleukins, so these are two main mechanisms, associated to Ag-NPs toxicity.
In CNS, ion channels have a key role in coordinating of cell viability and function. Sodium and potassium currents, beside the production of action potential, have an important role in releasing of neurotransmitters [45]. It was demonstrated that Nano-Ag (50–100 nm) decreases the intracellular Na+ concentration and disturbs the voltage current, so that it changes the action potential of hippocampal neurons [46].
Another mechanism is due to the glutamate, which is the major excitatory neurotransmitter in the brain. It was shown that Ag-NPs cause an increase in NMDA receptors. These receptors are responsible for the degeneration of glutamatergic neurons and impaired cell excitation. This process involves in the pathogenesis of depression [47].
In our study, some effects of prenatal exposure to Ag-NPs were more severe in females. So, probably sex differences in brain neurotransmitter and neurochemistry are related to sex-dependent changes in brain [48]. Also estrogen and progesterone enhance antioxidant mechanisms and exhibit neuroprotective and neuroregenerative activities [49]. It might be probably one of the reasons of sex differences of Ag-NPs toxicity.
Altogether, probably their effect may dependent on the time of exposure, duration of exposure, kind of maternal administration, used dosage, gender and fetus age. As the result, exposure to Ag-NPs increased depression like behaviours in the progenies specially males. Also, the main mechanism of Ag-NPs depression induction is probably increasing of the inflammatory factors, oxidative stress, and deficiency in the levels of monoaminergic transmitters. Therefore, the safety psychopharmacology application of nano silver in gestational period is still an open question. These findings are important for warning the women who are pregnant or planning to get pregnant.
Acknowledgements
This paper reports data obtained from a research project approved by Shahid Chamran University Research Council.
Financial support and sponsorship
Authors thank the Research Council of Shahid Chamran University of Ahvaz for its financial support.
Conflicts of interest
There are no conflicts of interest.
References
- Lövestam G, Rauscher H, Roebben G, Klüttgen BS, Gibson N, Putaud JP, et al. Considerations on a Definition of Nanomaterial for Regulatory Purposes. 1st ed. Luxembourg: Publication Office of European Union; 2010.
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005;113:823-39.
- Chen X, Schluesener HJ. Nanosilver: A nanoproduct in medical application. ToxicolLett 2008;176:1-12.
- Chernousova S, Epple M. Silver as antibacterial agent: Ion, nanoparticle, and metal. AngewChemInt Ed Engl 2013;52:1636-53.
- Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology 2008;17:438-47.
- Hritcu L, Stefan M, Ursu L, Neagu A, Mihasan M, Tartau L, et al. Exposure to silver nanoparticles induces oxidative stress and memorydeficits in laboratory rats. Cent Eur J Biol 2011;6:497-509.
- Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol 2011;6:321-8.
- Liu Y, Guan W, Ren G, Yang Z. The possible mechanism of silver nanoparticle impact on hippocampal synaptic plasticity and spatial cognition in rats. ToxicolLett 2012;209:227-31.
- Sun J, Zhang Q, Wang Z, Yan B. Effects of nanotoxicity on female reproductivity and fetal development in animal models. Int J MolSci 2013;14:9319-37.
- Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J MolSci 2011;12:6267-80.
- Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, et al. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010;31:8350-61.
- Ahmadi F, Kordestany AH. Investigation on silver retention in different organs and oxidative stress enzymes in male broiler fed diet supplemented with powder of nano silver. Am Eurasian J ToxicolSci 2011;3:28-35.
- Lee Y, Choi J, Kim P, Choi K, Kim S, Shon W, et al. A transfer of silver nanoparticles from pregnant rat to offspring. Toxicol Res 2012;28:139-41.
- Asharani PV, Lian Wu Y, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008;19:255102.
- Wang Z, Qu G, Su L, Wang L, Yang Z, Jiang J, et al. Evaluation of the biological fate and the transport through biological barriers of nanosilver in mice. Curr Pharm Des 2013;19:6691-7.
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 1st ed. New York: Oxford University Press; 2007.
- Ramos A. Animal models of anxiety: Do I need multiple tests? Trends PharmacolSci 2008;29:493-8.
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res 2005;162:127-34.
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245-55.
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. NeurosciBiobehav Rev 2005;29:571-625.
- Austin CA, Umbreit TH, Brown KM, Barber DS, Dair BJ, Francke-Carroll S, et al. Distribution of silver nanoparticles in pregnant mice and developing embryos. Nanotoxicology 2012;6:912-22.
- Powers CM, Slotkin TA, Seidler FJ, Badireddy AR, Padilla S. Silver nanoparticles alter zebrafish development and larval behavior: Distinct roles for particle size, coating and composition. NeurotoxicolTeratol 2011;33:708-14.
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp 2012;59:e3638.
- Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. CurrProtocNeurosci 2011;55:8.10A:8.10A.1–8.10A.14.
- Dhingra D, Valecha R. Evaluation of the antidepressant-like activity of Convolvulus pluricaulischoisy in the mouse forced swim and tail suspension tests. Med SciMonit 2007;13:BR155-61.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (NCS-R). JAMA 2003;289:3095-105.
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66-72.
- Shalam M, Shantakumar S, Narasu ML. Pharmacological and biochemical evidence for the antidepressant effect of the herbal preparation Trans-01. Indian J Pharmacol 2007;39:231-4.
- Delgado PL. Depression: The case for a monoamine deficiency. J Clin Psychiatry 2000;61Suppl 6:7-11.
- Michel TM, Frangou S, Thiemeyer D, Camara S, Jecel J, Nara K, et al. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder – A postmortem study. Psychiatry Res 2007;151:145-50.
- Powers CM, Badireddy AR, Ryde IT, Seidler FJ, Slotkin TA. Silver nanoparticles compromise neurodevelopment in PC12 cells: Critical contributions of silver ion, particle size, coating, and composition. Environ Health Perspect 2011;119:37-44.
- Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, et al. Distribution, translocation and accumulation of silver nanoparticles in rats. J NanosciNanotechnol 2009;9:4924-32
- Hoet PH, Brüske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J Nanobiotechnology 2004;2:12.
- Tang J, Xiong L, Zhou G, Wang S, Wang J, Liu L, et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J NanosciNanotechnol 2010;10:6313-7.
- Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011;32:9810-7.
- Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, et al. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Appl Surf Sci 2008;255:502-4.
- Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB, Alhadlaq HA. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. BiochemBiophys Res Commun 2010;396:578-83.
- Young IS, Woodside JV. Antioxidants in health and disease. J ClinPathol 2001;54:176-86.
- Wells PG, Bhuller Y, Chen CS, Jeng W, Kasapinovic S, Kennedy JC, et al. Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. ToxicolApplPharmacol 2005;207 2 Suppls:354-66.
- Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J Affect Disord 2012;139:230-9.
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med 2009;71:171-86.
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol 2006;27:24-31.
- Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, et al. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depressi on-like responses. PLoS One 2013;8:e64128.
- Ivani S, Karimi I, Tabatabaei SR. Biosafety of multiwalled carbon nanotube in mice: A behavioral toxicological approach. J ToxicolSci 2012;37:1191-205.
- Liu Z, Ren G, Zhang T, Yang Z. The inhibitory effects of nano-Ag on voltage-gated potassium currents of hippocampal CA1 neurons. Environ Toxicol 2011;26:552-8.
- Liu Z, Ren G, Zhang T, Yang Z. Action potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal CA1 neurons by silver nanoparticles. Toxicology 2009 29;264:179-84.
- Cortese -Krott MM, Münchow M, Pirev E, Hessner F, Bozkurt A, Uciechowski P, et al. Silver ions induce oxidative stress and intracellular zinc release in human skin fibroblasts. Free RadicBiol Med 2009;47:1570-7.
- Rhodes M, Creel T, Nord A. Sex differences in CNS neurotransmitter influences on behavior. Horm Brain Behav 2009;5:2747-85.
- Stein DG. Brain damage, sex hormones and recovery: A new role for progesterone and estrogen? Trends Neurosci 2001;24:386-91.