- *Corresponding Author:
- S. B. Patil
A. R. A. College of Pharmacy, Nagaon, Dhule-424 006, India.
E-mail: sbpatil_99@rediffmail.com
| Date of Submission | 5-Oct-2005 |
| Date of Decision | 7-June-2005 |
| Date of Acceptance | 9-Feb-2006 |
| Indian J Pharm Sci, 2006, 68 (1):64-67 |
Abstract
Mucoadhesive chitosan microspheres of amlodipine besylate were prepared for nasal administration with the aim of avoiding the first pass effect. A series of batches of microspheres were prepared by simple emulsification crosslinking method to optimize parameters like external phase (mixture of heavy and light liquid paraffin in the ratio of 1:1), stirring rate (1200 rpm), dioctyl sodium sulfosuccinate concentration (0.2% w/v), Chitosan:drug ratio (2:1), volume of crosslinking agent (glutaraldehyde, 1 ml) and time of crosslinking (3 h). The microspheres were evaluated for physical characteristics such as particle size, particle shape and surface morphology by scanning electron microscopy, drug entrapment efficiency, in vitro mucoadhesion, and in vitro drug release characteristics. The microspheres had a mean particle size of 36.47 + 3.39 mm, suitable for nasal administration. Electron microscopy revealed that microspheres were spherical with nearly smooth surface morphology. Application of in vitro drug release data to various kinetic equations indicated matrix diffusion controlled drug release from chitosan microspheres.
Introduction
Amlodipine besylate (AB), a calcium channel blocker, is a drug of choice in the treatment of hypertension and angina pectoris[1]. AB undergoes first pass metabolism by oral route and thus exhibits only 60-65% oral bioavailability. The present investigation was aimed at avoidance of first pass metabolism of AB by preparing chitosan microspheres for nasal administration. Mucoadhesive microparticle nasal delivery is an attractive concept, in that the drug can be entrapped inside particles to be released at the nasal mucosal surface, where the particles are adhered due to their bio/ mucoadhesiveness. Extensive works on microspheres using mucoadhesive polymers for drugs like pentazocine[2], insulin[3,4], FITC–dextran[5], gentamicin[6,7] are reported.
Chitosan is a polymer of choice, because it enhances the nasal absorption of low molecular weight molecules as well as peptides and proteins[8]. It is a mucoadhesive polymer having advantages as nontoxicity, biocompatibility and biodegradability[9]. The purpose of this study is to investigate the suitability of chitosan microspheres as nasal delivery system and also to study the influence of the process variables in the preparation of the microspheres.
Materials and Methods
AB was a gift sample from Baroda Pharma Pvt. Ltd., Vadodara. Chitosan was a gift sample from Central Institute of Fisheries Technology, Cochin. Glutaraldehyde solution (25%) was obtained from S. D. Fine Chemical Ltd. Mumbai. Dioctyl sodium sulfosuccinate (DOSS, Wilson Laboratories, Mumbai), liquid paraffin (heavy and light, Suvidhinath Laboratories, Vadodara) were procured from local suppliers. All other chemicals used were of AR grade.
Preparation of microspheres[10]
Chitosan (200 mg) was dissolved in 2% v/v acetic acid solution. The drug (100 mg) was added in it and the suspension was extruded through syringe in 100 ml of liquid paraffin (heavy and light 1:1 mixture) containing 0.2% w/v DOSS, with stirring on Remi three-blade stirrer at high speed. After 20 min of stirring, 1 ml of glutaraldehyde (25% solution, as crosslinking agent) was added and stirring was continued for 3 h. Microspheres obtained were filtered and washed several times with cyclohexane to remove oil, and finally washed with water to remove excess of glutaraldehyde. Washings were analyzed for drug contents. Microspheres were then air dried at room temperature.
Effect of process variables on microsphere properties
Chitosan microspheres were prepared at different stirring rates (600 rpm, 1200 rpm), with stabilizing agent (DOSS) at concentrations of 0.1, 0.2 and 0.3% w/v in the external phase, and with various chitosan:drug ratios 1:1, 2:1, 3:1, 4:1 and 5:1.
Particle size analysis
Particle size of the microspheres was determined by optical microscopy[11]. Average of 100 microspheres were used for the study and the mean particle size (arithmetic mean diameter) was considered to be the deciding factor in selecting optimum formulation conditions for each variable parameter studied.
Scanning electron microscopy (SEM) of microspheres
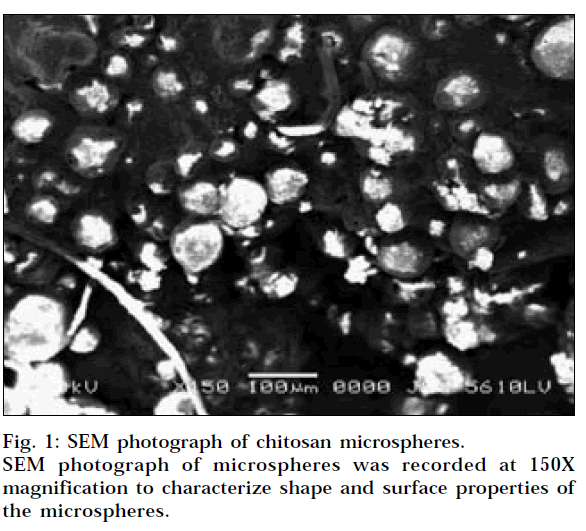
SEM of microspheres was recorded using Scanning Electron Microscope (Jeol, JSM 5610 LV, Japan) with a 20 kV accelerating voltage at 150x magnification.
Drug entrapment efficiency[12]
Weighed quantity of microspheres were crushed and suspended in methanol to extract the drug from microspheres. After 24 h, the filtrate was assayed spectrophotometrically at 361 nm for drug content against methanol as blank. Corresponding drug concentrations in the samples were calculated from the calibration plot generated by regression of the data taken in triplicate.
In vitro mucoadhesion
The in vitro mucoadhesion of microspheres was carried out by modifying the method described by Ranga Rao and Buri13 using sheep nasal mucosa. The dispersion (0.2 ml) of microspheres in water was placed on sheep nasal mucosa after fixing to the polyethylene support. The mucosa was then placed in the dessicator to maintain at >80% relative humidity and room temperature for 30 min to allow the polymer to hydrate and interact with the glycoprotein and also to prevent drying of the mucus. The mucosa was then observed under microscope, and the number of particles attached to the particular area was counted. After 30 min, the polyethylene support was introduced into a plastic tube cut in circular manner and held in an inclined position at an angle of 450. Mucosa was washed for 5 min with phosphate buffer saline pH 7.4 at the rate of 22 ml/min using a peristaltic pump; tube carrying solution was placed 2-3 mm above the tissue so that the liquid flowed evenly over the mucosa. Tissue was again observed under microscope to see the number of microspheres remaining in the same field area.
The adhesion number was found by the following equation: Na = N/N0x100, where Na is adhesion number, N0 is total number of particles in a particular area, and N is number of particles attached to the mucosa after washing.
In vitro release kinetics[14]
Microspheres equivalent to 5 mg of AB were weighed and suspended in 20 ml of phosphate buffer saline pH 7.4 and stirred at 60 rpm at 370. At specific time intervals, samples (1 ml) were withdrawn and filtered. Same volume (1 ml) of the phosphate buffer saline pH 7.4 was replaced after each sampling. The drug content in the sample was determined in the filtrate by the method described above.
Drug release pattern from microspheres
In order to understand the mechanism and kinetics of drug release, the results of the in vitro drug release study were fitted with various kinetic equations like zero order (% release vs t), first order (log % release vs t) and Higuchi model[15] (Mt/M∞ vs t). In order to define a model which will represent a better fit for the formulation, drug release data was further analyzed by Peppas equation[16], Mt/M∞ =ktn, where Mt is the amount of drug released at time t and M∞ is the amount released at time ∞, thus the Mt/M∞ is the fraction of drug released at time t, k is the kinetic constant and n is the diffusional exponent, a measure of the primary mechanism of drug release. R2 values were calculated for the linear curves obtained by regression analysis of the above plots.
Results and Discussion
The microspheres of AB were prepared by the emulsification crosslinking method using glutaraladehyde as crosslinking agent. The microspheres obtained under these conditions were found to be spherical and without aggregation, and median size ranged from 10 to 60 µm and are therefore suitable for nasal administration.
The effect of the various process variables like stirring rate, concentration of DOSS (stabilising agent) and chitosan:drug ratio on particle size of microspheres is presented in Table 1. Mean geometric particle size, percent drug entrapment and % in vitro mucoadhesion of different batches of microspheres prepared are tabulated in Table 2. Optimizations of various formulation parameters in preparation of AB microspheres were carried out. The heavy and light liquid paraffin (1:1) as external phase, DOSS (0.2% w/v) as stabilizing agent, and the stirring rate of 1200 rpm were found to be optimum to yield AB microspheres. Glutaraldehyde 25% aqueous solution was selected as crosslinking agent due to its high rate of crosslinking and easy removal of the unreacted free glutaraldehyde.
| Parameter | Median particle size diameter (μm) | |
|---|---|---|
| 1. | Stirring Rate | |
| Low (600 rpm) | 98.78±14.22 | |
| High (1200) | 35.67±6.38 | |
| 2. | DOSS Concentration | |
| 0.1% | 89.20±2.43 | |
| 0.2% | 36.00±4.27 | |
| 0.3% | – | |
| 3. | Chitosan:Drug Ratio | |
| 1:1 | 28.34±3.58 | |
| 2:1 | 36.47±3.39 | |
| 3:1 | 41.23±4.25 | |
| 4:1 | 43.92±3.4 | |
| 5:1 | 52.48±3.48 | |
Table 1: Effects Of Formulation Parameters On Particle Size Of Chitosan Microspheres
| Batch | Chitosan: | Particle | % drug | % in vitro |
|---|---|---|---|---|
| No. | drug ratio | size (μm) | entrapment | mucoadhesion |
| MS 1 | 1:1 | 23.34±3.58 | 40.8 | 61.21±4.21 |
| MS 2 | 2:1 | 36.47±3.39 | 72.57 | 65.12±3.50 |
| MS 3 | 3:1 | 41.23±4.25 | 65.0 | 67.29±4.93 |
| MS 4 | 4:1 | 43.92±6.20 | 68.0 | 69.55±2.26 |
| MS 5 | 5:1 | 52.48±3.48 | 55.65 | 70.43±1.89 |
Table 2: Physical Characteristics And Invitro Mucoadhesion Data Of Microspheres
With increase in chitosan concentration in the microspheres from batch MS1 to MS5, the particle size of microspheres increased, which may be due to the fact that increase in the concentration of polymer increases the crosslinking, and hence the matrix density of the microspheres increased, and that may result in the increase in the particle size of the microspheres. SEM of microspheres at magnification of 150X is presented in Fig.1, which revealed that microspheres were almost spherical in nature with slight smooth surface morphology.
In vitro mucoadhesion of microspheres was the most important aspect of present investigation. It was found that, for batches MS1 to MS5, as the amount of polymer was increased, the % in vitro mucoadhesion also increased. This may be due to the fact that, as the amount of polymer increased, the amino groups available for binding with the sialic acid residues in mucus layer also increase, and that results in the increase in the in vitro mucoadhesion of microspheres.
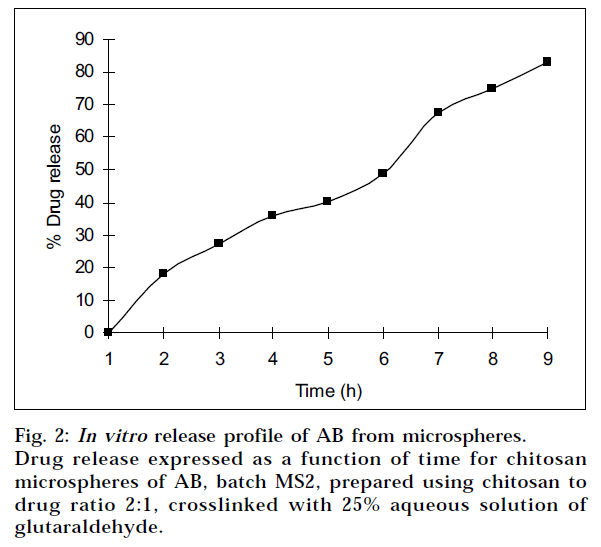
The in vitro drug release profiles for all batches are shown in Table 3. The release of active agent from the matrix involves initial swelling followed by diffusion of the drug. The fractional release (Mt/M∞) up to 60% of AB at time t is fitted to Peppas equation. In the present systems, the value for n was found to be in the range of 0.74 to 0.85 with a correlation coefficient close to 0.99, indicating that the release mechanism followed anomalous (non- Fickian) transport as well as case II transport. The optimized batch MS2 was having n = 0.74, indicating that the release mechanism followed is anomalous (non- Fickian) (Fig. 2). The relaxation rate and diffusion rates are comparable17.
| Time (h) | % Cumulative drug release | ||||
|---|---|---|---|---|---|
| MS1 | MS2 | MS3 | MS4 | MS5 | |
| 1 | 15.62 | 17.99 | 14.39 | 13.08 | 12.01 |
| 2 | 23.55 | 27.22 | 22.57 | 24.45 | 25.10 |
| 3 | 32.30 | 35.74 | 33.86 | 33.04 | 36.63 |
| 4 | 38.03 | 40.24 | 41.87 | 45.72 | 44.32 |
| 5 | 47.19 | 48.94 | 53.08 | 53.07 | 50.62 |
| 6 | 60.69 | 67.56 | 63.47 | 63.14 | 62.16 |
| 7 | 76.64 | 74.75 | 74.76 | 73.19 | 71.08 |
| 8 | 85.22 | 83.02 | 80.24 | 79.02 | 75.82 |
Table 3: In Vitro Cumulative Drug Release Data Of Chitosan Microspheres
In conclusion, these results indicate that the chitosan microspheres have potential to deliver AB following intranasal administration. Its possibility to avoid first pass metabolism of AB may ultimately show improvement of bioavailability than oral dosage, probably as a consequence of prolonged residence at the absorption site18.
Acknowledgements
The authors are also grateful to Baroda Pharma Pvt. Ltd., Vadodara, and Central Institute of Fisheries Technology, Cochin, for providing gift samples of AB and chitosan respectively.
References
- Robertson, R.M. and Robertson, D., In; Hardman, J.G., Limbird, L.E., Molinoff, P.E., Ruddon, R.W. and Gilman, A.G., Eds., Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th Edn., McGraw Hill, New York, 1996, 751.
- Sankar, C., Rani, M., Srivastava, A.K. and Mishra, B., Pharmazie, 2001, 56, 223.
- Illum, L., Jorgensen, H., Bisgaard, H., Krogsgard, O. and Rossing, N., Int. J. Pharm., 1987, 38, 189.
- Farraj, N.F., Johansen, B.R., Davis, S.S. and Illum, L., J. Control.Release, 1990, 13, 253.
- Kellaway, I.W. and Abd Ed-Hameed, M.D., Eur. J. Pharm,Biopharm., 1997, 44, 53.
- Illum, L., Farraj, N.F., Critcheley, H. and Davis, S.S., Int. J. Pharm., 1988, 46, 261.
- Lim, S.T., Forbe, B., Berry, D.J., Martin, G.P. and Brown, M.B., Int.J. Pharm., 2002, 231, 73.
- Illum L., Pharm. Res., 1998, 15, 1326.
- Felt, O., Buri, P. and Gurny R., Drug Dev. Ind. Pharm., 1998, 24, 979.
- Thanoo, B.C., Sunny M.C. and Jaykrishnan, A., J. Pharm.Pharmacol., 1992, 44, 283.
- Martin, A., Bustamante, P. and Chun, A.H.C., Eds., In; Physical Pharmacy, 4th Edn, B.J. Waverly Pvt. Ltd. New Delhi, 1996, 431.
- Singh, S. and Jain R., Indian Drugs, 1997, 34, 678.
- Rang Rao, K.V. and Buri, P.A., Int. J. Pharm, 1989, 52, 265.
- Niwa, T., Takenchi, H. and Hinto, T., Int. J. Pharm., 1995, 121, 45.
- Higuchi, T., J. Pharm, Sci., 1963, 52, 1145.
- Ritger, P.L. and Peppas, N.A., J. Control. Release, 1987, 5, 37.
- Korsemeyer, R.W., Gurny, R., Doelker, E., Buri, P. and Peppas, N. A.,Int. J. Pharm., 1983, 15, 25.
- El-Shafy, M. A., Kellaway, I. W., Taylor, G. and Dickinson, P. A., J.Drug Targett., 2000, 7, 355