- *Corresponding Author:
- F. H. Ge
School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou 510006, China
E-mail: gefahuan@163.com
| Date of Submission | 28 February 2017 |
| Date of Revision | 05 November 2017 |
| Date of Acceptance | 04 June 2018 |
| Indian J Pharm Sci 2018;80(4): 766-771 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A menthol/β-cyclodextrin inclusion complex was produced using supercritical CO2 while varying the temperature, pressure, co-solvent, inclusion time and depressurization time. The complex resulted was characterized with X-ray diffraction, scanning electron microscopy and differential scanning calorimetry. The characterization data confirmed the production of the inclusion complex at a maximum yield of 93.75 %. The dissolution rate of the complex was signiï¬?cantly higher than those of either untreated menthol or a physical mixture of menthol and β-cyclodextrin. This work thus demonstrated that supercritical CO2 could be successfully used to achieve inclusion complexes of menthol with β-CD.
Keywords
Supercritical fluid, inclusion complex, menthol, characterization, cyclodextrins
Essential oils are widely applied in traditional Chinese medicine and often account for the primary components. However, these oils are unstable because of their tendency to either volatilize or oxidize, which can affect both the quality and curative effects of the medicine. For this reason, cyclodextrins are sometimes added, both to enhance the stability of the essential oils and to improve their bioavailability [1]. Thus the final dosage form of menthol targeted for the market can be granules, capsules and tablets. In addition, cyclodextrin-menthol inclusion complexes can be used to producing functional nanofibers that contain fragrances/flavours with high temperature stability [2].
Cyclodextrins are torus-like molecules that have a hydrophilic outer surface and a lipophilic internal cavity, into which suitable molecules may enter to form cyclodextrin complexes. Several methods, such as co-precipitation, grinding, spray drying, and freeze drying, have been developed to form inclusion complexes [3]. Unfortunately, many of these methods have some shortcomings, including low efficiency of inclusion complexation, long processing times, high levels of residual organic solvent, and the need for an additional drying step. Over the last few years, the employment of supercritical CO2 (scCO2) as an abnormal inclusion medium has received increasing interest [3-9]. The method utilizes the unique properties of supercritical fluid (SCF), which has liquid-like density combined with viscosity and diffusivity intermediate between that of gas and liquid. Moreover, since the solvent is a dilute gas under ambient conditions, SCF-based process can produce a solvent-free final product [4].
The majority of reports involving supercritical fluids have focused on assessing the probability of gaining cyclodextrin complexes using all kinds of technologies. The most commonly used technology involves simply producing a physical mixture of the cyclodextrin and drug in static contact with scCO2 at constant pressure and temperature [3,5-9]. However, different methods have also developed, such as the supercritical antisolvent process [7], the controlled particle deposition process [5,8], and the aerosol solvent extraction system [10]. Up to now, though, there have been only a few investigations of the potential helpful effects of subsidiary substances on the supercritical process. For example, the effects of adding acidic compounds during the supercritical complexation of miconazole with different cyclodextrins were studied [11]. In addition, L-lysine was successfully used in combination with piroxicam and natural β-cyclodextrin (β-CD), and the addition of a little water had also been shown to have a positive effect on the inclusion efficiency [3,12].
In the present work, the primary objective was to prepare and characterize a menthol/β-CD inclusion complex. In contrast to the investigations described above [3,5-13], menthol, the main component of oleum menthae, is both lipophilic, neutral and smaller than ibuprofen [5,8], ketoprofen [3], resveratrol [6], flufennamic acid [13] and other common medications. In the inclusion experiments reported herein, the effects of pressure, temperature, inclusion time, and depressurization time were investigated and the inclusion complex was characterized by scanning electron microscopy (SEM), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and dissolution testing.
Menthol was supplied by the Ji’an City Natural Medical Oil Plant (Jiangxi Province, China) and β-CD was obtained from the Shandong Binzhou Zhiyuan Bio-Technology Co., Ltd. (Shandong Province, China). High purity CO2 (99.9 %) was purchased from the Guangzhou Gas Factory Co., Ltd. (Guangdong, China). All other chemicals were of analytical grade.
Approximately 13 g sample composed of a mixture of menthol and β-CD with the molar ratio of 1:1 was placed individually into a filter paper cylinder set inside the extraction vessel of a HA221-50-06 SCF extraction apparatus (Huaxing Supercritical Fluid Extraction Co., Jiangsu Province, China). The instrument was operated in the static mode and each sample was maintained under a scCO2 atmosphere at constant pressure and temperature for a time. Finally, the vessel was reduced pressure and the sample was dried under vacuum at 313K. The inclusion complex yield was measured with the same differential solubility method [14] that had been previously applied to measure the included/free drug ratio in piroxicam/cyclodextrin inclusion complex, since the method was readily extended to other drug/ cyclodextrin systems [3,15].
This method utilised differences in solubility of the drug and the cyclodextrin in acetonitrile. When acetonitrile is mixed with the treated sample, the non-included drug dissolved, while the included drug remained trapped. The amount of the free drug can thus be measured. If a mixture of water-acetonitrile (1:1 v/v) is used instead of pure acetonitrile, the cyclodextrin and the complex completely dissolved, allowing the total amount of the drug to be measured [3]. The inclusion yield (η) can then be measured using the following Eqn. 1: η = 100× (1–free menthol content/total menthol content).
Samples were determined for the free and total drug amount using a GC-9790 gas chromatograph equipped with an FID detector (Zhejiang Fuli Analytical Instruments Co., Ltd., China). The injector and detector temperatures were 523 and 573 K, respectively, and nitrogen was used as the carrier gas at a 2.0 ml min−1 flow-rate. A 1.0 μl sample was injected under split conditions with a split ratio of 30:1. The oven temperature was initially set at 323 K, then programmed to rise at 5 K/min to 413 K, followed by a rise at 1 K/min to 428 K and a rise at 10 K/min to 553 K. Cedrol was used as the internal standard when quantifying menthol. The calibration curves for menthol were linear over the concentration range of 67.2-336.0 μg/ml, with the correlation coefficients (R2) of 0.9998.
Micrographs were obtained with a SEM (Quanta 400, FEI Company, USA). Samples were first coated with gold using a sputter system (E5100, Bio-Rad, München, Germany). DSC was achieved with a comprehensive thermal analyser (HCT-3, Beijing Henven Scientific Instrument Plant, China). Samples of 4-8 mg were heated in an aluminium pan (40 μl) under a nitrogen gas flow of 20 ml min-1. A heating rate of 10 K min-1 was applied to the maximum temperature of 570 K. The powder XRD patterns were obtained with an X-ray diffractometer (Empyrean, Panalytical Company, Holland). The patterns were typically gathered over the 2θ range of 5-45°.
Drug release studies were carried out carried out at 310 K for untreated menthol, its unprocessed physical mixture with β-CD, and the inclusion complex, employing a test described in the Chinese Pharmacopoeia (Volume IV, General rule 0931; 2015). The dissolution tests were conducted in a vessel containing 900 ml of a pH 1HCl solution and rotating at 100 rpm. The sample (250 mg) was added to the chamber and 5 ml aliquots of the dissolution fluid were taken following 5 and 10 min and then at further 10 min intervals up to 90 min. These aliquots were filtered through a membrane (Sarstedt, pore size 0.45 μm). Each aliquot was replaced with an equal volume of fresh medium. The filtrates were assayed using a gas chromatograph equipped with a FID detector.
As shown in Table 1, samples 1 to 3 were prepared with different co-solvents. The inclusion yield was very low without any co-solvent or with 5 % ethanol, while the addition of a little water had a positive effect on the inclusion efficiency. From a qualitative point of view, it appeared that water addition was essential to complexation. Similar result was also reported by Sauceau et al. [12] and Banchero [3]. This might occur because addition of water to the scCO2 decreases the hydrophobic interactions between the menthol and the scCO2 (both of which are hydrophobic), thus helping the menthol to enter the β-CD.
| Sample no. | Co-solvent (w/w) |
P(MPa) | T(K) | Density of CO2 (kg/m3)a | Inclusion time (h) | Depressure time (min) | Inclusion yield, ? (%) |
|---|---|---|---|---|---|---|---|
| 1 | - | 20 | 313 | 841 | 2 | 10 | <5 |
| 2 | 5 % Ethanol | 20 | 313 | 841 | 2 | 10 | 7.60 |
| 3 | 5 % H2O | 20 | 313 | 841 | 2 | 10 | 60.43 |
| 4 | 5 % H2O | 10 | 313 | 632 | 2 | 10 | 31.65 |
| 5 | 5 % H2O | 15 | 313 | 782 | 2 | 10 | 45.35 |
| 6 | 5 % H2O | 25 | 313 | 881 | 2 | 10 | 89.72 |
| 7 | 5 % H2O | 30 | 313 | 911 | 2 | 10 | 83.24 |
| 8 | 5 % H2O | 20 | 323 | 786 | 2 | 10 | 46.38 |
| 9 | 5 % H2O | 20 | 333 | 726 | 2 | 10 | 36.40 |
| 10 | 5 % H2O | 20 | 343 | 662 | 2 | 10 | 33.58 |
| 11 | 5 % H2O | 20 | 313 | 841 | 0.5 | 10 | 85.96 |
| 12 | 5 % H2O | 20 | 313 | 841 | 1 | 10 | 69.58 |
| 13 | 5 % H2O | 20 | 313 | 841 | 4 | 10 | 90.08 |
| 14 | 5 % H2O | 20 | 313 | 841 | 2 | 1 | 93.75 |
| 15 | 5 % H2O | 20 | 313 | 841 | 2 | 5 | 76.42 |
| 16 | 5 % H2O | 20 | 313 | 841 | 2 | 30 | 19.27 |
Table 1: Operating Conditions and Inclusion Yield Obtained for Systems with ß-CD (n=3)
The results for samples 3 and 4 to 7 demonstrated that the inclusion yields increased with increasing pressure up to 25 MPa, and then decreased at 30 MPa. The density of the scCO2 increases as the pressure increases, which may help dissolve the menthol. As the scCO2 permeated into the pores of the β-CD, the menthol would have precipitated inside the pores. However, when the density of the scCO2 becomes overly high at 30 MPa, the hydrophobic interactions between the menthol and the scCO2 are too pronounced, thus restricting the ability of menthol to enter the β-CD.
From samples 3 and 8 to 10 it was evident that the inclusion yields decreased along with the increase in temperature from 313 to 343 K. As the temperature increased, the density of the CO2 decreased and this might reduce the dissolution of menthol in the scCO2 and thus restrict precipitation of the menthol inside the β-CD pores. In addition, increasing the temperature may also shift the complexation equilibrium towards the free form, because temperature increases molecular movement and so reduces the stability of the inclusion complex.
From samples 3 and 11 to 13 it can be concluded that the inclusion yield first decreased, and then increased as the inclusion time was increased from 0.5 to 4 h. As the melting point of menthol is 317 K, the compound was likely molten from 313 to 343 K because scCO2 lowers the melting points of some organic compounds [16]. Thus, in the case of the physical mixtures of menthol and β-CD, the menthol could readily dissolve in the scCO2 and precipitate inside the β-CD. However, this was a dynamic process and, over time, the menthol could also move out of the pores. In this manner, the menthol could redissolve in the scCO2 to decrease the inclusion yield. The inclusion yield is the result of complexation and decomplexation. It would expect that after a certain time dynamic equilibrium would occur, meaning that that yield would increase in time until it remain the same.
Depressurization time is that the pressure gradually released during the period of time. The inclusion yields increased with reductions in the depressurization time from samples 14 to 16. This may have resulted from loss of menthol from the inclusion complex during depressurization.
SEM, DSC and XRD were all used to characterize the product to confirm the formation of an inclusion complex between menthol and β-CD. Figure 1 presented the SEM images of unprocessed menthol, unprocessed β-CD, an untreated physical mixture of menthol and β-CD and the complex formed using scCO2. The untreated physical mixture was similar to the unprocessed β-CD because the menthol was volatilized during the SEM observation process. The image of the β-CD inclusion complex, however, showed obvious differences. These particles exhibited irregular shapes compared with the pure material, suggesting the presence of different crystalline morphologies following the complexation.
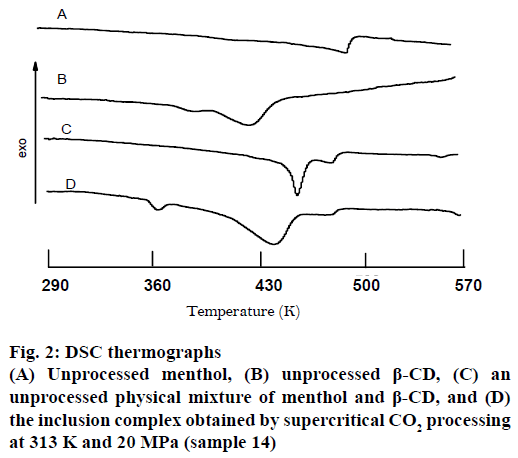
As shown in Figure 2, the thermogram of pure menthol did not show a regular melting peak. This could be that menthol absorbed the heat for volatilization during heating. Pure crystalline menthol exhibited a peak at approximately 490 K, which corresponded to thermal decomposition. While the DSC plot for pure β-CD exhibited a small endothermic peak about 380 K and a broad endothermic peak about 428 K, corresponding to free water and included water loss during heat, respectively. The plot obtained from the unprocessed physical mixture presented two endothermic peaks at about 455 and 480 K, respectively, attributed to the included water loss of the β-CD and thermal decomposition of menthol. And the peak corresponding to free water loss during heat was shielded by menthol volatilization. In contrast, in the complex the two endothermic peaks shifted to 440 and 475 K, respectively, indicating the almost complete encapsulation of menthol in the β-CD. And the peak at about 360 K corresponded to free water loss during heat. However, it should be noted that the change of the endothermic peaks might also indicate the transformation of the menthol into the amorphous state rather than the production of an inclusion complex, or possibly a combination of these two phenomena.
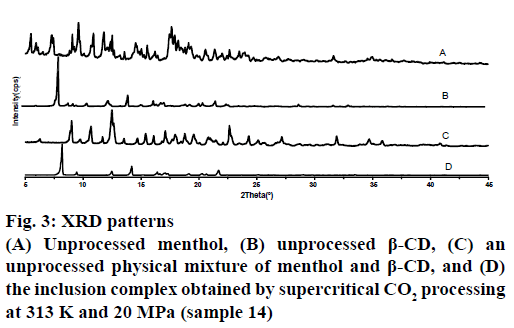
Figure 3 showed the XRD patterns obtained from unprocessed menthol, unprocessed β-CD, an untreated physical mixture of menthol and β-CD and the complex formed using scCO2. The patterns of the unprocessed menthol and β-CD exhibited a series of intense peaks corresponding to their crystallinity. As well, the pattern of the physical mixture presented peaks similar to the superposition of the patterns of the unprocessed menthol and β-CD. By comparison, the pattern generated by the complex produced at 20 MPa and 313 K contained peaks similar to those of pure β-CD. Generally speaking, the absence of menthol diffraction patterns in the complex might be caused either by the formation of an inclusion complex or by the transformation of menthol from a crystalline to an amorphous state. But a former study based on rapid expansion from a supercritical solution experiments performed with scCO2 under similar conditions observed the formation of crystalline particles [17,18]. Therefore, in accord with the results obtained by DSC, the disappearance of the menthol diffraction patterns in the case of the complex provided further evidence for the formation of the inclusion complex.
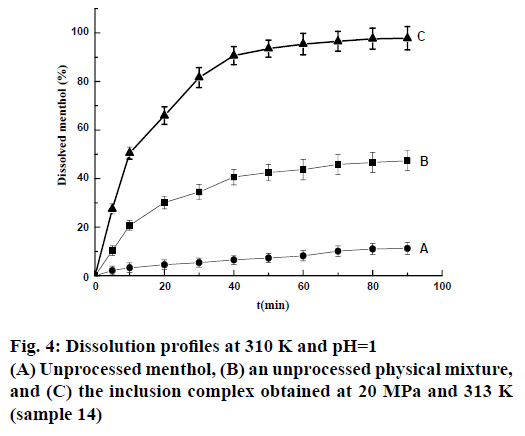
Drug release studies were performed in a solution at pH=1 to evaluate the extent to which the loading process improved the water solubility of menthol. The unprocessed menthol showed rather slow dissolution (Figure 4) in this medium, with only approximately 11.2 wt. % dissolved after 1.5 h. The addition of β-CD evidently had a positive effect because the untreated physical mixture exhibited higher dissolution rates than the unprocessed menthol. A significant improvement in dissolution, leading to 97.8 wt. % dissolved after 1.5 h, was observed for the inclusion complex.
In the work, there was a maximum inclusion yield of 93.75 %. SEM, DSC and XRD data all confirmed the formation of an inclusion complex between menthol and β-CD. These results provided evidence that scCO2 can be used to synthesize inclusion complexes of menthol with β-CD. Further studies are needed to evaluate feasibility of processing these complexes into appropriate pharmaceutical preparations and examining the stability of the resulting formulations.
Acknowledgements
The authors thank The Hunan Provincial Natural Science Foundation of China (No. 2015JJ3111, 2017JJ2240), The Project of Scientific Research of Hunan Provincial Education Department (No. 15A175), Science and Technology Plan Project of Chenzhou city (No. CKJ2016020), and Ministry of Health of Hunan Province (No. B2016092) for financial support.
Conflict of interest
The authors declare no conflicts of interest.
References
- Marques HMC. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr J 2010;25:313-26.
- Uyar T, Nur Y, Hacaloglu J, Besenbacher F. Electrospinning of functional poly(methyl methacrylate) nanofibers containing cyclodextrin-menthol inclusion complexes. Nanotechnology 2009;20(12):183-87.
- Banchero M, Manna L. The use of lysine to enhance the supercritical complexation of ketoprofen and cyclodextrins. J Supercrit Fluids 2012;67:76-83.
- Naylor A, Lewis AL, Illum L. Supercritical fluid-mediated methods to encapsulate drugs: recent advances and new opportunities. Ther Deliv 2011;2:1551-65.
- Hussein K, Türk M, Wahl MA. Drug loading into ß-cyclodextrin granules using a supercritical fluid process for improved drug dissolution. Eur J Pharm Sci 2008;33:306-12.
- Zhou R, Wang F, Guo Z, Zhao YL. Preparation and characterization of resveratrol/hydroxypropyl-ß-cyclodextrin inclusion complex using supercritical antisolvent technology. J Food Process Eng 2012;35:677-86.
- Lee CW, Kim SJ, Youn YS, Widjojokusumo E, Lee YH, Kim J, et al. Preparation of bitter taste masked cetirizine dihydrochlori/ß-decyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J Supercrit Fluids 2010;55:348-57.
- Türk M, Upper G, Steurenthaler M, Hussein Kh, Wahl MA. Complex formation of Ibuprofen and ß-Cyclodextrin by controlled particle deposition (CPD) using SC-CO2. J Supercrit Fluids 2007;39:435-43.
- Sultana T, Jung JM, Hong SS, Lee WK, Gal YS, Kim HG, et al. Characteristic profiles of the inclusion complex of omeprazole/peracylated-ß-cyclodextrin formed in supercritical carbon dioxide. J Incl Phenom Macrocycl Chem 2012;72:207-12.
- Lee SY, Jung II, Kim JK, Lim GB, Ryu JH. Preparation of itraconazole/HP-ß-CD inclusion complexes using supercritical aerosol solvent extraction system and their dissolution characteristics. J Supercrit Fluids 2008;44:400-08.
- Barillaro V, Bertholet P, Henry de Hassonville S, Ziémons E. Evrard B, Delattre L, et al. Effect of acidic ternary compounds on the formation of miconazole/cyclodextrin inclusion complexes by means of supercritical carbon dioxide. J Pharm Pharm Sci 2004:7:378-88.
- Sauceau M, Rodier E, Fages J. Preparation of inclusion complex of piroxicam with cyclodextrin by using supercritical carbon dioxide. J Supercrit Fluids 2008;47:326-32.
- Nunes AVM, Almeida APC, Marques SR, Sampaio de Sousa AR, Casimiro T, Catarina MMD. 2002. Processing triacetyl-ß-cyclodextrin in the liquid phase using supercritical CO2. J Supercrit Fluids 2008;54:357-61.
- Van Hees T, Peil G, De Hassonville SH, Ecrad B, Delattre L. Determination of the free/included piroxicam ratio in cyclodextrin complexes: comparison between UV spectrophotometry and differential scanning calorimetry. Eur J Pharm Sci 2002;15:347-53.
- Al-Marzouqui AH, Shehatta I, Jobe B, Dowaidar A. Phase solubility and inclusion complex of itraconazole with ß-cyclodextrin using supercritical carbon dioxide. J Pharm Sci 2006;95:292-304.
- He J, Wang, B. Acetone influence on glass transition of poly-methyl-methacrylate and poly-styrene in compressed carbon dioxide. Ind Eng Chem Res 2009;48:5093-97.
- Türk M, Hils P, Helfgen B, Lietzow R, Schaber K. Micronization of pharmaceutical substances by rapid expansion of supercritical solutions (RESS): experiments and modeling. Part Part Syst Char 2002;19:327-35.
- Martin HJ. Characterization of poorly soluble drug nanoparticles prepared by the RESS method for improving bioavailability [dissertation]. Tübingen, Germany: University of Tübingen; 2003.