- *Corresponding Author:
- T. Phaechamud
Department of Pharmaceutical Technology, Faculty of Pharmacy, Silpakorn University, Nakhon, Pathom-73000, Thailand
E-mail: thawatchaienator@gmail.com
| Date of Submission | 21 July 2009 |
| Date of Revision | 18 November 2009 |
| Date of Acceptance | 4 February 2010 |
| Indian J Pharm Sci, 2010, 72 (2): 173-183 |
Abstract
The aim of this research was to investigate the technique for preparation of coated valproic acid and sodium valproate sustained-release matrix tablets. Different diluents were tested and selected as the effective absorbent for oily valproic acid. Effect of the amount of absorbent and hydroxypropylmethylcellulose on drug release from valproic acid-sodium valproate matrix tablets prepared with wet granulation technique was evaluated in pH change system. Colloidal silicon dioxide effectively adsorbed liquid valproic acid during wet granulation and granule preparation. The amounts of colloidal silicon dioxide and hydroxypropylmethylcellulose employed in tablet formulations affected drug release from the tablets. The drug release was prominently sustained for over 12 h using hydroxypropylmethylcellulose-based hydrophilic matrix system. The mechanism of drug release through the matrix polymer was a diffusion control. The drug release profile of the developed matrix tablet was similar to Depakine Chrono; , providing the values of similarity factor (f2) and difference factor (f1) of 85.56 and 2.37, respectively. Eudragit; L 30 D-55 was used as effective subcoating material for core matrix tablets before over coating with hydroxypropylmethylcellulose film with organic base solvent. Drug release profile of coated matrix tablet was almost similar to that of Depakine Chrono; .
Keywords
Coated matrix, preparation technique, sodium valproate, sustained-release, valproic acid
Matrix diffusion is a suitable system in producing oral sustained release dosage form, especially tablets. Matrix tablet can be achieved by using appropriate type and concentration of a matrix substance, followed by general manufacturing process mainly including granulation and compression. Hydroxypropylmethylcellulose (HPMC) is the major hydrophilic carrier material used for the preparation of oral controlled drug delivery systems. One of its most important characteristics is the high gelation velocity and viscosity, which has a significant effect on the release kinetics of the incorporated drug [1,2]. It was proven that HPMC at high concentration promoted the drug release approaching to a zero order release kinetic because of its gelation properties [2]. Colloidal silicon dioxide such as Aerosil® 200 has been used in several pharmaceutical applications such as a moisture adsorbent, free-flow agent and glidant in the tablet manufacturing [3]. In theophylline-loaded lipid microparticles, Aerosil® 200 was employed as a thickening and a suspending agent [4].
Valproic acid (VA) and sodium valproate (VAS) are anticonvalsants widely used for treatment of simple and complex absence seizures. Physical characteristic of VA are as follows; clear, colorless to pale yellow, slightly viscous liquid, and sparingly soluble in water. The solubility data are 1.27 mg/ml in water and 1.25 mg/ml in 0.1N HCl. Boiling point of VA is 221-222°. VA is a very stable compound since no degradation is observed by the action of heat, light, and strong aqueous alkali, or acid [5]. VAS is a white crystalline, very hygroscopic powder and very soluble in water and alcohol [6]. One gram of VAS is soluble in 0.4 ml of water and also in 1.5 ml of ethanol. VAS was extremely stable when it was refl uxed in water, 1.0 N hydrochloric acid, or 1.0 N sodium hydroxide for 3 h. Also, it was very stable when subjected to heat at 110o for 10 days and to sunlight for 30 days in the dry environment. The pKa values of VA and VAS are 4.6 and 4.8, respectively [5].
VA and VAS have been used in combination because there are minor differences in the pharmacokinetics of the formulation and accessibility in market [7]. VA and VAS are available in different dosage forms; capsule, tablet, enteric-coated tablet, sprinkle, liquid, intravenous, suppository and controlled-release formulations [8]. Sustained-release formulation of the combination between VA and VAS reduces the fluctuation in plasma drug concentrations, thus minimizing or preventing plasma peak-related adverse events, and allows prolongation of the dosing interval enabling a once or twice daily administration with inherent benefits in terms of patient compliance [9]. Due to oily characteristic of VA, tablet formulation is diffi cult to prepare. Divalproex sodium, a compound containing an equal proportion (on a molar basis) of sodium valproate and valproic acid, dissociates into valproate ion in the gastrointestinal tract [10]. Divalproex sodium requires twice or three times daily administration. Once-daily administration of divalproex sodium extended-release tablets may potentially be used to sustain plasma valproic acid concentrations within the usually accepted therapeutic ranges for various indications in children and aldolescent [11]. Controlled-release of divalproate sodium tablet could provide desired nearly constant therapeutic plasma concentration over the entire 24 h dosing interval [10]. A relatively good correlation was observed between the absorption profiles and the dissolution profiles of the developed 200 mg and 400 mg VAS sustained-release tablet by a membranecontrolled system [12]. An addition of citric acid in the film coat exerted a plasticizing effect on the enteric polymer film and improved film formation and polymer coalescence. As citric acid was greater than 10% (w/w) in the enteric coated VAS pellets, a decrease in drug content was observed due to the conversion of sodium valproate to the volatile compound, valproic acid [13]. However, technique concentrating on matrix preparation and coating of VA-VAS tablet has not been reported.
The purpose of this research was to study the technique for the preparation of coated VA and VAS sustained-release matrix tablets, using HPMC as matrix former by wet granulation technique and to compare drug release of the developed tablets to that of a commercial product, Depakine Chrono®. The effect of excipients on physical properties and drug release from matrix tablet was also investigated.
Materials and Methods
Valproic acid (Lot 041101) and sodium valproate (Lot 040901E) were purchased from Hunan Xiangzhong Pharmaceutical Co., Ltd., Shaoyang Hunan, China. Valproic acid reference standard (Lot 123K3748, Sigma, Taufkirchen, Germany) was used as received. Colloidal silicon dioxide (Aerosil® 200, Degussa, Dusseldorf, Germany), hydroxypropylmethylcellulose (Methocel® K 15 M, Dow Chemical, The heeren, Sigapore) and microcrystalline cellulose (Avicel® PH 102, FMC Biopolymer, Philadelphia, USA) were used as matrix components. Isopropyl alcohol (Shell Chemicals, Sereya, Singapore) was used as the granulating liquid. Povidone (Plasdone® K 90, ISP technologies, Texas, USA) and magnesium stearate (Nof corporation, Tokyo, Japan) were used as binder and lubricant, respectively. The coating material was hydroxypropylmethylcellulose (Pharmacoat® 615, Shin-Etsu chemical Co., Ltd, Tokyo, Japan) and Eudragit® 30D-55 which was purchased from Rama Production, Bangkok, Thailand. Triethyl citrate (Lot AG CH9470, Fluka Chemical, Buch, Switzerland) was used as plasticizer. Titanium dioxide (Sensient, Scarlino, Italy) and talcum (Super®-1250) (Shengtai Chem Co., Ltd., Guangdong, China) were also added as opacifier in coating material. Isopropyl alcohol (Shell Chemicals, Sereya, Singapore) and methylene chloride (DOW Chemical, The heeren, Singapore) were used as solvents in the coating process. Calcium carbonate (Fujian Sannong Calcium Carbonate Co., Ltd., Sanming, Fujiang, China), corn starch (Weifang S Co., Ltd., Shahengtai Medicine Co., Ltd., Shandong, China) and dibasic calcium phosphate (Yichang Shenfa Foreign Trade Co., Ltd., Shanghai, China) were used as received.
Adsorption of VA with some excipients
VA is an oily liquid which is difficult for applying in tablet preparation. Colloidal silicon dioxide, talc, microcrystalline cellulose, calcium carbonate, corn starch and dibasic calcium phosphate were individually tested for VA adsorption by mixing with VA 145 mg. Each of these excipients was gradually weighed for mixing with VA using mortar and pestle until VA was completely adsorbed with no liquid residue left (n=3).
Preparation and evaluation of matrix granule
The matrix granules were prepared by wet granulation method. VA was gradually adsorbed on colloidal silicon dioxide (Aerosil® 200) using the mortar and pestle. The amount of colloidal silicon dioxide used in this study was varied (7, 9, 10, 15 and 20 % by weight). Microcrystalline cellulose and hydroxypropylmethylcellulose (Methocel® K15M) of 0, 5, 7.5, 10, 12.5, 15 and 20 % by weight were dried, mixed and screened through a 20-mesh sieve, then mixed with the active ingredient. Wet granules were prepared by adding PVP-K 90 in isopropyl alcohol solution (3% w/w of total weight of core tablet formulation) into powder mixture, sheared by the pestle and screened through a 12-mesh sieve. The granules were tray dried at 60° using a hot air oven for 3 h. The dried granules were screened through a 20-mesh sieve before the evaluation of flow property and compressibility. The humidity in a granule-preparation room was regulated around 50 % RH. The bulk and tapped densities of the granules were determined in triplicate using the test for apparent volume, and the Carr’s index was calculated. The amount of ingredients used in each formulation (presented as SR1 to SR 12) was shown in the Table 1.
| Ingredient | Formula (amount per tablet, mg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR1 | SR2 | SR3 | SR4 | SR5 | SR6 | SR7 | SR8 | SR9 | SR10 | SR11 | SR12 | |
| VA | 145 | 145 | 145 | 145 | 145 | 145 | 145 | 145 | 145 | 145 | 145 | - |
| VAS | 333 | 333 | 333 | 333 | 333 | 333 | 333 | 333 | 333 | 333 | 333 | 500 |
| Colloidal silicon dioxide | 49 | 63 | 70 | 105 | 140 | 63 | 63 | 63 | 63 | 63 | 63 | 63 |
| HPMC | 70 | 70 | 70 | 70 | 70 | - | 35 | 52.5 | 87.5 | 105 | 140 | 105 |
| Povidone | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| Microcrystalline cellulose | 68 | 54 | 47 | 12 | - | 124 | 89 | 71.5 | 36.5 | 19 | - | - |
| Magnesium stearate | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Talc | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Total | 700 | 700 | 700 | 700 | 723 | 700 | 700 | 700 | 700 | 700 | 716 | 703 |
Table 1: Formula of Valproic Acid and Sodium Valproate Sustained-Release Matrix Tablets
Preparation of core matrix tablet
VA and VAS sustained release tablets were HPMCbased hydrophilic matrix system. After the dried granules were screened through a 20-mesh sieve, they were mixed with magnesium stearate and talcum. Then the core matrix tablets were compressed using single punch tablet machine with a caplet punch (Yeoheng, Bangkok, Thailand). The 200 tablets per batch size were prepared for tablet evaluation. The process for scale up the core matrix tablet was similar to the above mention, except the amount of tablet was 2,000 tablets per batch size. The humidity in a core-matrix-tablet preparation room was regulated around 50 % RH.
Preparation of fi lm coated matrix tablet
The VA and VAS sustained release core tablets were coated with fi lm coater (model 0603/1017, N. R. Industries Co., Ltd., Bangkok, Thailand) using HPMC-based fi lm with different thickness by varying spraying duration (1, 2, 3 and 4 h). The coating solution was prepared by adding 10% HPMC, 5% talcum and 5% titanium dioxide into a mixture of 1:1 isopropyl alcohol and methylene chloride. The conditions for coating were as follows: inlet air temperature, 60°; atomizing air pressure, 300,000 Pa; pan speed, 8 rpm and coating time, 1, 2, 3 and 4 h.
Chromatographic condition of HPLC analysis
A Shimadazu HPLC system model SPDM10Avp consisting of pump, LC-10Advp (Liquid chromatograph), autosampler, SIL-10Advp, column heater, CTO-10Asvp (column oven) and detector, SPD-M10Avp (diode array detector), injection valve equipped with auto-injector, variable wavelength detector set at 220 nm and 20 microclines loop injection valve (Shimadzu, Kyoto, Japan) was used for determination of drug content. For analysis, a reversed-phase Innersole ODS 3 C18 (5 μm) 4.6×150 mm column was eluted by using a mixture of acetronitrile and a 0.32% W/V solution of potassium dihydrogen orthophosphate (60:40) adjusted to pH 3 with orthophosphoric acid as the mobile phase with a fl ow rate of 1 ml per minute and a detection wavelength at 220 nm. Quantification of VA was carried out by measuring the peak areas in relation to those of standard chromatograph analyzed under the same conditions. The VAS was converted to the free acid at this pH during the HPLC analysis. The HPLC analysis was validated for accuracy, linearity and precision before used. The correlation coeffi cients from the system validation for accuracy and linearity were 0.9998 and 0.9998, respectively. The precision was expressed in terms of relative standard deviation (%RSD) values. RSD values for precision were less than 2.0%, indicating a good repeatability.
Evaluation of matrix tablets
The hardness of tablets was determined using a hardness tester (model TBH210TD, Pharmatest, Ontario, Canada). The tablet thickness was measured using a thickness tester (Teclock, Kyoto, Japan). Friability of prepared tablets was evaluated using a friability tester (Yeo Heng, Bangkok, Thailand). In this study, the tablets were prepared by controlling the weight within 700±5% mg per tablet, hardness in the range of 127-147 N and friability no more than 0.1%. The suitable formulations were chosen for scale up and film coating. Content uniformity was determined using a HPLC method. Dissolution profiles of the prepared VA and VAS sustained release tablets were compared with that of Depakine Chrono®. In vitro dissolution testing of VA and VAS sustained release tablets was determined using a USP apparatus II dissolution tester (model VK7010, Vankel, NJ, USA), operating at 100 rpm. Dissolution test was performed in 500 ml of 0.1 N HCl for 45 min followed by 900 ml of 0.05 M phosphate buffer pH 5.5 containing 0.5% SLS, the medium temperature was maintained at 37±0.5°. The 10 ml of dissolution medium were withdrawn at 30 min, 1, 2, 3, 4, 5, 6, 8, 10 and 12 h. The medium was replenished with 10 ml of fresh buffer each time. Each sample was fi ltered through Nylon fi lter 0.45 micron. The samples were assayed by HPLP under the above mentioned analysis condition. The obtained dissolution profi les were compared with that of the Depakine Chrono®.
Determination of surface morphology of matrix tablet
The surface and cross-sectional topography of the prepared matrix tablets and Depakine Chrono® were determined using a scanning electron microscope (SEM) (Maxim 200 Camscan, Cambridge, England) operated at an accelerating voltage of 20 KeV. The samples were stuck on a metal stub using carbon double adhesive and sputter coated with gold before test.
Evaluation of similarity factor and difference factor of release profi les
The similarity and difference of release profi les of the developed formulation was compared to that of the commercial formulation in terms of similarity factor (f2) and difference factor (f1) using the following eqns; f2 = 50×log [{1+1/n Σt=1 |Rt– Tt|2}-0.5×100 ]..1 and f1= [{Σt=1 |Rt– Tt|}/{ Σt=1Rt}]×100..2, where Rt and Tt are the percent drug dissolved at each time point for the sample and reference products, respectively, n is the number of dissolution sample times, and t is the time sample index [14]. The two curves are considered to be similar when f2 value is close to 100 (50-100). Release profi les are considered to be different when f1 value is close to 15, generally f1 value of less than 15 (0-15) indicates similarity between the profi les.
Dissolution profi le fi tting
Least square fitting the experimental dissolution data (cumulative drug release >10% and up to 80%) to the mathematical equations (power law, first order, Higuchi’s and zero order) was carried out using a nonlinear computer programme, Scientist for Windows, version 2.1 (MicroMath Scientific Software, Salt Lake City, UT, USA). The coeffi cient of determination (r2) was used to indicate the degree of curve fi tting. Goodness-of-fi t was also evaluated using the Model Selection Criterion (MSC) [15], given are observed and calculated values of the i-th point, respectively, and wi is the weight that applies to the i-th point, n is number of points and p is number of parameters.

Results and Discussion
The amount of different excipients that could adsorb 145 mg VA was 30±2.58, 180±1.67, 260±2.55, 370±1.96, 600±2.33 and 760±2.68 mg for colloidal silicon dioxide, talc, microcrystalline cellulose, calcium carbonate, corn starch and dibasic calcium phosphate, respectively. Colloidal silicon dioxide demonstrated the good adsorbent for VA, because of its fine particle about 7-40 nm in size, anomalous surface area and high silanol groups on the surface particle [16]. The silanol groups of colloidal silicon dioxide should potentially form a network structure through interparticular hydrogen bonds between the carboxyl groups of VA. Such bonding between colloidal silicon dioxide and lipid has been previously mentioned [4].
Owing to high moisture absorption ability of sodium valproate [6], the humidity in the preparation room was controlled to be less than 50% RH. The Carr’s index of each granule formulation was in a range of 5-15, corresponding to the excellent fl owability. The angle of repose of each granule formulation was less than 25, indicating the excellent flowability, except the angle of repose of SR11 which was in the range of 25-30, corresponding to good flowability (data not shown).
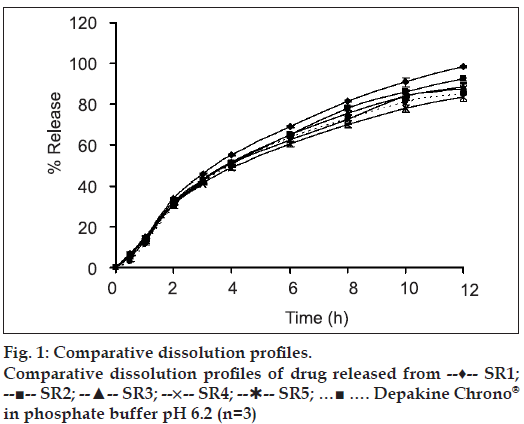
Weight, hardness and friability of each matrix tablet were carefully controlled in a range of conditions during the tablet preparation. The label amount of drug in the prepared tablets was varied from 98.48±1.55% to 106.8±2.45%. Effect of colloidal silicon dioxide on physical properties of matrix tablets was evident. The core matrix tablet of SR2 which contained 9% colloidal silicon dioxide exhibited good appearance, non-sticking and good compressibility. On the other hand, the core matrix tablet containing less than 9% colloidal silicon dioxide exhibited sticking tablets. As the concentration of Aerosil® 200 was increased from 7% to 20% by weight, the percent cumulative drug release was slightly decreased (fig. 1).
Silanol groups on the particle surface of Aerosil® 200 could interact via hydrogen bond with each other to form connecting bridge. The binding ability of colloidal silicon dioxide particles promoted the drug adsorption on the surfaces [17] and the drug release was retarded. The adsorption of ketoprofen to colloidal silicon dioxide and thereafter the retardation of drug release from gel system have been reported [18]. In addition, some investigators also reported the gelation properties of colloidal silicon dioxide [19]. Due to the –OH groups on the microparticle surface, Aerosil® 200 could form a great number of hydrogen bonds with dissolution medium. The gelation ability was greater when the concentration of Aerosil® 200 was increased, therefore the adsorbed VA could gradually diffuse from the gel layer to dissolution medium and the drug release was slightly prolonged.
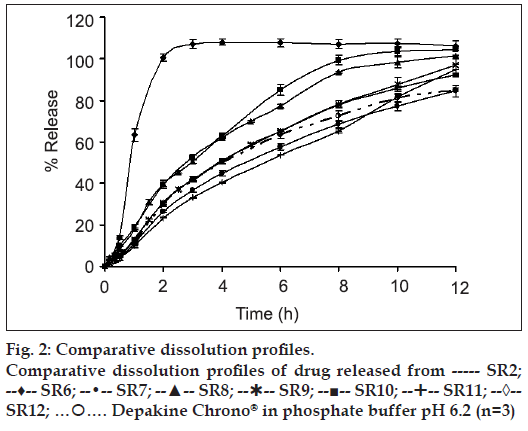
The HPMC-based hydrophilic matrix system could prolong the drug release. As the concentration of HPMC K15 M was increased from 0 % (SR 14) to 20 % (SR 11), the drug release rate was gradually decreased (fig. 2). After the core matrix tablet initially contacted with the dissolution medium (0.1 N HCl solution), VAS, which was a water soluble drug depositing on surface matrix tablet, could be rapidly dissolved and converted to VA. Then, water penetrated the matrix, leading to polymer swelling and drug dissolution. Therefore, the drug could gradually diffuse from the matrix. With a higher polymer concentration, the resultant gel layer would be more viscous [19] and the tightness of the swollen hydrogel network was increased [20]. Therefore, VA diffusion through a gel layer to a dissolution medium was decreased. A similar result was reported on the tetracycline hydrochloride released from hydrophilic matrix systems containing HPMC K4M [21].
Dissolution profiles of all developed matrix tablets were compared to that of the commercial product, Depakine Chrono®, as presented in figs. 1 and 2 and Table 2. The similarity factor (f2) values were found to be greater than 50 for most of the developed formula, except SR6, SR7, SR8 and SR12 containing 0%, 5%, 7.5% and 15% HPMC K 15 M by weight, respectively, and VAS 500 mg. Therefore, the dissolution profi les of SR6, SR7, SR8 and SR12 were different from that of Depakine Chrono®.
| Formula | Difference factor (f1) | Similarity factor (f2) |
|---|---|---|
| SR 1 | 13.31 | 56.75 |
| SR 2 | 5.82 | 71.20 |
| SR 3 | 4.79 | 79.05 |
| SR 4 | 4.36 | 79.92 |
| SR 5 | 3.66 | 81.71 |
| SR 6 | 87.51 | 16.44 |
| SR 7 | 31.51 | 38.61 |
| SR 8 | 25.40 | 43.67 |
| SR 9 | 6.88 | 64.72 |
| SR 10 | 6.81 | 70.31 |
| SR 11 | 12.45 | 57.25 |
| SR 12 | 39.04 | 34.14 |
Table 2: Difference factor (f1) and similarity factor (f2) of dissolution profiles for depakine chrono® and different core matrix tablets
Difference factor (f1) values were found to be less than 15 for most of the developed formulations, except SR6, SR7, SR8 and SR12 (Table 2). SR 6 released the drug rapidly since it lacked the swellable matrix agent. SR7 and SR8 contained low concentration of swellable matrix agent, therefore the low gel layer formation and gel strength promoted a rapid erosion of the matrix [21] resulting in a rapid diffusion of drug through the dissolution medium occurred. The drug release from SR12 was faster than SR10 and Depakine Chrono®. Since VAS is water soluble and is not adsorbed by Aerosil® 200, the conversion of VAS to VA could not promote the adsorption the employed Aerosil® 200. Therefore, VAS could convert to VA rapidly and diffuse through a gel layer to the dissolution medium.
The most suitable formulae were SR3 and SR10 since the difference factor (f1) values were 4.79 and 6.81, respectively, and the values of similarity factor (f2) were 79.05 and 70.31, respectively. These systems were chosen for scale up and film coating studies. The dissolution profi le of each scale-up core formulation was compared to that of pre-scale up core formulation. The physical properties of core matrix tablets after scale up were not different from the prescale up core tablets (data not shown).
SR3 was more suitable than SR4 and SR5 although the difference factor (f1) of SR3 was greater than that of SR4 and SR5 and the similarity factor (f2) of SR3 was less than that of SR4 and SR5. Because the amount of Aerosil® 200 for SR4 and SR5 was rather high and bulky, the tablet preparation was diffi cult. The dissolution profile of SR3 core after scale up was similar to that of the pre-scale up core. The drug release of SR10 core after scale up was slightly faster than that of the pre-scale up core.
Both scale-up cores (SR3 and SR10) demonstrated the dissolution profi les similar to Depakine Chrono® (Table 3). The values of difference factor (f1) were 6.53 and 2.37, and the values of similarity factor (f2) were 68.42 and 85.56, respectively, for SR3 and SR10. Drug release from the scale up SR3 core was faster than that of the scale up SR10 core. Since the content of swellable matrix agent of the scale up SR3 core was less than that of the scale up SR10 core, therefore, the diffusion path length for the drug diffusion of the former was shorter [22].
| Formula | Difference factor | Similarity factor |
|---|---|---|
| (f1) | (f2) | |
| SR 3 core scale up | 6.53 | 68.42 |
| SR 3 subcoat | 7.37 | 64.78 |
| SR 3 film 1 h | 11.72 | 56.62 |
| SR 3 film 2 h | 13.61 | 54.25 |
| SR 3 film 3 h | 13.32 | 54.23 |
| SR 3 film 4 h | 15.23 | 51.19 |
| SR 10 core scale up | 2.37 | 85.56 |
| SR 10 subcoat | 2.24 | 85.99 |
| SR 10 film 1 h | 6.23 | 72.19 |
| SR 10 film 2 h | 5.79 | 71.49 |
| SR 10 film 3 h | 6.74 | 69.30 |
| SR 10 film 4 h | 7.25 | 67.24 |
Table 3: Difference factor (f1) and similarity factor (f2) of dissolution profiles for depakine chrono® and different core after scale up and film coating
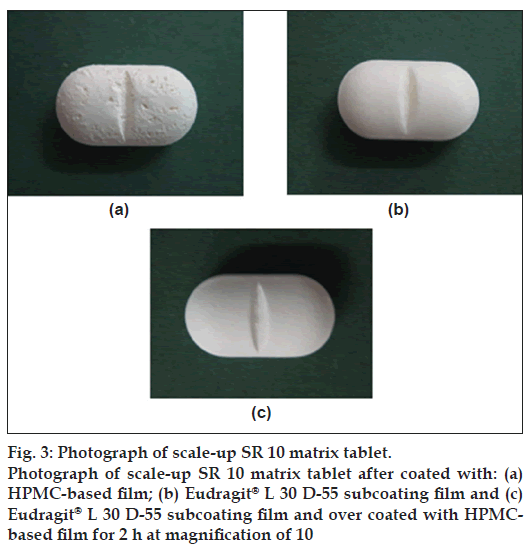
There was the cratering defect which exhibited on tablet surface after coating with HPMC (fig. 3a). Therefore, the Eudragit® L 30 D-55 subcoating of 0.5% by weight of total core tablet weight was performed by controlling the duration at 1 h of spraying. Triethyl citrate was added as a plasticizer at the concentration of 15% w/w of dry polymer weight. Subcoating with Eudragit® L 30 D-55, an aqueous acrylic coating dispersion, has been employed for soft gelatin capsule [23]. After the subcoating process, the subsequent HPMC-based film coating could be performed without the appearance of crater. Coated matrix exhibited the smooth and homogeneous fi lm after over coating with HPMC-based film.
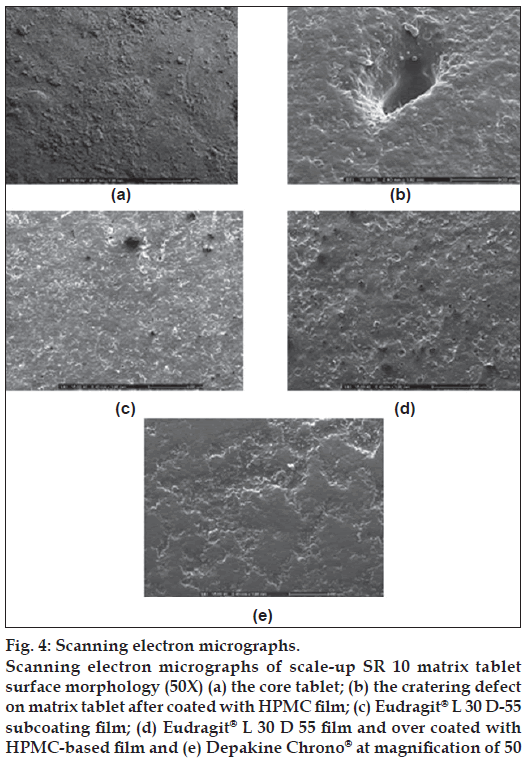
The cratering defect was evident when the directed spraying HPMC-based fi lm was used (fig. 4b). This defect of film coating was volcanic-like craters on tablet surface. Because the coating solution penetrated the surface of the tablet, often at the crown where the surface was more crater, the localized disintegration of the core and disruption of the coating was exhibited [24]. This might be in line with an essentiality of VA which could be very soluble in organic coating solution in HPMC-based fi lm. Therefore, a previous subcoating was applied to protect the penetration of HPMC-based coating solution into a core matrix tablet in this study. Figs. 3b and 4c present the smooth subcoating with Eudragit® L 30 D-55 and the lack of cratering defect. The surface of Depakine Chrono® fi lm (fig. 4e) was rather smooth and similar to that of the prepared matrix tablet as shown in fig. 4d. Scale-up core of SR10 matrix tablets were coated with Eudragit® L 30 D-55 and over coated with HPMC-based fi lm at different thicknesses by varying the spraying duration of HPMC solution (1, 2, 3 and 4 h). The thickness of fi lm was increased as the duration of fi lm coating was increased as presented in fig. 5a to 5e. The thin layer of Eudragit® L 30 D-55 subcoating was evident (fig. 5a). The fi lm thickness of Depakine Chrono® (fig. 5f) was comparable to that of the matrix tablet coated with Eudragit® L 30 D-55 and over coated with HPMC-based fi lm for 2 h (fig. 5c).
Figure 4: Scanning electron micrographs.
Scanning electron micrographs of scale-up SR 10 matrix tablet surface morphology (50X) (a) the core tablet; (b) the cratering defect on matrix tablet after coated with HPMC fi lm; (c) Eudragit® L 30 D-55 subcoating film; (d) Eudragit® L 30 D 55 fi lm and over coated with HPMC-based fi lm and (e) Depakine Chrono® at magnifi cation of 50
Figure 5: Scanning electron micrographs.
Scanning electron micrographs of cross-section of scale-up SR 10 matrix tablets after different coating (100X) (a) Eudragit® L 30 D-55 subcoating fi lm; (b) Eudragit® L 30 D-55 and over coated with HPMCbased
film for 1 h; (c) 2 h; (d) 3 h; (e) 4 h and (f) Depakine Chrono® at the magnifi cation of 100
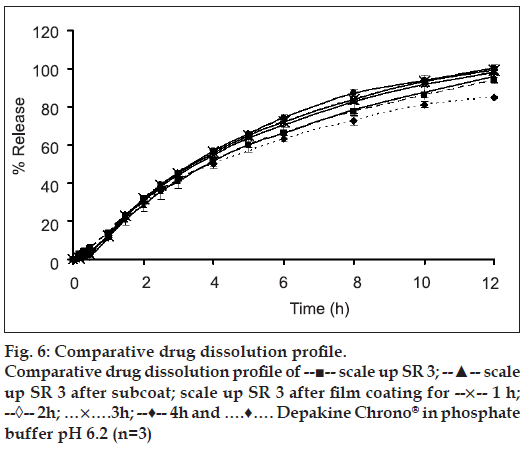
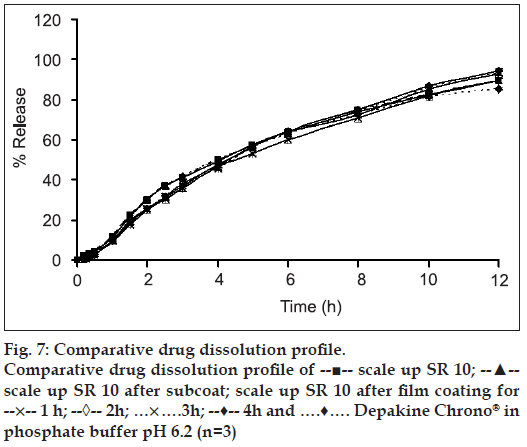
The slight lag time in release profi les (figs. 6 and 7) was the time required for the dissolution medium to diffuse through the coating layer and for the dissolved drug molecules to diffuse outward across film coating [25]. The subcoating with Eudragit® L 30 D-55 did not affect the drug release considering from drug release profi les of both the scale-up core tablets and the Eudragit® L 30 D-55 subcoating tablets. The drug release rates of over coated HPMC-based fi lm of SR3 were greater than that of SR10 due to the amount of HPMC K15M and microcrystalline cellulose in the core of each formula. SR10 contained rather high amount HPMC K15M, therefore the drug release was more retarded from the effect of added polymer. After the tablets contacted with the dissolution medium, water penetration between microcrystalline cellulose in the tablet and a local swelling occurred. Water was trapped in microcrystalline cellulose as a result of adsorption and capillary effects. Then, the crystalline framework burst and microcrystalline cellulose fragmented into smaller particles [26]. The amount of microcrystalline cellulose in SR3 formula was higher than that in SR10. Therefore, the drug release of SR3 was faster than SR10. The similarity factor (f2) was greater than 50 for all the fi lm coated formula and the difference factor (f1) was less than 15 for most of fi lm coated formula except SR3 fi lm coated at 4 h which was 15.23 indicating the different drug release profi les from Depakine Chrono® (Table 3).
There was a tendency of the slight increment of drug release rate as the over coated HPMC-based fi lm thickness was increased. It was possible that a drug or a core component migrated in or onto an applied film during coating. It has been reported earlier that coating conditions could affect the water penetration to the substrate during the coating process and subsequently the migration of water soluble components of the tablet core to the film coating. If components of the core migrate into the film layer during the early stages of the coating process, it could lead to heterogeneous film formation [27]. Drug migration into polymeric film coat has been previously reported [28,29]. Since spraying period was short, it might not completely cover the surface of the core matrix tablets. However, the slight increase in drug release from the tablet after over coating with HPMC-based film might be due to the property of VA which could be very soluble in organic coating solution in HPMC-based fi lm that supported the drug migration on polymer fi lm. Although the core matrix tablets were coated with Eudragit® L 30 D-55 which was generally used for enteric film coating, it was possible that the migrated drug could be released into 0.1 N hydrochloric acid.
From curve fitting, the drug release from tablets containing HPMC K 15 M was a diffusion control. The best fi t model was the Higuchi’s model since r2 and MSC from curve fi tting were apparently higher than those of the first order and zero order curve fi ttings (Table 4). The estimate parameters from curve fi tting to power law equation were presented in Table 5. The high value of a model selection criteria (MSC) indicated the high degree of goodness-of-fit with power law equation.
| Tablet | First order | Higuchi's | Zero order | |||
|---|---|---|---|---|---|---|
| r2 | MSC | r2 | MSC | r2 | MSC | |
| Depakinechrono | 0.9903 | 3.97 | 0.9986 | 5.88 | 0.9361 | 2.08 |
| SR 1 | 0.9936 | 4.25 | 0.9997 | 7.34 | 0.9519 | 2.23 |
| SR 2 | 0.9946 | 4.55 | 0.9993 | 6.55 | 0.9620 | 2.60 |
| SR 3 | 0.9945 | 4.54 | 0.9994 | 6.77 | 0.9480 | 2.29 |
| SR 4 | 0.9877 | 3.83 | 0.9973 | 5.33 | 0.9411 | 2.26 |
| SR 5 | 0.9910 | 4.14 | 0.9960 | 4.94 | 0.9342 | 2.15 |
| SR 6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SR 7 | 0.9920 | 3.83 | 0.9998 | 7.39 | 0.9788 | 2.85 |
| SR 8 | 0.9944 | 4.69 | 0.9987 | 6.15 | 0.9608 | 2.74 |
| SR 9 | 0.9550 | 2.6 | 0.9654 | 2.86 | 0.9911 | 4.23 |
| SR 10 | 0.9970 | 5.25 | 0.9992 | 6.58 | 0.9587 | 2.61 |
| SR 11 | 0.9989 | 6.03 | 0.9975 | 5.18 | 0.9920 | 4.03 |
| SR 12 | 0.9700 | 2.84 | 0.9967 | 5.05 | 0.9581 | 2.51 |
| SR 3 scale up core | 0.9967 | 5.26 | 0.9980 | 5.75 | 0.962 | 2.83 |
| SR 3 subcoating | 0.9906 | 4.23 | 0.9958 | 5.02 | 0.9637 | 2.87 |
| SR 3 film 1 h | 0.9853 | 3.72 | 0.9956 | 4.94 | 0.9714 | 3.05 |
| SR 3 film 2 h | 0.9846 | 3.68 | 0.9964 | 5.14 | 0.9702 | 3.01 |
| SR 3 film 3 h | 0.9810 | 3.46 | 0.9964 | 5.12 | 0.9680 | 2.94 |
| SR 3 film 4 h | 0.9707 | 3.03 | 0.9933 | 4.51 | 0.9724 | 3.09 |
| SR 10 scale up core | 0.9934 | 4.58 | 0.9995 | 7.17 | 0.9498 | 2.55 |
| SR 10 subcoating | 0.9930 | 4.52 | 0.9994 | 7.03 | 0.9494 | 2.54 |
| SR 10 film 1 h | 0.9954 | 4.87 | 0.9978 | 5.63 | 0.9714 | 3.05 |
| SR 10 film 2 h | 0.9902 | 4.18 | 0.9917 | 4.35 | 0.9594 | 2.76 |
| SR 10 film 3 h | 0.9924 | 4.38 | 0.9961 | 5.04 | 0.9792 | 3.37 |
| SR 10 film 4 h | 0.9894 | 4.05 | 0.9956 | 4.93 | 0.9766 | 3.25 |
Table 4: Comparison of degree of goodness-of-fit between different release models and dissolution data
| Tablet | k ± SD ×10-3 | tl ± SD (min) | n ± SD | MSC |
|---|---|---|---|---|
| Depakinechrono | 41.6111 ± 3.4633 | 51.42 ± 1.72 | 0.4731 ± 0.0144 | 6.29 |
| SR 1 | 42.5115 ± 2.1818 | 46.31 ± 1.14 | 0.4849 ± 0.0091 | 7.78 |
| SR 2 | 31.2783 ± 1.0793 | 44.72 ± 0.93 | 0.5287 ± 0.0058 | 8.50 |
| SR 3 | 42.1782 ± 0.8880 | 46.82 ± 0.55 | 0.4751 ± 0.0036 | 9.20 |
| SR 4 | 46.0594 ± 0.7651 | 47.56 ± 0.49 | 0.4480 ± 0.0027 | 9.36 |
| SR 5 | 49.4263 ± 2.5105 | 49.34 ± 1.37 | 0.4418 ± 0.0084 | 7.04 |
| SR 6 | n.d. | n.d. | n.d. | n.d. |
| SR 7 | 39.2524 ± 1.8993 | 39.94 ± 0.99 | 0.5246 ± 0.0089 | 9.11 |
| SR 8 | 50.2509 ± 4.1183 | 44.92 ± 2.20 | 0.4751 ± 0.0145 | 6.32 |
| SR 9 | 7.0828 ± 4.5132 | 18.44 ± 18.38 | 0.8026 ± 0.1042 | 4.27 |
| SR 10 | 28.1273 ± 1.3173 | 46.39 ± 1.38 | 0.5251 ± 0.0076 | 7.67 |
| SR 11 | 16.4512 ± 1.7424 | 38.13 ± 5.34 | 0.6042 ± 0.0165 | 8.18 |
| SR 12 | 69.7833 ± 11.2683 | 47.47 ± 3.17 | 0.4545 ± 0.0310 | 5.17 |
| SR 3 scale up core | 30.2013 ± 2.5710 | 44.58 ± 2.52 | 0.5360 ± 0.0144 | 6.35 |
| SR 3 subcoating | 26.7757 ± 2.4023 | 46.70 ± 2.47 | 0.5586 ± 0.0152 | 6.26 |
| SR 3 film 1 h | 27.0354 ± 2.4638 | 46.10 ± 2.16 | 0.5690 ± 0.0160 | 6.48 |
| SR 3 film 2 h | 28.7964 ± 2.3300 | 46.38 ± 1.91 | 0.5618 ± 0.0142 | 6.68 |
| SR 3 film 3 h | 29.2102 ± 2.3905 | 48.14 ± 1.83 | 0.5599 ± 0.0145 | 6.57 |
| SR 3 film 4 h | 25.5681 ± 1.7129 | 48.62 ± 1.45 | 0.5874 ± 0.0118 | 7.06 |
| SR 10 scale up core | 37.0145 ± 1.8662 | 49.76 ± 1.29 | 0.4955 ± 0.0087 | 6.99 |
| SR 10 subcoating | 36.8874 ± 1.9845 | 49.91 ± 1.37 | 0.4953 ± 0.0093 | 6.85 |
| SR 10 film 1 h | 28.7273 ± 4.5260 | 60.53 ± 5.78 | 0.5319 ± 0.0260 | 5.69 |
| SR 10 film 2 h | 23.9260 ± 4.2564 | 48.22 ± 4.65 | 0.5663 ± 0.0302 | 4.87 |
| SR 10 film 3 h | 23.3955 ± 4.0753 | 53.38 ± 6.66 | 0.5721 ± 0.0284 | 5.82 |
| SR 10 film 4 h | 24.4961 ± 4.8292 | 56.61 ± 7.33 | 0.5681 ± 0.0322 | 5.50 |
Table 5: Estimate Parameters from Curve Fitting with Power Law Equation
The values of exponent (n) for most of the formulations were shown in Table 5. For a matrix tablet, a cylindrical geometry was considered; n takes values in the range of 0.45-0.89 for anomalous transport [22]. The high water uptake, leading to higher swelling of the tablet, supported the anomalous release mechanism of VA. The n value of the optimized formulation (scale-up SR 10 core) was found to be 0.4955 while that of the marketed formulation was 0.4731, indicating the Fickian diffusion or nearly tended to Fickian diffusion (n=0.45). While the matrix tablet came into contact with a dissolution medium, the macromolecular chains of HPMC swelled at the tablet surface and formed a gel layer around a dry-like core. Drug diffusion occurred at the core-gel interface then through this gel [26]. The erosion of the swollen layer and the dissolution of the matrix itself were also observed. The drug release data were explored for the release mechanisms that followed. For the controlled or sustained release formulations, the diffusion, swelling and erosion were the three most important rate-controlling mechanisms. The drug release from the polymeric system was mostly occurred by diffusion and was best described by the Fickian diffusion. In conclusion, the VA and VAS sustained-release matrix tablets were prepared using HPMC as a matrix former which could prolong the drug release for 12 h. Aerosil® 200 effectively adsorbed oily VA and slightly infl uenced the drug release of the matrices. The drug release from optimized formulation followed the Higuchi’s kinetics while the mechanism of drug release was the Fickian diffusion, controlled by diffusion through a swollen matrix. Eudragit® L 30 D-55 was used as subcoating material for scale-up core matrix tablets before over coating with HPMC-based fi lm. The similarity factor (f2) and difference factor (f1) values of drug release profi le of scale-up SR10 after fi lm coating were greater than 50 and were less than 15, respectively, supporting the similar release to that of Depakine Chrono®.
Acknowledgements
This research work was kindly supported by Government-Industries Cooperation Project, Division of Research and Evaluation, Commission on Higher Education, Ministry of Education, Thailand and Faculty of Pharmacy, Silpakorn University. I would like to express my appreciation to Mrs. Kamnuam Kongsupalak, Dr. Wanchai Chongcharoen, Dr. Wisit Tangkeangsirisin and Dr. Nalinee Poolsup for their help and expertise.
References
- Conti S, Maggi L, Segale L, Machiste E, Conte U, Grenier P, etal. Matrices containing NaCMC and HPMC 2: Swelling and releasemechanism study. Int J Pharm 2007;333:143-51.
- Hardy IJ, Cook WG, Melia CD. Compression and compaction properties of plasticised high molecular weight hydroxypropylmethylcellulose (HPMC) as a hydrophilic matrix carrier. Int J Pharm 2006;311:26-32.
- Jonat S, Albers P, Gray A, Schmidt PC. Investigation of the glidant properties of compacted colloidal silicon dioxide by angle of repose and X-ray photoelectron spectroscopy. Eur J Pharm Biopharm 2006;63:356-9.
- Albertini B, Passerini N, Gonzalez-Rodrıguez ML, Perissutti B, Rodriguez L. Effect of Aerosil® on the properties of lipid controlled release microparticles. J Control Release 2004;100:233-46.
- Chang ZL. Sodium valproate and valproic acid. In: Florey K, editor. Analytical Profile of Drug Substance. Vol. 8. New York: Academic Press; 1979. p. 529-54.
- Trapani G, Cutrignelli A, Latrofa A, Franco M, Serra M, Pisu MG, etal. Valproic acid-hydrophilic cyclodextrin complexes and valproic acid-solid dispersions: Evaluation of their potential pharmaceutical use. Drug DevInd Pharm 2004;30:53-64.
- Lin MC, Kou K, Chen CC, Wu S-M, Wu H-L. Simple and sensitive fluorimetric liquid chromatography method for the determination of valproic acid in plasma. J Chromatogr B AnalytTechnol Biomed Life Sci 2004;810:169-72.
- Loscher W. Valproate: A reappraisal of its pharmacodynamic properties and mechanisms of action. ProgNeurobiol 1999;58:31-59.
- Doughty J, Baker GA, Jacoby A, Lavaud V. Compliance and satisfaction with switching from an immediate-release to sustained-release formulation of valproate in people with epilepsy. Epilepsy Behav 2003;4:710-6.
- Qui Y, Garren J, Samara E, Cao G, Abraham C, Cheskin HS, et al. Once-a-day controlled-release dosage form of divalproex sodium II: Development of a predictive in vitro drug release method. J Pharm Sci 2003;92:2317-25.
- Dutta S, Zhang Y, Conway JM, Sallee FR, Biton V, Reed MD, et al.Divalproex-ER pharmacokinetics in older children and adolescents. PediatrNeurol 2003;30:330-7.
- Fujisaki Y, Tsukune T, Funyu M, Okumura M, Ukigaya T, Sugibayash K. Development of sustained-release tablets containing sodium valproate: In vitro and in vivo correlation. Drug Develop Ind Pharm 2006;32:207-17.
- Bruce LD, Petereit HU, Beckert T, McGinity JW. Properties of enteric coated sodium valproate pellets. Int J Pharm 2003;246:85-96.
- Polli JE, Rekhi GS, Augsburger LL, Shah VP. Method to compare dissolution profiles and a rationale for wide dissolution specifications for metoprololtartate tablets. J Pharm Sci 1997;86:690-700.
- MicroMath Scientist Handbook Rev. 7EEF, MicroMath. Salt Lake City 1995. p. 467.
- Rowe RC, Sheskey PJ, Weller PJ. Handbook of Pharmaceutical Excipients. 4th ed. Washington Dc: APhA Publications; 2003. p. 161-3.
- Sherriff M, Enever RP. Rheological and drug release properties of oil gels containing colloidal silicon dioxide. J Pharm Sci 1979;68:842-5.
- Gallagher SJ, Trottet L, Heard CM. Ketoprofen: Release from, permeation across and rheology of simple gel formulation that simulate increasing dryness. Int J Pharm 2003;8:37-45.
- Heng PW, Chan LW, Easterbrook MG, Li X. Investigation of the influence of mean HPMC particle size and number of polymer particles on the release of aspirin from swellable hydrophilic matrix tablets. J Control Release 2001;76:39-49.
- Streubel A, Siepmann J, Bodmeier R. Floating matrix tablets based on low density foam powder: Effects of formulation and processing parameters on drug release. Eur J Pharm Sci 2003;18:37-45.
- Jamzad S, Tutunji L, Fassihi R. Analysis of macromolecular changes and drug release from hydrophilic matrix systems. Int J Pharm 2005;292:75-85.
- Chavanpatil MD, Jain P, Chaudhari S, Shear R, Vavia PR. Novel sustained release, swellable and bioadhesivegastroretentive drug delivery system for ofloxacin. Int J Pharm 2006;316:86-92.
- Felton LA, Haase MM, Shah NH, Zhang G, Infeld MH, Malick AW, etal. Physical and enteric properties of soft gelatin capsules coated withEudragit® L 30 D-55. Int J Pharm 1995;113:17-24.
- Rowe RC. Defects in aqueous film coated tablets, In: McGinity JW, editor. Drug and the pharmaceutical aqueous polymeric coatings for pharmaceutical dosage forms. 2nd ed. New York: Marcel Dekker, Inc; 1997. p. 419-28.
- Dashevsky A, Wagner K, Kolter A, Bodmeier R. Physicochemical and release properties of pellets coated with Kollicoat® SR 30 D, a new aqueous polyvinyl acetate dispersion for extended release. Int J Pharm 2005;290:15-23.
- Chambin O, Champion D, Debray C, Rochai-Gonthier MH, Le Meste M, Pourcelot Y. Effects of different cellulose derivatives on drug release mechanism studied at a preformulation stage. J Control Release 2004;95:101-8.
- Ruotsalainen M, Heinamaki J, Guo H, Laitinen N, Yliruusi J. A novel technique for imaging film coating defects in the film-core interface and surface of coated tablets. Eur J Pharm Biopharm 2003;56:381-8.
- Phaechamud T, Koizumi T, Ritthidej GC. Chitosan citrate as film former: Compatibility with water-soluble anionic dyes and drug dissolution from coated tablet. Int J Pharm 2000;198:97-111.
- Sauer D, Zheng W, Coots LB, McGinity JW. Influence of processing parameters and formulation factors on the drug release from tablets powder-coated Eudragit L 100-55. Eur J Pharm Biopharm 2007;67:464-75.