- *Corresponding Author:
- Liya Jiang
Department of Anesthesiology, Chunan County Hospital of Traditional Chinese Medicine, Chunan, Zhejiang 311700, China
E-mail: m15923026913_1@163.com
| Date of Received | 15 January 2022 |
| Date of Revision | 24 October 2022 |
| Date of Acceptance | 05 September 2023 |
| Indian J Pharm Sci 2023;85(5):1309-1320 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Propofol, an anesthetic agent with known anticancer properties in various tumor types, holds promise as a potential therapeutic option for glioma. However, the specific functions and underlying mechanisms of propofol in glioma tumorigenesis remain poorly understood. We collected twenty-one glioma tissue samples for analysis. In vitro experiments, including 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide, flow cytometry, Western blot, transwell and wound healing assays, were performed to assess the effects of propofol on glioma cells. We also examined the expression levels of long noncoding ribonucleic acid SET binding factor 2-antisense strand 1, microRNA-373-3p, and pyruvate dehydrogenase kinase 3 through quantitative reverse transcription-polymerase chain reaction and Western blotting. Propofol demonstrated inhibitory effects on glioma cell viability by promoting cell apoptosis and inducing cell cycle arrest. Additionally, propofol suppressed the migratory and invasive capacities of glioma cells. We observed an increased expression of SET binding factor 2-antisense strand 1 in glioma, which was attenuated upon propofol treatment. Furthermore, overexpression of SET binding factor 2-antisense strand 1 weakened the inhibitory effects of propofol on glioma progression. MicroRNA-373-3p was identified as a target of SET binding factor 2-antisense strand 1, and its up-regulation counteracted the functional impact of SET binding factor 2-antisense strand 1 in glioma cells treated with propofol. Additionally, we found that microRNA-373-3p targeted pyruvate dehydrogenase kinase 3 and knockdown of microRNA-373-3p reversed propofol-mediated suppression of glioma progression by increasing pyruvate dehydrogenase kinase 3 expression. Our findings suggest that propofol exerts inhibitory effects on glioma cell oncogenic phenotypes through the SET binding factor 2-antisense strand 1/microRNA- 373-3p/pyruvate dehydrogenase kinase 3 axis. This study sheds light on the potential application of propofol in glioma prevention and provides valuable insights into the molecular mechanisms underlying its antitumor properties in this challenging malignancy.
Keywords
Curcumin, microRNA-145-5p, ischemia/reperfusion, inflammatory response, oxidative stress, interleukin
Glioma, the most common and aggressive primary brain tumor, poses a formidable challenge to modern medicine due to its high morbidity and mortality rates[1]. Despite extensive research efforts, effective therapeutic strategies for glioma remain limited, emphasizing the urgent need to explore novel and innovative therapeutic avenues. Recently, there has been growing interest in repurposing existing drugs for cancer treatment, given their established safety profiles and welldocumented pharmacological properties[2,3].
One such candidate is propofol, a widely used intravenous anesthetic, which has demonstrated potential anticancer properties in various tumor types[4].
Emerging evidence suggests that propofol exhibits remarkable antitumor effects in several cancer models by influencing critical cellular pathways, including proliferation, migration, invasion and apoptosis[5,6]. Propofol could induce cytotoxicity to play an inhibitive role in glioma[6]. More specifically, the previous study suggests that propofol was able to damage cell proliferative and invasive capacities in glioma[7]. Nevertheless, the mechanism underlying propofol in glioma progression needs more exploration.
Propofol could induce the long noncoding RNAs (lncRNAs) dysregulation to implicate the treatment of tumors[8]. lncRNAs are the RNA transcripts with a length of >200 nucleotides, which have essential roles in glioma by stimulating messenger Ribonucleic Acid (mRNA) expression via acting as the ceRNAs for microRNAs (miRNAs)[9]. The lncRNA SET Binding Factor 2-Antisense Strand 1 (SBF2-AS1) plays a promoting role in multiple tumors, like colorectal, live, and pancreatic cancers[10-12]. Moreover, SBF2-AS1 could promote the development and drug resistance in glioma cells[13,14]. However, how and whether SBF2-AS1 could be implicated in the mechanism mediated by propofol remains unclear.
miR-373-3p was discovered to function in different tumors, such as prostate, tongue squamous cell and pancreatic cancers[15-17]. Previous studies have suggested that low levels of miR-373-3p indicated the poor outcome of glioma and it could inhibit the malignancy of glioma[18,19]. The Pyruvate Dehydrogenase Kinase (PDK) takes part in the development of tumors[20]. PDK3 belongs to the PDK family, and the inhibition of PDK3 could induce cell death of glioma cells[21]. Moreover, PDK3 promoted the survival and mobility of glioma cells[22]. Interestingly, the star base and TargetScan exhibited the complementary sites between SBF2- AS1 or PDK3 and miR-373-3p. Thus, we assumed there might be a SBF2-AS1/miR-373-3p/PDK3 axis in glioma.
However, the precise molecular mechanisms underlying these effects in glioma cells have not been fully elucidated. To address this gap in knowledge, we investigated the potential involvement of noncoding RNAs (ncRNAs), specifically the lncRNA SBF2-AS1 and miRNA, miR-373-3p, in mediating propofol inhibitory actions on glioma oncogenic phenotypes. In this research article, we present compelling experimental data that highlight the regulatory role of the SBF2-AS1/miR-373-3p axis in glioma progression and how propofol modulates this axis to exert its antitumor effects. Our findings provide new insights into the molecular mechanisms by which propofol can potentially serve as an effective therapeutic option for glioma, complementing existing treatment modalities.
The objectives of this study were threefold; to evaluate the expression levels of SBF2-AS1 and miR-373-3p in glioma tissues and their correlation with clinic pathological features, to investigate the impact of propofol treatment on glioma cell proliferation, migration, invasion, and apoptosis, and to delineate the regulatory interactions between SBF2-AS1, miR-373-3p, and their downstream target gene PDK3 in the context of propofolmediated glioma inhibition.
Materials and Methods
Clinical samples:
The glioma tissues and normal brain tissues were harvested via surgical resection and stored at -80° which were recruited from Chun'an County Hospital of Traditional Chinese Medicine. We have got the permission from the Ethics Committee of Chun'an County Hospital of Traditional Chinese Medicine.
Cell culture and exposure to propofol:
Human NHA astrocytes and glioma cell lines (A-172 and LN-229) (Bena Culture Collection, Beijing, China) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Thermo Fisher, Wilmington, Delaware, United States of America (USA)) containing 100 U/ml of antibiotics and 10 % Fetal Bovine Serum (FBS) (Thermo Fisher) at 37° in 5 % Carbon dioxide (CO2). Cells were treated with 0, 5 and 10 μg/ml of propofol for the indicated time for functional analysis.
Cell transfection:
SBF2-AS1 overexpression vector was constructed using pcDNA3.1 vector (Thermo Fisher) with empty pcDNA3.1 vector as the negative control (vector). The PDK3 siRNA (si-PDK3), miR-373- 3p mimic, inhibitor (anti-miR-373-3p,) and the controls (si-NC, miR-NC, or anti-miR-NC) were synthesized by Genomeditech (Shanghai, China). Then, 1 μg of vectors or 40 nm of oligonucleotides was used with the Lipofectamine™ 2000 (Thermo Fisher). 24 h later, cells were harvested for propofol treatment.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR):
As per the instructions of Trizol kit (Solarbio, Beijing, China) and TaqMan complementary Deoxyribonucleic Acid (cDNA) synthesis kit (Thermo Fisher), total RNA was isolated for reverse transcription, followed by qRT-PCR on an ABI PRISM 7500 system with Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) and U6 as endogenous references. The 2-ΔΔCt method was applied for the calculation of molecule expression[23],
Western blot:
The total protein was extracted via 1 % protease inhibitor-supplemented Radioimmunoprecipitation Assay (RIPA) lysis buffer (Thermo Fisher). Following the detection of protein concentration via the Bicinchoninic Acid (BCA) method, immunoblotting was conducted as described before[24]. The antibodies included; the primary antibodies anti-Cyclin Dependent Kinase (CDK)- 2 (ab235941, 1:3000), anti-CDK4 (ab137675, 1:1000), anti-Matrix Metalloproteinase (MMP)- 2 (ab97779, 1:2000), anti-MMP9 (ab137867, 1:2000 dilution), anti-PDK3 (ab154549, 1:3000), anti-GAPDH (ab9485, 1:2000) and secondary antibodies Immunoglobulin G (IgG) labeled via horseradish peroxidase (ab205718, 1:10000) were provided via Abcam (Cambridge, United Kingdom (UK)). The BeyoECL Plus kit (Beyotime, Shanghai, China) was used for visualizing protein signaling.
3-[4,5-Dimethylthiazol-2-yl]-2,5-Diphenyl Tetrazolium Bromide (MTT) assay:
A-172 and LN-229 cells (1×104 cells per well) were incubated with propofol for 48 h in the 96- well plates. Next, the culture medium was discarded, and replaced with 200 μl fresh culture medium, followed by adding with 20 μl MTT solution (Solarbio) in each well. After 4 h, flipping the board over and snapping it upside down, and gently deducting the supernatant culture in the well. After 150 μl Dimethyl Sulfoxide (DMSO) reaction, the absorbance was examined at 570 nm.
Flow cytometry:
After the treatment of propofol for 48 h, A-172 and LN-229 cells were (1×106 cells) fixed with 75 % ethanol, followed by 30 min incubation with RNase A (1 mg/ml) and Propidium Iodide (PI) (20 μg/ml) (Solarbio). Cells distribution was analyzed via a flow cytometer (Agilent, Hangzhou, China).
For cell apoptosis analysis, cell in different group were suspended in 400 μl of Membrane Linker V Binding Buffer, then an Annexin V-Fluorescein Isothiocyanate (FITC)/PI apoptosis detection kit (Solarbio) was exploited to stain with 1×105 A-172 and LN-229 cells, then apoptotic rate was tested via a flow cytometer.
Wound healing assay:
When the glioma cells in 6-well plates cultured until 80 % confluence, a straight scratch was made by a 200 μl pipette tip. Following 24 h culture, the wound was imaged at 0 h and 24 h (100×), and cell migratory ability was tested in accordance to the wound closure rate.
Transwell assay:
About 1×105 A-172 and LN-229 cells in serum- free DMEM were put into the upper chambers of Matrigel-coated transwell chambers (Costar, Corning, New York, USA) with 500 μl 10 % serum- supplemented medium in the lower chambers. 24 h later, invasive cells were texted via a microscope with three random fields after crystal violet staining.
Dual-luciferase reporter assay:
The targets were searched via Starbase or Target- Scan. Wild Type (WT) or Mutant (MUT) SBF2- AS1 and PDK3 3′ Untranslated Regions (3′ UTRs) were built using psiCHECK-2 vector. Then the constructs (SBF2-AS1 WT/MUT or PDK3 3’UTR WT/MUT) with miR-NC or miR-373-3p were co-transfected into A-172 and LN-229 cells, and the binding intensity was examined 48 h later.
Statistical analysis:
The data were expressed as mean±standard deviation. Student’s t-tests or one-way Analysis of Variance (ANOVA) was used to compare differences. The difference was statistically significant when p<0.05.
Results and Discussion
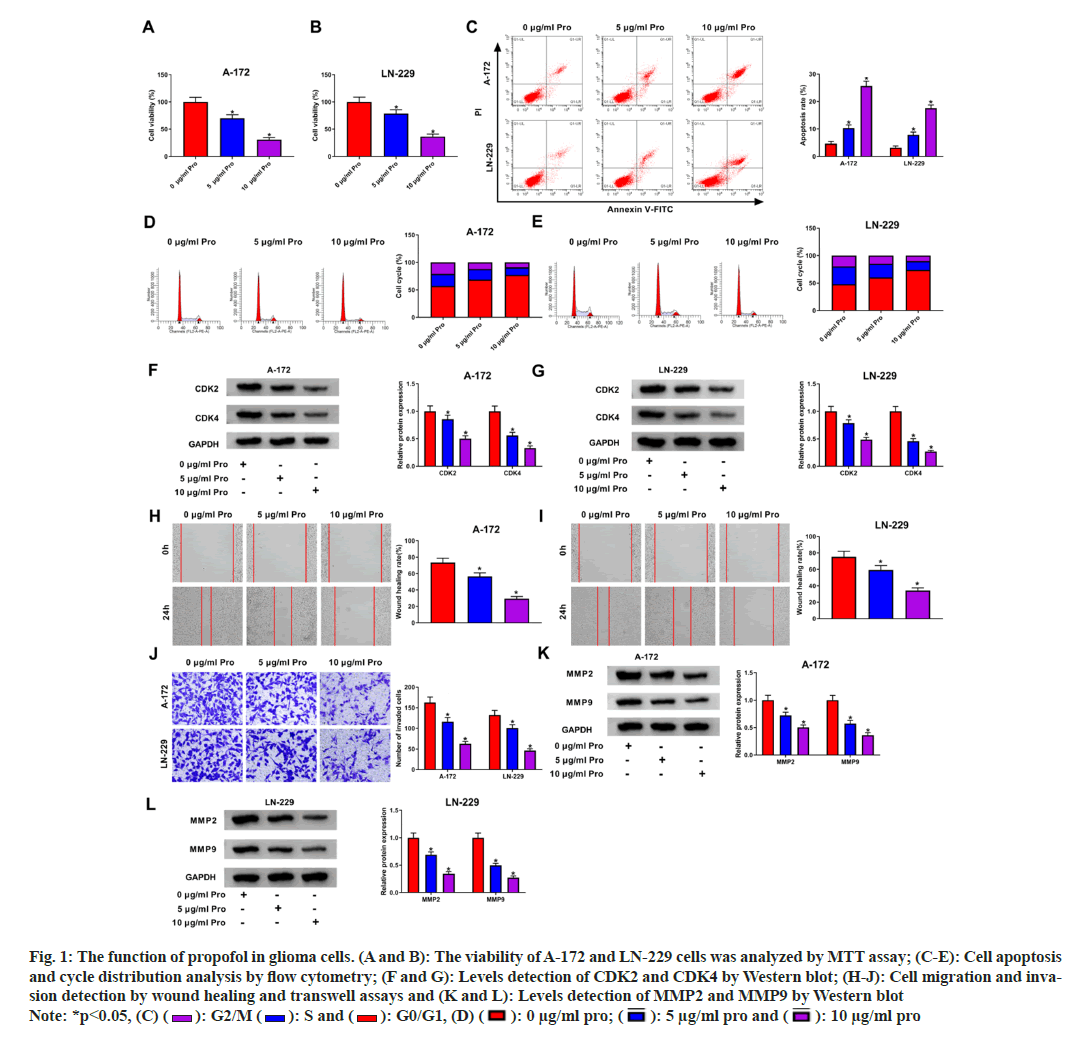
Fig. 1A and fig. 1B illustrate the concentration Fig. 1A and fig. 1B illustrate the concentration dependent inhibitory effects of propofol on the viability of A-172 and LN-229 cells. Moreover, treatment with increasing concentrations of propofol induced a significant rise in apoptosis rates, as depicted in fig. 1C. Additionally, propofol treatment led to cell cycle arrest at the G0/G1 phase, as evidenced by the data presented in fig. 1D and fig. 1E. Furthermore, the levels of cell cycle proteins CDK2 and CDK4 were markedly reduced upon propofol treatment, as shown in fig. 1F and fig. 1G. Remarkably, a concentration-dependent decrease in the migratory and invasive capabilities of A-172 and LN-229 cells was observed following exposure to different concentrations of propofol, as represented in fig. 1H-fig. 1J. Concomitantly, propofol exposure led to reduced protein levels of MMP2 and MMP9, as depicted in fig. 1K and fig. 1L.
Fig. 1: The function of propofol in glioma cells. (A and B): The viability of A-172 and LN-229 cells was analyzed by MTT assay; (C-E): Cell apoptosis and cycle distribution analysis by flow cytometry; (F and G): Levels detection of CDK2 and CDK4 by Western blot; (H-J): Cell migration and invasion detection by wound healing and transwell assays and (K and L): Levels detection of MMP2 and MMP9 by Western blot Note: *p<0.05, (C) ( ): G2/M (
): G2/M ( ): S and (
): S and ( ): G0/G1, (D) (
): G0/G1, (D) ( ): 0 μg/ml pro; (
): 0 μg/ml pro; ( ): 50 μg/ml pro and (
): 50 μg/ml pro and ( ): 10 μg/ml pro
): 10 μg/ml pro
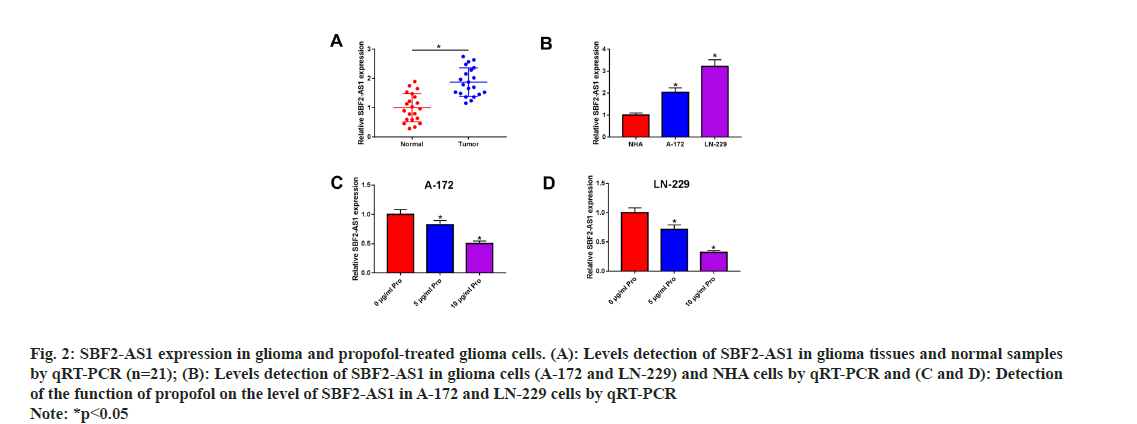
Subsequently, the response of SBF2-AS1 to propofol was examined. Fig. 2A illustrates that SBF2-AS1 expression was notably elevated in glioma tissues compared to normal tissues (n=21). Furthermore, A-172 and LN-229 cells exhibited significantly higher levels of SBF2-AS1 compared to NHA cells, as depicted in fig. 2B. To investigate the effect of propofol on SBF2-AS1 expression, we conducted dose-dependent experiments in A-172 and LN-229 cells. As presented in fig. 2C and fig. 2D, propofol treatment resulted in a dosedependent reduction in SBF2-AS1 levels. These findings suggest a potential association between SBF2-AS1 and the propofol-mediated inhibition of glioma development.
Fig. 2: SBF2-AS1 expression in glioma and propofol-treated glioma cells. (A): Levels detection of SBF2-AS1 in glioma tissues and normal samples by qRT-PCR (n=21); (B): Levels detection of SBF2-AS1 in glioma cells (A-172 and LN-229) and NHA cells by qRT-PCR and (C and D): Detection of the function of propofol on the level of SBF2-AS1 in A-172 and LN-229 cells by qRT-PCR Note: *p<0.05
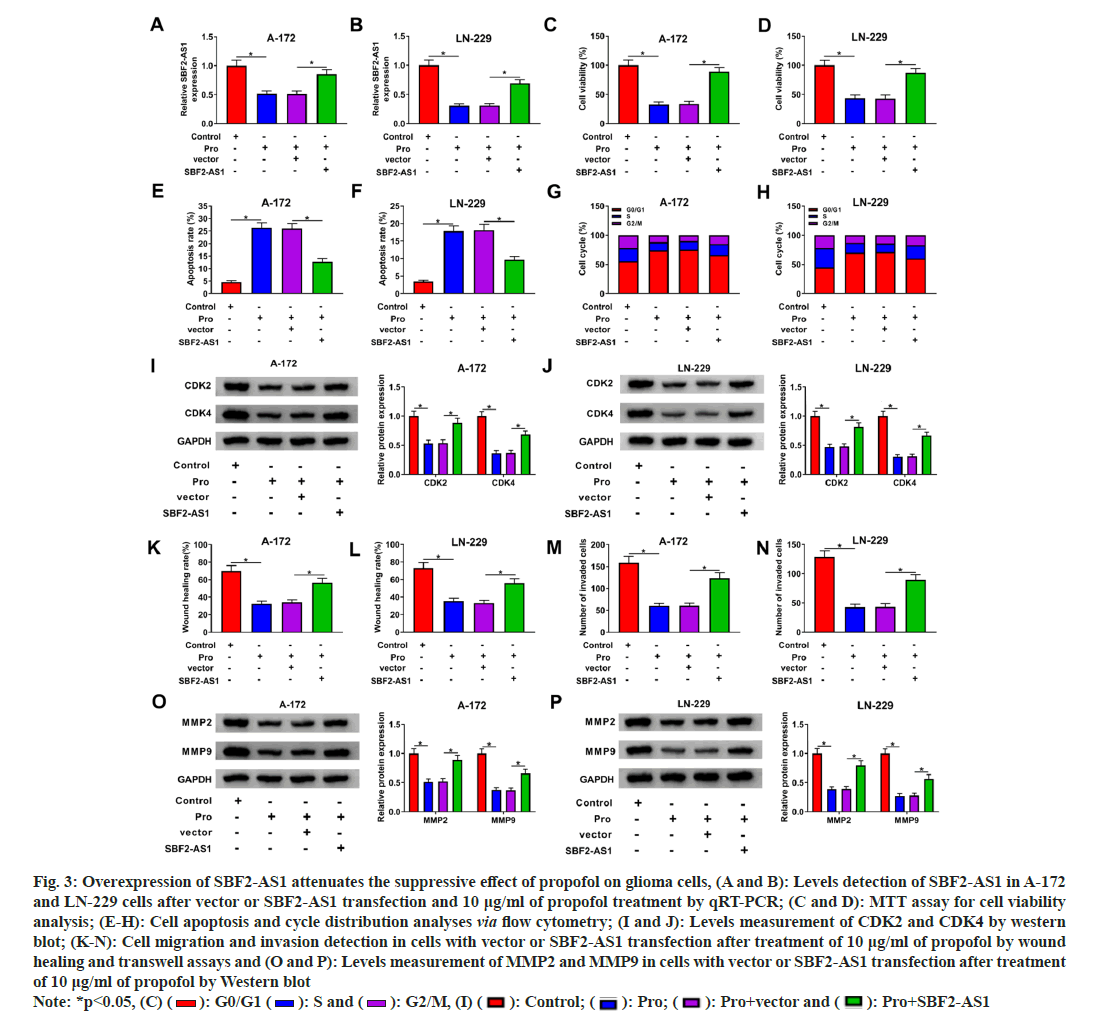
Later, we investigated the role of SBF2-AS1 in propofol-mediated anti-glioma effects. Glioma cells were transfected with SBF2-AS1 overexpression vector or control vector, followed by propofol treatment. Propofol exposure led to a significant decrease in SBF2-AS1 levels in A-172 and LN-229 cells, while transfection with the SBF2- AS1 overexpression vector effectively restored SBF2-AS1 abundance in the presence of propofol (fig. 3A and fig. 3B). MTT assays demonstrated that propofol-challenged glioma cells exhibited significantly reduced viability after SBF2-AS1 transfection (fig. 3C and fig. 3D). Moreover, overexpression of SBF2-AS1 provided protection against propofol-induced apoptosis in A-172 and LN-229 cells (fig. 3E and fig. 3F). Additionally, propofol-induced cell cycle arrest at the G0/G1 phase was weakened upon overexpression of SBF2-AS1 (fig. 3G and fig. 3H).
Fig. 3: Overexpression of SBF2-AS1 attenuates the suppressive effect of propofol on glioma cells, (A and B): Levels detection of SBF2-AS1 in A-172 and LN-229 cells after vector or SBF2-AS1 transfection and 10 μg/ml of propofol treatment by qRT-PCR; (C and D): MTT assay for cell viability analysis; (E-H): Cell apoptosis and cycle distribution analyses via flow cytometry; (I and J): Levels measurement of CDK2 and CDK4 by western blot; (K-N): Cell migration and invasion detection in cells with vector or SBF2-AS1 transfection after treatment of 10 μg/ml of propofol by wound healing and transwell assays and (O and P): Levels measurement of MMP2 and MMP9 in cells with vector or SBF2-AS1 transfection after treatment of 10 μg/ml of propofol by Western blot Note: *p<0.05, (C) ( ): G0/G1 (
): G0/G1 ( ): S and (
): S and ( ): G2/M, (I) (
): G2/M, (I) ( ): Control; (
): Control; ( ): Pro; (
): Pro; ( ): Pro+vector and (
): Pro+vector and ( ): Pro+SBF2-AS1
): Pro+SBF2-AS1
Furthermore, Western blot analysis revealed that restoration of SBF2-AS1 attenuated the suppressive effects of propofol on CDK2 and CDK4 protein levels (fig. 3I and fig. 3J). Moreover, upregulation of SBF2-AS1 rescued the migratory and invasive capabilities of A-172 and LN-229 cells following propofol treatment (fig. 3K-fig. 3N). Additionally, the increased expression of SBF2- AS1 counteracted propofol-mediated suppression of MMP2 and MMP9 protein levels (fig. 3O and fig. 3P).
Collectively, these results suggest that SBF2- AS1 plays a crucial role in the propofol-mediated anti-glioma effects, influencing cell viability, apoptosis, cell cycle progression, migration, and invasion in glioma cells.
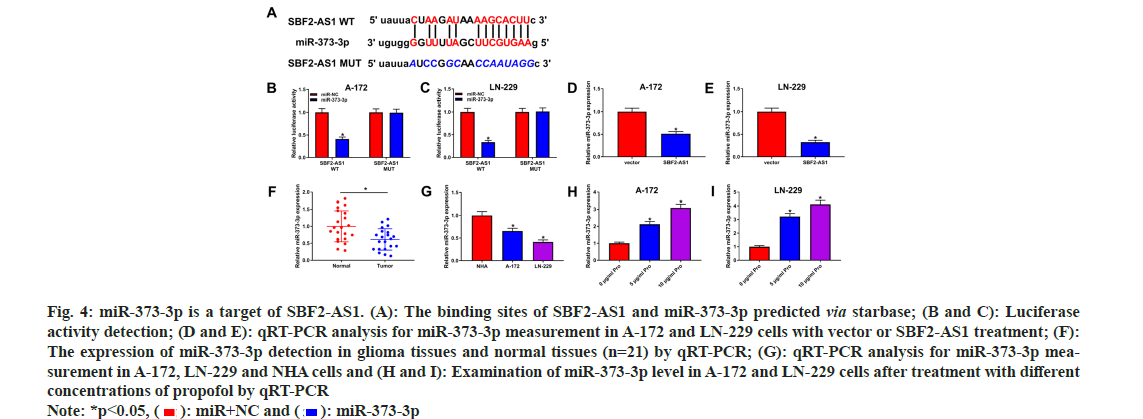
lncRNA have been identified as functional decoys for miRNAs. To explore the potential targeted relationship between SBF2-AS1 and miR-373- 3p, StarBase analysis was performed, revealing a predicted interaction (fig. 4A). Subsequently, a dual-luciferase reporter assay confirmed this interaction, showing that introduction of the miR- 373-3p mimic led to a significant reduction of luciferase activity in the SBF2-AS1 WT group, while no effect was observed in the SBF2-AS1 MUT group (fig. 4B and fig. 4C).
Fig. 4: miR-373-3p is a target of SBF2-AS1. (A): The binding sites of SBF2-AS1 and miR-373-3p predicted via starbase; (B and C): Luciferase activity detection; (D and E): qRT-PCR analysis for miR-373-3p measurement in A-172 and LN-229 cells with vector or SBF2-AS1 treatment; (F): The expression of miR-373-3p detection in glioma tissues and normal tissues (n=21) by qRT-PCR; (G): qRT-PCR analysis for miR-373-3p measurement in A-172, LN-229 and NHA cells and (H and I): Examination of miR-373-3p level in A-172 and LN-229 cells after treatment with different concentrations of propofol by qRT-PCR Note: *p<0.05, ( ): miR+NC and (
): miR+NC and ( ): miR-373-3p
): miR-373-3p
Furthermore, overexpression of SBF2-AS1 resulted in a significant decrease in miR-373-3p levels (fig. 4D and fig. 4E). Consistently, both glioma tissues and cells exhibited decreased levels of miR-373-3p (fig. 4F and fig. 4G). Strikingly, propofol treatment prominently increased the abundance of miR-373-3p in A-172 and LN-229 cells (fig. 4H and fig. 4I). These findings suggest a direct regulatory association between SBF2- AS1 and miR-373-3p and indicate that propofol treatment may influence miR-373-3p expression levels in glioma cells.
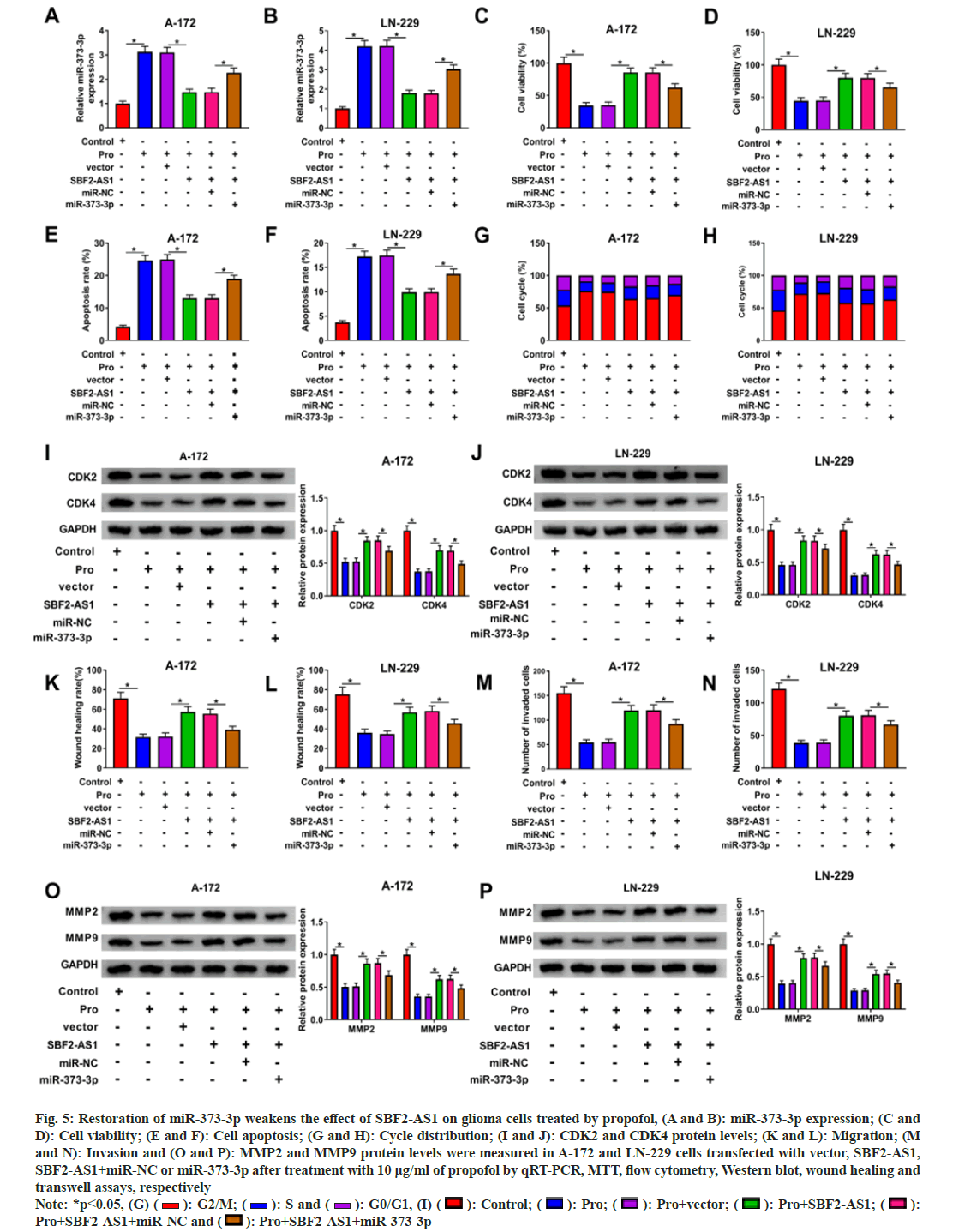
To investigate whether the functional impact of SBF2-AS1 relied on miR-373-3p, we conducted rescue analyses. In the presence of propofol, SBF2- AS1 over expression led to a notable reduction in miR-373-3p levels, which were subsequently restored by the introduction of the miR-373-3p mimic (fig. 5A and fig. 5B).
Fig. 5: Restoration of miR-373-3p weakens the effect of SBF2-AS1 on glioma cells treated by propofol, (A and B): miR-373-3p expression; (C and D): Cell viability; (E and F): Cell apoptosis; (G and H): Cycle distribution; (I and J): CDK2 and CDK4 protein levels; (K and L): Migration; (M and N): Invasion and (O and P): MMP2 and MMP9 protein levels were measured in A-172 and LN-229 cells transfected with vector, SBF2-AS1, SBF2-AS1+miR-NC or miR-373-3p after treatment with 10 μg/ml of propofol by qRT-PCR, MTT, flow cytometry, Western blot, wound healing and transwell assays, respectively Note: *p<0.05, (G) ( ): G2/M; (
): G2/M; ( ): S and (
): S and ( ): G0/G1, (I) (
): G0/G1, (I) ( ): Control; (
): Control; ( ): Pro; (
): Pro; ( ): Pro+vector; (
): Pro+vector; ( ): Pro+SBF2-AS1; (
): Pro+SBF2-AS1; ( ): Pro+SBF2-AS1+miR-NC and (
): Pro+SBF2-AS1+miR-NC and ( ): Pro+SBF2-AS1+miR-373-3p
): Pro+SBF2-AS1+miR-373-3p
Functional assays revealed that the up-regulation of miR-373-3p counteracted SBF2-AS1-mediated promotion of cell viability and inhibition of apoptosis in propofol-treated A-172 and LN-229 cells (fig. 5C-fig. 5F). Moreover, overexpression of miR-373-3p increased the proportion of cells arrested at the G0/G1 phase in propofol-treated cells with SBF2-AS1 overexpression (fig. 5G and fig. 5H). Furthermore, miR-373-3p overexpression attenuated SBF2-AS1-induced upregulation of CDK2 and CDK4 expression in A-172 and LN-229 cells treated with propofol (fig. 5I and fig. 5J).
Additionally, the significant increase in the migratory and invasive capabilities of propofoltreated A-172 and LN-229 cells, mediated by SBF2- AS1 overexpression, was mitigated upon miR-373- 3p overexpression (fig. 5K-fig. 5N). Moreover, the MMP2 and MMP9 proteins upregulated by SBF2- AS1 were significantly reduced by miR-373-3p overexpression in propofol-treated A-172 and LN- 229 cells (fig. 5O and fig. 5P).
These rescue analysis suggest that the functional role of SBF2-AS1 in glioma cells is dependent on miR-373-3p and highlight the regulatory interplay between SBF2-AS1 and miR-373-3p in the context of propofol treatment.
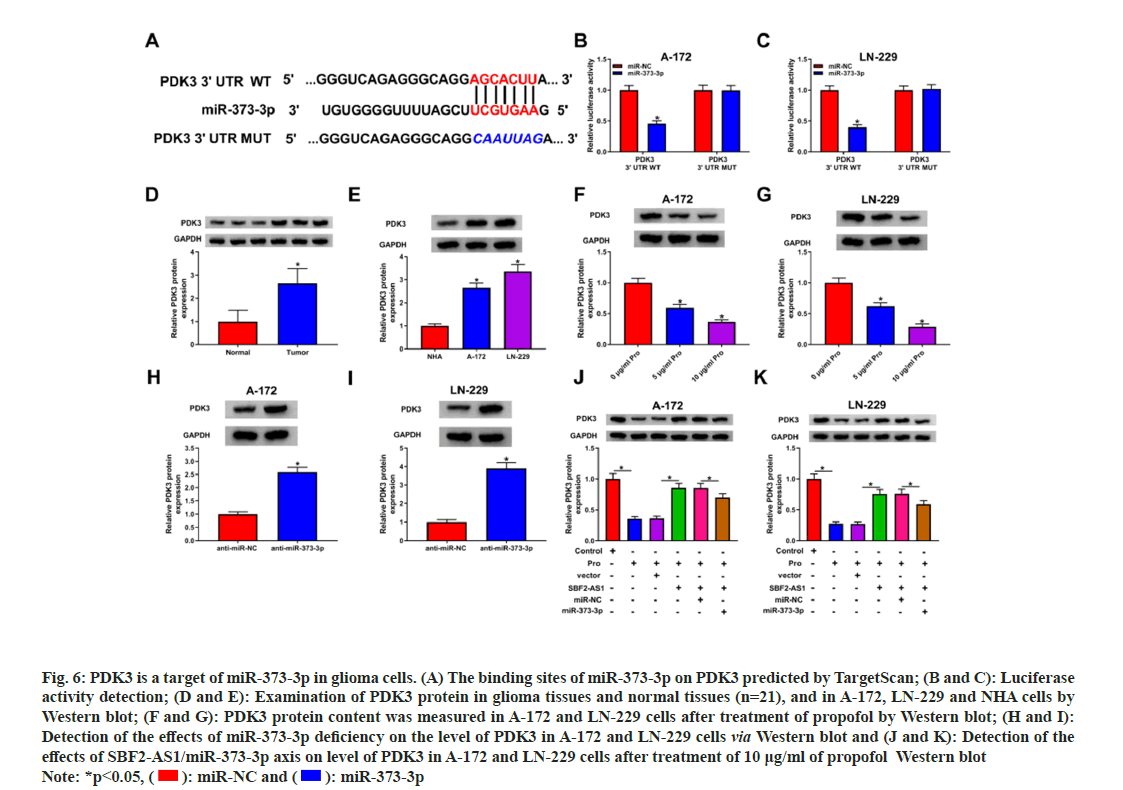
Subsequently, we utilized TargetScan to predict the complementary sequences of miR-373-3p on the PDK3 gene (fig. 6A). The dual-luciferase reporter assay in glioma cells with PDK3 3’UTR WT revealed decreased luciferase activity upon the addition of the miR-373-3p mimic (fig. 6B and fig. 6C). Glioma tissues and cells exhibited higher protein expression levels of PDK3 (fig. 6D and fig. 6E). However, exposure to propofol significantly reduced the protein level of PDK3 in glioma cells (fig. 6F and fig. 6G). Moreover, knockdown of miR-373-3p notably promoted PDK3 protein expression (fig. 6H and fig. 6I). Furthermore, the abundance of PDK3 protein was significantly elevated after overexpressing SBF2-AS1 in propofol-treated glioma cells. However, this effect was attenuated after the addition of miR-373-3p (fig. 6J and fig. 6K). These findings suggest that SBF2-AS1 can enhance PDK3 expression through the action of miR-373-3p.
Fig. 6: PDK3 is a target of miR-373-3p in glioma cells. (A) The binding sites of miR-373-3p on PDK3 predicted by TargetScan; (B and C): Luciferase activity detection; (D and E): Examination of PDK3 protein in glioma tissues and normal tissues (n=21), and in A-172, LN-229 and NHA cells by Western blot; (F and G): PDK3 protein content was measured in A-172 and LN-229 cells after treatment of propofol by Western blot; (H and I): Detection of the effects of miR-373-3p deficiency on the level of PDK3 in A-172 and LN-229 cells via Western blot and (J and K): Detection of the effects of SBF2-AS1/miR-373-3p axis on level of PDK3 in A-172 and LN-229 cells after treatment of 10 μg/ml of propofol Western blot Note: *p<0.05, ( ): miR-NC and (
): miR-NC and ( ): miR-373-3p
): miR-373-3p
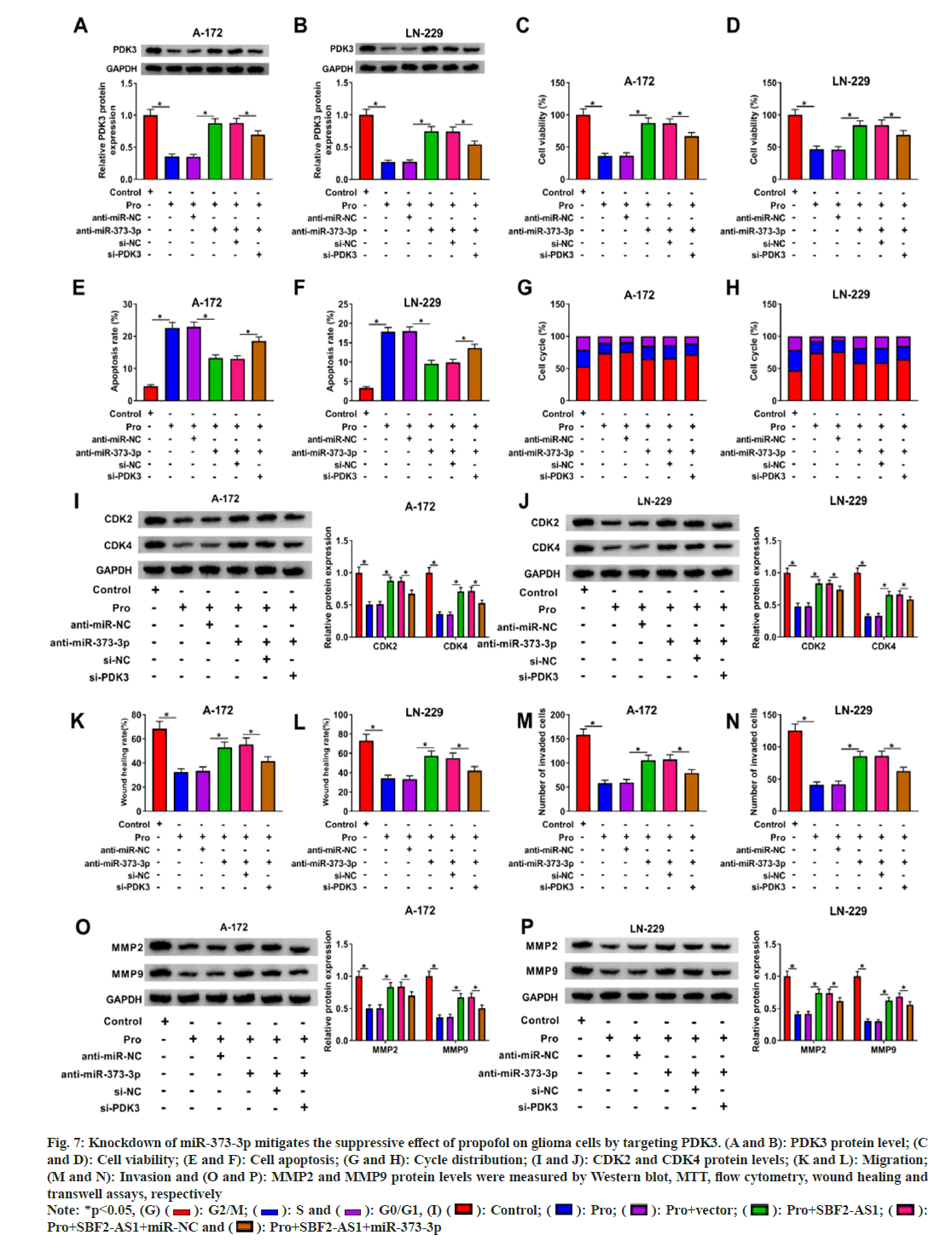
Later, we investigated the functional role of miR-373-3p in glioma through its interaction with PDK3. In propofol-treated A-172 and LN- 229 cells, knockdown of miR-373-3p led to a significant up-regulation of PDK3 levels, which was subsequently attenuated by transfection with si-PDK3 (fig. 7A and fig. 7B). Functional analysis demonstrated that deficiency of miR- 373-3p alleviated the suppression of cell viability, migration and invasiveness caused by propofol in A-172 and LN-229 cells (fig. 7C-fig. 7P). However, these effects were reversed upon silencing PDK3. These findings indicate that inhibition of miR- 373-3p could increase PDK3 expression, thereby attenuating propofol-induced inhibition of glioma progression. Thus, miR-373-3p's regulatory role in glioma appears to be mediated through its interaction with PDK3, highlighting a potential therapeutic target for propofol anti-glioma effects.
Fig. 7: Knockdown of miR-373-3p mitigates the suppressive effect of propofol on glioma cells by targeting PDK3. (A and B): PDK3 protein level; (C and D): Cell viability; (E and F): Cell apoptosis; (G and H): Cycle distribution; (I and J): CDK2 and CDK4 protein levels; (K and L): Migration; (M and N): Invasion and (O and P): MMP2 and MMP9 protein levels were measured by Western blot, MTT, flow cytometry, wound healing and transwell assays, respectively Note: *p<0.05, (G) ( ): G2/M; (
): G2/M; ( ): S and (
): S and ( ): G0/G1, (I) (
): G0/G1, (I) ( ): Control; (
): Control; ( ): Pro; (
): Pro; ( ): Pro+vector; (
): Pro+vector; ( ): Pro+SBF2-AS1; (
): Pro+SBF2-AS1; ( ): Pro+SBF2-AS1+miR-NC and (
): Pro+SBF2-AS1+miR-NC and ( ): Pro+SBF2-AS1+miR-373-3p
): Pro+SBF2-AS1+miR-373-3p
Glioma poses a significant challenge as a prevalent brain disorder, and its treatment remains a formidable task[25]. The impact of anesthetic techniques on tumor outcomes has been increasingly recognized[26,27]. Consequently, we delved into the function of propofol in glioma cells and, for the first time, established its association with the SBF2-AS1/miR-373-3p/PDK3 axis.
Following propofol treatment, we observed a consistent inhibition of glioma cell viability, which is in line with previous findings[7,28]. Cell cycle progression and apoptosis play pivotal roles in determining cell viability. Through flow cytometry analysis, this study revealed that propofol induced cell cycle arrest and promoted cell apoptosis. Furthermore, CDK2 and CDK4 are crucial biomarkers in the cell cycle process, and their inhibition indicates cell cycle arrest[29,30]. Our assessment of these protein levels further confirmed propofol-induced cell cycle arrest. Additionally, MMP9 and MMP2 are key proteins implicated in glioma cell invasiveness and migration[31,32]. By evaluating the levels of MMP2 and MMP9 and conducting transwell assays, our results demonstrated that propofol suppressed the invasive and migratory capacities of glioma cells.
Emerging evidence has suggested that lncRNAs play a role in mediating the anticancer effects of propofol in glioma[8]. In our study, we observed an enhancement of SBF2-AS1 content in glioma tissues. Moreover, we found that propofol treatment led to a decrease in SBF2-AS1 levels in glioma cells, implying that SBF2-AS1 may be involved in the mechanism by which propofol exerts its effects in glioma. Previous studies have indicated an oncogenic role of SBF2-AS1 in glioma[13,14], and in our investigation, we also discovered that SBF2- AS1 attenuated the therapeutic effects of propofol.
Furthermore, SBF2-AS1 has been reported to function as a miRNA sponge, influencing tumor behavior[12,33]. For the first time, we validated that SBF2-AS1 interacts with miR-373-3p in glioma. miR-373-3p has been shown to possess anticancer properties in glioma[34,35], and consistent with this, we observed lower levels of miR-373-3p in glioma tissues and cells. Notably, propofol treatment increased the expression of miR-373- 3p. Importantly, the restoration of miR-373-3p alleviated the oncogenic function of SBF2-AS1 in propofol-treated glioma cells. Additionally, we explored and confirmed that miR-373-3p targets PDK3, which was found to be elevated in glioma and appeared to have a potential carcinogenic role. Moreover, silencing PDK3 abated the promoting effect of miR-373-3p knockdown in propofoltreated cells, further implicating the role of PDK3 in glioma progression. We further demonstrated that SBF2-AS1 modulates PDK3 expression through miR-373-3p. Taken together, our findings indicate that propofol exerts its repression of glioma cell oncogenic phenotypes through the SBF2-AS1/ miR-373-3p/PDK3 axis. This study highlights the intricate regulatory network involving lncRNAs, miRNAs and target genes in propofol-mediated anti-glioma effects.
In conclusion, propofol played a suppressive role in glioma via modulating SBF2-AS1/miR-373- 3p/PDK3 axis, indicating a potential application of propofol in glioma prevention. This study elucidate the molecular mechanisms underlying the antitumor properties of propofol and its potential to disrupt glioma oncogenic phenotypes that could pave the way for the development of more targeted and efficient therapeutic approaches for glioma patients. Moreover, by exploring the intricate interplay between ncRNAs and established oncogenic pathways, this research contributes to the growing body of knowledge aimed at harnessing existing drugs for cancer treatment and offers new prospects for personalized medicine strategies in glioma therapy.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet 2018;392(10145):432-46.
[Crossref] [Google Scholar] [PubMed]
- Tatipamula VB, Thonangi CV, Dakal TC, Vedula GS, Dhabhai B, Polimati H, et al. Potential anti-hepatocellular carcinoma properties and mechanisms of action of clerodane diterpenes isolated from Polyalthia longifolia seeds. Sci Rep 2022;12(1):9267.
[Crossref] [Google Scholar] [PubMed]
- Polimati H, Pragada RR, Thuan NH, Tatipamula VB. Hepatoprotective potential of bioflavonoids. Studies Nat Prod Chem 2022;72:259-85.
- Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, et al. Prevention of pain on injection of propofol: Systematic review and meta-analysis. BMJ 2011;342:d1110.
[Crossref] [Google Scholar] [PubMed]
- Sahinovic MM, Struys MM, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet 2018;57(12):1539-58.
[Crossref] [Google Scholar] [PubMed]
- Hsu SS, Jan CR, Liang WZ. Evaluation of cytotoxicity of propofol and its related mechanism in glioblastoma cells and astrocytes. Env Toxicol 2017;32(12):2440-54.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Xu W, Zhu J. Propofol suppresses proliferation and invasion of glioma cells by upregulating microRNA-218 expression. Mol Med Rep 2015;12(4):4815-20.
[Crossref] [Google Scholar] [PubMed]
- Jiang S, Liu YA, Huang L, Zhang F, Kang R. Effects of propofol on cancer development and chemotherapy: Potential mechanisms. Eur J Pharmacol 2018;831:46-51.
[Crossref] [Google Scholar] [PubMed]
- Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer 2018;17(1):61.
- Shen J, Zhang J, Jiang X, Wang H, Pan G. LncRNA HOX transcript antisense RNA accelerated kidney injury induced by urine-derived sepsis through the miR-22/high mobility group box 1 pathway. Life Sci 2018;210:185-91.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Gu Y, Han P, Li Z, Zhao JL, Gao MZ. Long noncoding RNA SBF2?AS1 promotes colorectal cancer proliferation and invasion by inhibiting miR?619?5p activity and facilitating HDAC3 expression. J Cell Physiol 2019;234(10):18688-96.
[Crossref] [Google Scholar] [PubMed]
- Hua YQ, Zhu YD, Xie GQ, Zhang K, Sheng J, Zhu ZF, et al. Long non-coding SBF2-AS1 acting as a competing endogenous RNA to sponge microRNA-142-3p to participate in gemcitabine resistance in pancreatic cancer via upregulating TWF1. Aging 2019;11(20):8860-78.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Zheng J, Liu X, Xue Y, Shen S, Zhao L, et al. Transcription factor NFAT5 promotes glioblastoma cell-driven angiogenesis via SBF2-AS1/miR-338-3p-mediated EGFL7 expression change. Front Mol Neurosci 2017;10:301.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Yin J, Lu C, Wei Y, Zeng A, You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J Exp Clin Cancer Res 2019;38(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Qiu X, Zhu J, Sun Y, Fan K, Yang DR, Li G, et al. TR4 nuclear receptor increases prostate cancer invasion via decreasing the miR-373-3p expression to alter TGFβR2/p-Smad3 signals. Oncotarget 2015;6(17):15397.
[Crossref] [Google Scholar] [PubMed]
- Weng J, Zhang H, Wang C, Liang J, Chen G, Li W, et al. miR-373-3p targets DKK1 to promote EMT-induced metastasis via the Wnt/-catenin pathway in tongue squamous cell carcinoma. Biomed Res Int 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Hu W, Liu Q, Pan J, Sui Z. miR-373-3p enhances the chemosensitivity of gemcitabine through cell cycle pathway by targeting CCND2 in pancreatic carcinoma cells. Biomed Pharmacother 2018;105:887-98.
[Crossref] [Google Scholar] [PubMed]
- Jing SY, Jing SQ, Liu LL, Xu LF, Zhang F, Gao JL. Down-expression of miR-373 predicts poor prognosis of glioma and could be a potential therapeutic target. Eur Rev Med Pharmacol Sci 2017;21(10):2421-5.
[Google Scholar] [PubMed]
- Gao Y, Yu H, Liu Y, Liu X, Zheng J, Ma J, et al. Long non-coding RNA HOXA-AS2 regulates malignant glioma behaviors and vasculogenic mimicry formation via the miR-373/EGFR axis. Cell Physiol Biochem 2018;45(1):131-47.
[Crossref] [Google Scholar] [PubMed]
- Roche TA, Hiromasa Y. Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia and cancer. Cell Mol Life Sci 2007;64(7):830-49.
[Crossref] [Google Scholar] [PubMed]
- Han JE, Lim PW, Na CM, Choi YS, Lee JY, Kim Y, et al. Inhibition of HIF1α and PDK induces cell death of glioblastoma multiforme. Exp Neurobiol 2017;26(5):295.
[Crossref] [Google Scholar] [PubMed]
- Xie Z, Li X, Chen H, Zeng A, Shi Y, Tang Y. The lncRNA-DLEU2/miR-186-5p/PDK3 axis promotes the progress of glioma cells. Am J Transl Res 2019;11(8):4922.
[Google Scholar] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001;25(4):402-8.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Wu J, Li Y, Jiang Y, Wang L, Chen Y, et al. Circ_0000527 promotes the progression of retinoblastoma by regulating miR-646/LRP6 axis. Cancer Cell Int 2020;20:301.
[Crossref] [Google Scholar] [PubMed]
- Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol 2019;16(8):509-20.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Pan S, Jiang W, Xue F, Zhu X. Effects of propofol on the development of cancer in humans. Cell Prolif 2020;53(8):e12867.
[Crossref] [Google Scholar] [PubMed]
- Gao X, Mi Y, Guo N, Luan J, Xu H, Hu Z, et al. The mechanism of propofol in cancer development: An updated review. Asia Pac J Clin Oncol 2020;16(2):e3-11.
[Crossref] [Google Scholar] [PubMed]
- Wang XY, Li YL, Wang HY, Zhu M, Guo D, Wang GL, et al. Propofol inhibits invasion and proliferation of C6 glioma cells by regulating the Ca2+ permeable AMPA receptor-system xc− pathway. Toxicol In Vitro 2017;44:57-65.
[Crossref] [Google Scholar] [PubMed]
- Roskoski Jr R. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol Res 2019;139:471-88.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Li J, Chen J, Shan Q, Dai H, Xie H, et al. MCM family in HCC: MCM6 indicates adverse tumor features and poor outcomes and promotes S/G2 cell cycle progression. BMC Cancer 2018;18(1):200.
[Crossref] [Google Scholar] [PubMed]
- Jian Y, Xu CH, Li YP, Tang B, Xie SH, Zeng EM. Down-regulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by up-regulating RECK. Biosci Rep 2019;39(8):BSR20182226.
[Crossref] [Google Scholar] [PubMed]
- Miao F, Cui C, Zuo D, Zhang H, Mei P, Chen H, et al. Rap2B promotes cell adhesion, proliferation, migration and invasion of human glioma. J Neuro Oncol 2019;143(2):221-9.
[Crossref] [Google Scholar] [PubMed]
- Tian YJ, Wang YH, Xiao AJ, Li PL, Guo J, Wang TJ, et al. Long noncoding RNA SBF2-AS1 act as a ceRNA to modulate cell proliferation via binding with miR-188-5p in acute myeloid leukemia. Artificial Cells Nanomed Biotechnol 2019;47(1):1730-7.
[Crossref] [Google Scholar] [PubMed]
- Li C, Feng S, Chen L. MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway. Mol Cell Biochem 2021;476:699-713.
[Crossref] [Google Scholar] [PubMed]
- Zhou XY, Liu H, Ding ZB, Xi HP, Wang GW. lncRNA SNHG16 promotes glioma tumorigenicity through miR-373/EGFR axis by activating PI3K/AKT pathway. Genomics 2020;112(1):1021-9.
[Crossref] [Google Scholar] [PubMed]