- *Corresponding Author:
- Qi Xiong Li
Department of Pharmacology, City College, Wuhan University of Science and Technology, Hubei Wuhan 430083, China
E-mail: xiongqili54321@163.com
| Date of Received | 25 April 2020 |
| Date of Revision | 30 September 2020 |
| Date of Acceptance | 04 January 2021 |
| Indian J Pharm Sci 2021;83(1):69-75 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Urinary N-acetyl-β-D-glucosaminidase is a marker of early tubular damage. Therefore, the current study was to investigate effects and its underling mechanisms of valsartan on gentamicin induced renal tubular injury through urinary N-acetyl-β-D-glucosaminidase parameter variety in rats. Animals were divided into four groups consisting of 12 rats each. The study lasted for 10 d. Rats were treated in two batches on the 6th and 11th d of the experiment, with 6 rats in each group. Control group rats were administered with distilled water (10 ml/kg) daily via an intragastric gavage; gentamicin group rats were given gentamicin 100 mg/kg/d intraperitoneally; valsartan (10 mg/kg/d, intragastric gavage) +gentamicin group; valsartan (20 mg/kg/d, intragastric gavage) +gentamicin group. Rats treated with gentamicin showed significant elevation in the activity and expression of urinary N-acetyl-β-D-glucosaminidase as compared with the control group; the activities of superoxide dismutase, glutathione peroxidase and catalase were lower while malondialdehyde was higher in kidney tissues; urinary protein content was not changed; serum creatinine and blood urea nitrogen were increased. Moreover, pathological damage of the renal tubules was serious in gentamicin treated rats. Valsartan significantly inhibited activity and expression of urinary N-acetyl-β-D-glucosaminidase in a dose dependent manner; dramatically increased superoxide dismutase, glutathione peroxidase and catalase activities and markedly decreased malondialdehyde levels in kidney tissues; renal tubular structural damages were also effectively ameliorated by valsartan. These results show that changes of urinary N-acetyl-β-Dglucosaminidase levels can reflect the extent of renal tubular injury, that valsartan has protective role on gentamicin-induced renal tubular injury by down-regulation urinary N-acetyl-β-D-glucosaminidase, and its down-regulation urinary N-acetyl-β-D-glucosaminidase effect may be due to its antioxidant properties in kidney tissues.
Keywords
Valsartan, gentamicin, urinary N-acetyl-β-D-glucosaminidase, expression, rat
N-acetyl-β-D-glucosaminidase (NAG) is a widely distributed lysosomal enzyme in the renal proximal tubular cells. NAG is released from lysosomes when proximal tubule cells are destroyed, therefore, NAG appears in the renal tubules and is excreted from the urine called urine N-acetyl-β-D-glucosaminidase (U-NAG)[1,2]. Increased NAG enzymatic activity in urine has been found to be associated with the extent of tubular damage[1,3,4]. Although albuminuria and U-NAG are known as progression markers of kidney damages, U-NAG as an early renal tubular damage marker, is known to be more sensitive to renal damage than albuminuria in kidney damages, as its level increases before the onset of microalbuminuria during tubular damage progression[5-8].
Valsartan is an effective oral antihypertensive drug[9]. Our recent study showed that valsartan plays an important role in doxorubicin induced glomerular toxicity through its antioxidant properties[10]. However, it is not known whether valsartan has an effect on the increase in U-NAG levels in proximal tubule cell damage caused by aminoglycoside antibiotics. If affected, does this effect reflect the protective extent to the proximal tubule cells damage? Therefore, we investigated the relationship and its underling mechanisms between valsartan and U-NAG levels in gentamicin (GM)- induced proximal tubular toxicity in rats.
Materials and Methods
Reagents and chemicals
Valsartan was purchased from Weifang Fine Chemical Co. Ltd. (Shanghai, China). GM was purchased from Shangdong Dezhou Pharmaceutical Factory (Dezhou, China). U-NAG, urinary protein, serum creatinine (SCr), blood urea nitrogen (BUN), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) Kit were purchased from Beijing Zhongshan Biotechnology Co. Ltd. (Beijing, China). Goat anti-mouse IgG and antibody against β-actin and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals and treatments
48 adult male Sprague-Dawley rats weighing 220 ±15 g were selected for this study. All the experimental procedures were approved by the Wuhan University Animal Care Committee. The animals were maintained in standard cages, at controlled temperature 25±2°, humidity (40- 60 %) and 12/12 h light/dark cycle with free access to food and water. The animals and standard laboratory animal forage were supplied by Wuhan University Experimental Research Centre, Wuhan, China.
Animals were divided into four groups consisting of 12 rats each, the study lasted for 10 d. Rats were treated in two batches on the 6th and 11th d of the experiment, with 6 rats in each group. control group rats were administered with distilled water (10 ml/kg) daily via an intragastric gavage; GM group rats were given GM 100 mg/kg/d intraperitoneally; valsartan (10 mg/kg/d, intragastric gavage)+GM group; (4) valsartan (20 mg/ kg/d, intragastric gavage)+GM group. Valsartan was given separately 2 h before GM. At the 6th and 11th d after valsartan administration, urines were collected for 24 h to measure the urinary protein, U-NAG activity and expression and all animals were euthanized after sodium pentobarbital (45 mg/kg) anesthetization in 6th and 11th d respectively. Blood samples were collected to measure SCr and BUN concentrations. Kidneys from both sides were rapidly removed and sectioned at 4 μm thickness for histological examination. Blood samples were collected and centrifuged at 200 g for 5 min at +4° for subsequent measurement of SCr and BUN. Kidneys were removed rapidly, sectioned for histological analysis. The remaining kidney tissues were homogenized in Tris hydrochloride (Tris HCL) buffer (0.05 mol/l Tris–HCl, 1.15 % KCl, pH 7.4), using a Polytron homogeniser. The homogenate was centrifuged at 18,000×g (+4°) for 30 min, the supernatant was utilized for biochemical analysis.
Biochemical analysis
Urinary protein content was measured according to the sulfosalicylic acid colorimetric method[11]. Determination of U-NAG index activity was done by the following method: urine samples were centrifuged at 1000 rpm for 5 min at 4° then the enzymatic activity was measured by a spectrophotometric, colorimetric method. NAG index was measured using an end point spectrophotometric reaction, whereas the urinary concentration of creatinine was determined by a spectrophotometric, kinetic reaction using the Jaffe method. Urinary NAG index was calculated by the following equation: NAG index (U/g)=U-NAG activity (U/L)/U-Cr concentration (g/L)[4,12]. SCr concentration was measured using a kinetic spectrophotometric fixedtime method at 510 nm (Jaffe reaction), while BUN concentration was also determined using a kinetic spectrophotometric fixed-time method at 450 nm. The concentrations of MDA were determined by the reaction with thiobarbituric acid[13]. SOD activity determination is based on the inhibition of pyrogallol autooxidation[14]. GPx was measured by the enzymatic method[15]. CAT activity determination method was determinated by the rate constant of the Hydrogen peroxide (H2O2) decomposition rate at 240 nm[16]. Total protein content was determined using Lowry method[17].
Western blot analysis
U-NAG expression was measured using western blot with the following groups: control group; GM; valsartan 10 mg/kg/d+GM group; valsartan 20 mg/kg/d+GM group. Briefly, urine samples were centrifuged at 1000 rpm for 5 min at 4° then urine samples were washed in Phosphate-buffered saline (PBS) and then lysed in 1 ml of 1 % Nonidet P-40, 25 mm Tris-HCl, 150 mm Sodium chloride (NaCl), 10 mm Ethylenediaminetetraacetic acid (EDTA), pH 8.0, containing a 1:50 dilution of a protease inhibitor cocktail for 30 min on ice. Samples were centrifuged at 14 000 g for 5 min to pellet cell debris. Samples (20 μg) were mixed with sodium dodecyl sulfate– polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled for 5min, electrophoresed on a 10 % SDS polyacrylamide gel and electro-blotted onto Hybond-ECL nitrocellulose membrane. The membrane was blocked in PBS containing 5 % skimmed milk powder and 0.02 % Tween 20. To detect NAG, the membrane was incubated for1 h with goat polyclonal antibody (Santa Cruz, California, USA) to NAG. After washing, the membrane was incubated with a 1:20 000 dilution of peroxidase conjugated goat anti-mouse IgG in PBS containing 1% normal goat serum and 1% fetal calf serum. The blotting was then developed using the ECL detection kit to produce a chemiluminescence signal, which was captured on x-ray films. Equal loading of proteins was confirmed based on immunoblotting with an antibody against β-actin[18,19].
Histopathological examination
At 6th and 11th d of the experiment, the kidneys from both sides were rapidly removed, immersed in 4 % formaldehyde for fixation and embedded in paraffin for sectioning. A microtome was used to cut sections at 4 μm thickness and the sections were mounted on a glass slide and stained with haematoxylin/eosin (HE). The slides were examined using an Olympus BX 51 microscope and pictures were taken with an Olympus UC 30 digital camera and processed using the Olympus Stream Basic program.
Statistical analysis
Results were expressed as mean±standard deviation (SD); the differences between groups were analyzed by one way analysis of variance and by the Student- Neumann-Keuls’ t-test. Statistical significance was defined as p<0.05.
Results and Discussion
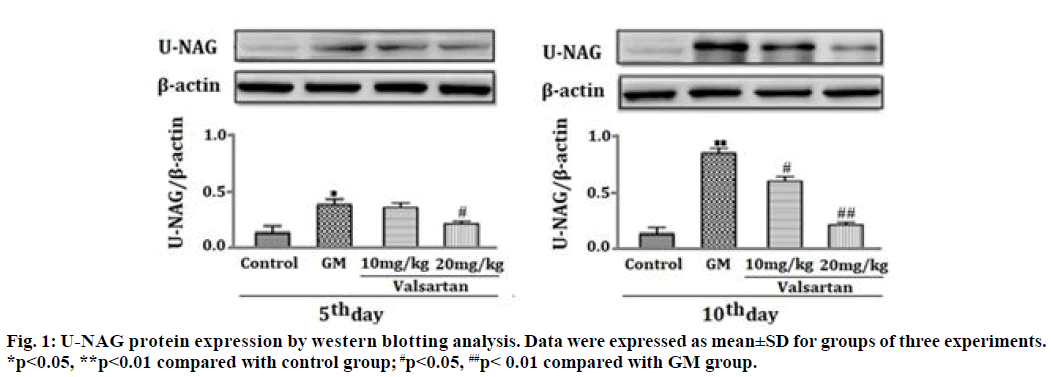
As shown in Table 1, U-NAG activity in GM-induced-renal tubular injury for 5 and 10 d was significantly increased as compared with the control group (p<0.01) and this U-NAG activity was significantly suppressed in a dose dependent manner by co-administration of valsartan (10 and 20 mg/kg) (p<0.05 and p<0.01 respectively). Fig. 1 analysis showed that the level of protein expression of U- NAG at 5 and 10 d after GM treatment was significantly increased as compared with the control group (p<0.05, p<0.01). However, up-regulation of U-NAG protein expression was significantly decreased in a dose dependent manner after treatment with valsartan for 5 and 10 d at both doses (10 mg/kg, 20 mg/kg) (p<0.05, p<0.01).
| Treatment Groups | 5th d | 10th d |
|---|---|---|
| U-NAG (U/g creatinine) | U-NAG (U/g creatinine) | |
| Control | 20.16±0.22 | 20.12±0.82 |
| GM 100mg/kg | 39.78±2.42** | 62.46±4.40** |
| Valsartan (10mg/kg)+GM | 29.46±2.12# | 26.28±6.36## |
| Valsartan (20mg/kg)+GM | 20.40±1.60## | 20.83±4.60## |
Data given are the mean±standard deviations (n=6). **p<0.01 compared with the control group, #p<0.05 compared with GM group, ##p<0.01 compared with GM group.
Table 1: Effects of Valsartan on 24 H U-Nag Activity in GM-Induced Renal Tubular Injury
As demonstrated in Table 2, urinary protein content at 5 and 10 d after GM treatment were not changed, however, at 10 d after GM treatment, Scr and BUN were significantly increased (p<0.01) (Table 3). These changes in SCr and BUN were significantly reduced by co-administration of valsartan (10 and 20 mg/kg) (p<0.05 and p<0.01, respectively). The activities of SOD, GPx and CAT were lower while MDA was higher in GM-induced-renal tubular injury group than those in the control at 5 and 10 d (p<0.01), (Table 4 and 5). Valsartan for 5 and 10 d at both doses (10 mg/kg, 20 mg/kg) was significantly decreased MDA and markedly increased the activities of SOD, GPx and CAT when compared with rats treated with GM alone (p< 0.05, p<0.01), (Table 4 and 5).
| Treatment Groups | 5th d | 10th d |
|---|---|---|
| Urinary protein/m (mg/d) | Urinary protein/m (mg/d) | |
| Control | 0.46±0.06 | 0.44±0.04 |
| GM 100mg/kg | 0.48±0.22 | 0.48±0.43 |
| Valsartan (10mg/kg)+GM | 0.44±0.10 | 0.42±0.34 |
| Valsartan (20mg/kg)+GM | 0.42±0.08 | 0.46±0.20 |
Data given are the mean±standard deviations (n=6). No statistical significance compared with the control group and GM group.
Table 2: Effects of Valsartan on 24 H Urinary Protein Content in GM-Induced Renal Tubular Injury
| Treatment Groups | 5th d | 10th d | 5th d | 10th d | |
|---|---|---|---|---|---|
| SCr (µmol /L) | SCr (µmol /L) | BUN (nmol/L) | BUN (nmol/L) | ||
| Control | 44.24±8.30 | 48.64±6.22 | 4.36±0.24 | 4.68±0.32 | |
| GM 100mg/kg | 46.18±6.34 | 108.42±18.36**a | 5.12±0.46 | 27.26±3.54**a | |
| Valsartan (10mg/kg)+GM | 46.44±5.88 | 84.28±8.28#b | 4.46±0.68 | 17.44±1.82#b | |
| Valsartan (20mg/kg)+GM | 44.32±4.26 | 54.38±4.42##c | 4.68±0.64 | 5.48±1.26##c | |
Data given are the mean±standard deviations (n=6). **p<0.01 compared with the control group, #p<0.05 compared with GM group, ##p<0.01 compared with GM group.
Table 3: Effects of Valsartan on SCR and Bun Contents in GM-Induced Renal Tubular Injury
| Treatment Groups | 5th d | 10th d | 5th d | 10th d |
|---|---|---|---|---|
| MDA (nM/mg prot) | MDA (nM/mg prot) | SOD(U/mg prot) | SOD (U/mg prot) | |
| Control | 0.40±0.02 | 0.64±0.02 | 74.2±1.7 | 68.4 ± 0.32 |
| GM 100mg/kg | 3.84±0.08** | 8.82±0.06** | 34.0±3.3** | 26.2±0.34** |
| Valsartan (10mg/kg)+GM | 3.66±0.04 | 2.24±0.24## | 42.8±3.2 | 62.4±0.12## |
| Valsartan (20mg/kg)+GM | 2.42±0.06# | 1.22±0.22## | 69.4±4.0# | 65.8±0.28## |
Data given are the mean±standard deviations (n=6). **p<0.01 compared with the control group, #p<0.05 compared with GM group, ##p<0.01 compared with GM group.
Table 4: Effects of Valsartan on Kidney MDA Contents and Sod Activity in GM-Induced Renal Tubular Injury
| Treatment Groups | 5th d | 10th d | 5th d | 10th d |
|---|---|---|---|---|
| GPx (μM/min/mg prot) | GPx (μM/min/mg prot) |
CAT mM/min/mg prot |
CAT mM/min/mg prot |
|
| Control | 114±22 | 118±21 | 53.0±4.0 | 68.0±4.2 |
| GM 100mg/kg | 45±13** | 32±18** | 36.2±6.6** | 25.6±5.4** |
| Valsartan (10mg/kg)+GM | 68±14# | 84±28## | 33.8±4.4 | 46.8±3.2## |
| Valsartan (20mg/kg)+GM | 74±39# | 108±12## | 57.4±2.6# | 64.8±2.7## |
Data given are the mean±standard deviations (n=6). **p<0.01 compared with the control group, #p<0.05 compared with GM group, ##p<0.01 compared with GM group.
Table 5: Effects of Valsartan on Kidney GPx and CAT Activity in GM-Induced Renal Tubular Injury
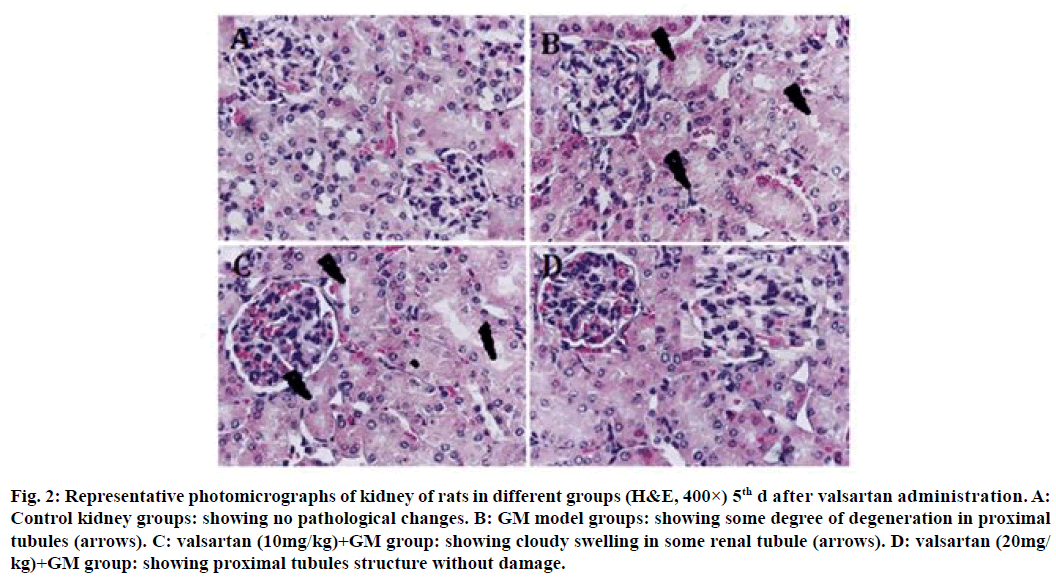
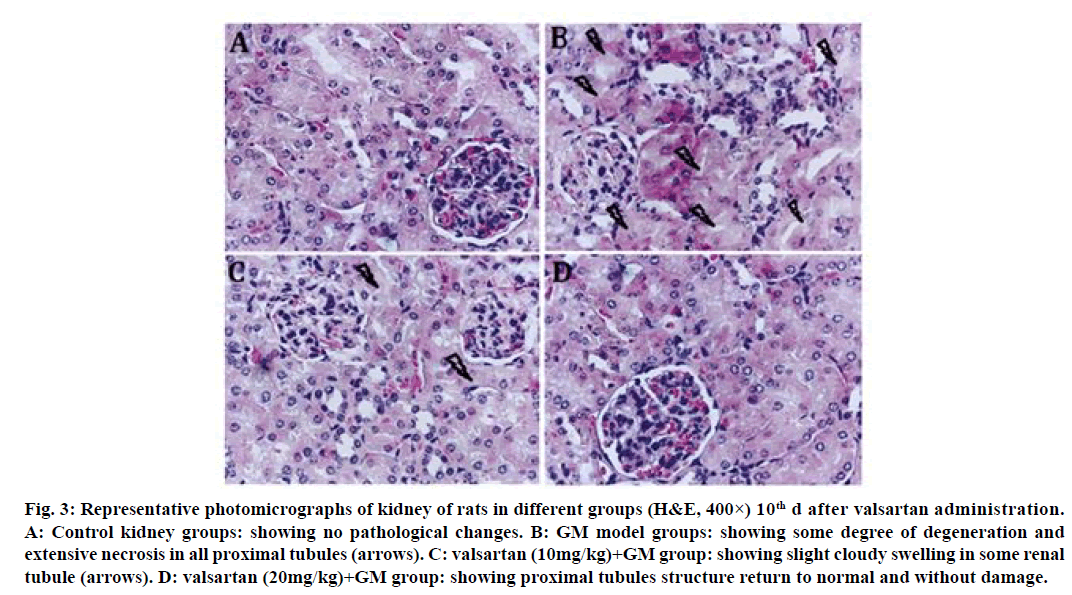
Histopathology confirmed that the control group maintained normal kidney morphology after treatment for 5 d with valsartan (fig. 2A); GM model groups showed some degree of degeneration in proximal tubules (fig. 2B); valsartan (10 mg/kg) showed no significant improvement on GM induced tubular pathological injury (fig. 2C), whereas valsartan (20 mg/kg) showed proximal tubules structure without damage (fig. 2D). The GM group revealed a degree of degeneration and extensive necrosis of all proximal tubules, and significant inflammatory cell infiltration after treatment for 10 d with valsartan (fig. 3B); valsartan (10 mg/kg) showed slight cloudy swelling in some renal tubule (fig. 3C), valsartan (20 mg/kg) showed proximal tubules structure return to normal and without damage (fig. 3D).
Figure 2: Representative photomicrographs of kidney of rats in different groups (H&E, 400×) 5th d after valsartan administration. A: Control kidney groups: showing no pathological changes. B: GM model groups: showing some degree of degeneration in proximal tubules (arrows). C: valsartan (10mg/kg)+GM group: showing cloudy swelling in some renal tubule (arrows). D: valsartan (20mg/ kg)+GM group: showing proximal tubules structure without damage.
Figure 3: Representative photomicrographs of kidney of rats in different groups (H&E, 400×) 10th d after valsartan administration.
A: Control kidney groups: showing no pathological changes. B: GM model groups: showing some degree of degeneration and
extensive necrosis in all proximal tubules (arrows). C: valsartan (10mg/kg)+GM group: showing slight cloudy swelling in some renal
tubule (arrows). D: valsartan (20mg/kg)+GM group: showing proximal tubules structure return to normal and without damage.
The kidneys are often affected by the toxic effects of exogenous compounds or drugs. The first clinical response to renal toxicity is the presence of enzymes in the urine, such as NAG derived from the renal tubules. Excretion of NAG in proximal tubule cells has been proposed as an early index of proximal tubule cell injury[4,20]. Elevated U-NAG levels are significantly associated with proximal tubule cell damage, while albuminuria is not related. Albuminuria is an early marker of glomerular damage[1,21]. Most of the renal injuries induced by drugs are due to alteration of the proximal tubule cells[22], among them, aminoglycosides are the most common drugs[20]. GM is an aminoglycoside antibiotic, widely used for the treatment and prevention of life-threatening gram negative bacterial infections. However, 30 % of patients treated with GM show some degree of nephrotoxicity, limiting its clinical usage[23,24]. Our previous studies showed that U-NAG levels was significant increase in GM-treated rats[25]. The results from this study showed that U-NAG activity and protein expression were significantly increased in GM-treated rats. The change of U-NAG level was earlier than changes of SCr and BUN, but was consistent with tubular histopathology damage in degree and time. Urinary protein content was not changed. Our research further proves that U-NAG can be used as an early renal tubular damage marker, especially for GM tubular damage. It can be seen that the urinary protein is not associated with GM proximal tubule cell damage. The activities of SOD, GPx and CAT were significantly reduced while MDA was significantly increased in kidney tissues in rats treated with GM. Our previous research also showed that GM has oxidative damage to kidney tissues[25].
The results of our current study indicated that valsartan significantly inhibited activity and expression of U-NAG in GM-treated rats; meanwhile, GM-induced renal tubular structural damages were also effective ameliorated by valsartan, U-NAG level reduction and renal tubular pathology morphological change is consistent. In addition, valsartan significantly increased the activity of SOD, GPx and CAT in kidney tissues and significantly reduced MDA levels. Its antioxidant effect may be related to reducing renal tubular damage and inhibiting NAG secretion.
Based on the above, our findings show that changes in U-NAG level are associated with the extent of tubular damage in GM treated rats. Valsartan reducing U-NAG levels and is consistent with improving the extent of tubular damage. Therefore, valsartan can reflect the improvement of renal tubular injury by affecting the activity and expression of rat U-NAG. Clinically, valsartan is beneficial effects for the prevention and treatment of GM-induced nephrotoxicity.
Acknowledgements:
The authors wish to thank the Research Funds of City College, Wuhan University of Science and Technology (Grant NO. 2020CYYBKY001) for financial support. Qi Xiong Li and Xiao Ye Jiang contributed equally to this work.
Conflicts of interest:
The authors declare no conflicts of interest.
References
- Lee M, Hong N, Lee Yh, Kang ES, Cha BS, Lee BW. Elevated N-acetyl-β-D-glucosaminidase, a urinary tubular damage marker, is a significant predictor of carotid artery atherosclerosis in type1 diabetes, independent of albuminuria: A cross-sectional study. J Diabetes Complicat 2018;32(8):777-83.
- Kim YD, Yim DH, Eom SY, Moon SI, Park CH, Kim GB, et al. Temporal changes in urinary levels of cadmium, N-acetyl-β-d-glucosaminidase and β2-microglobulin in individuals in a cadmium-contaminated area. Environ Toxicol Pharmacol 2015;39(1):35-41.
- Sheira G, NoreldinN, Tamer A, Saad M. Urinary biomarker N-acetyl-beta-Dglucosaminidase can predict severity of renal damage in diabetic nephropathy. J Diabetes Metab Disord 2015;14(1):1-5.
- Price RG. Measurement of N-acetyl-h-D-glucosaminidase and its isoenzymes in urine Methods and clinical applications. Eur J Clin Chem Clin Biochem 1992;30:693-705.
- Han E, Kim MK, Lee YH, Kim HS Lee BW. Association between nonalbumin proteinuria and renal tubular damage of N-acetyl-β-D-glucosaminidase and its clinical relevance in patients with type 2 diabetes without albuminuria. J Diabetes Complicat 2019;33(3):255-60.
- Kim SR, Lee YH, Lee SG, Kang ES, Cha BS, Kim JH, et al. Urinary N-acetyl-β-D-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Medicine 2016;95(27):e4114.
- Myśliwiec M, Balcerska A, Zorena K, Myśliwska J, Lipska BS, Wiśniewski P, et al. Serum and urinary cytokine homeostasis and renal tubular function in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2006;19(12):1421-7.
- Tassi C, Mancuso F, Feligioni L, Mararge M, Capodicasa E. Expression modes of N-acetyl-beta-Dglucosaminidase in patients with chronic renal insufficiency. Clin Chim Acta 2004;346(2):129-33.
- Criscione L, Gasparo M, Bühlmayer P, Whitebread S, Ramjoué HP, Wood J. Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype. Br J Pharmacol 1993;110(2):761-71.
- Liu HX, Li J, Li QX. Therapeutic effect of valsartan against doxorubicin-induced renal toxicity in rats. Iran Basic Med Sci 2019;22(3):251-54.
- Salant DJ, Ybulsky AV. Experimental glomerulonephritis. Meth Enzymol 1988;162:421-61.
- Price RG. Urinary h-N-acetylhexosaminidase (NAG) as an indicator of renal disease. Curr Probl Clin Biochem 1979;1(9):150-63.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
- Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide-dismutase. J Biol Chem 1972;247(10):3170-5.
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984;105:114-20.
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Song YJ, Li J, Xie XF, Wang H, Li QX. Effects of amlodipine on TGF-β-induced Smad2, 4 expressions in adriamycin toxicity of rat mesangial cells. Arch Toxicol 2011;85(6):663-8.
- Li J, Tie CR, Li QX, Zheng F. Amlodipine prevents adriamycin-induced toxicity in cultured rat mesangial cells by up-regulation of Smad6, Smad7 expression. Environ Toxicol Pharmacol 2014;38(1):251-6.
- Tulkens PM. Nephrotoxicity of aminoglycoside antibiotics. Toxicol Lett 1989;46:107-23.
- Hong CY, Chia KS. Markers of diabetic nephropathy. J Diabetes Complicat 1998;12:43-60.
- Robic D, Bens M, Loko F, Vandewalle A, Bourbouze R. N-Acetyl-/3-D-glucosaminidase (NAG) isoenzymes in primary cultures of rabbit kidney proximal tubule cells: a cellular model for studies on nephrotoxicity? Toxicology 1995;103(1):37-44.
- Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: some recent research. Food Chem Toxicol 2003;41(11):1447-52.
- Olbricht CJ, Fink M, Gudjahr E. Alteration in lysosomal enzymes of the proximal tubule in gentamicin nephrotoxicity. Kidney Int 1991;39(4):639-46.
- Li J, Li QX, Xie XF, Ao Y, Tie CR, Song RJ. Differential roles of dihydropyridine calcium antagonist nifedipine, nitrendipine and amlodipine on gentamicin-induced renal tubular toxicity in rats. Eur J Pharmacol 2009;620(1-3):97104.