- *Corresponding Author:
- Shweta Arora, J. Ali
Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, Hamdard Nagar, New Delhi-110 062, India
E-mail: dawn.foster@yale.edu
| Date of Submission | 10 May 2006 |
| Date of Revision | 10 January 2006 |
| Date of Acceptance | 03-September-2012 |
| Indian J Pharm Sci,2006, 68 (3): 295-300 |
Abstract
Pulsatile systems are gaining a lot of interest as they deliver the drug at the right site of action at the right time and in the right amount, thus providing spatial and temporal delivery and increasing patient compliance. These systems are designed according to the circadian rhythm of the body. The principle rationale for the use of pulsatile release is for the drugs where a constant drug release, i.e., a zero-order release is not desired. The release of the drug as a pulse after a lag time has to be designed in such a way that a complete and rapid drug release follows the lag time. Various systems like capsular systems, osmotic systems, single- and multiple-unit systems based on the use of soluble or erodible polymer coating and use of rupturable membranes have been dealt with in the article. It summarizes the latest technological developments, formulation parameters, and release profiles of these systems. Products available as once-a-daily formulation based on Pulsatile release like Pulsincap®, Ritalin®, and Pulsys® are also covered in the review. These systems are beneficial for the drugs having chronopharmacological behaviour where night time dosing is required and for the drugs having high first-pass effect and having specific site of absorption in GIT. Drugs used in asthmatic patients and patients suffering from rheumatoid arthritis are also discussed along with many other examples.
Circadian rhythm regulates many body functions in humans, viz., metabolism, physiology, behaviour, sleep patterns, hormone production, etc. It has been reported that more shocks and heart attacks occur during morning hours [1]. The level of cortisol is higher in the morning hours, and its release is reported to decline gradually during the day. Blood pressure is also reported to be high in the morning till late afternoon, and then drops off during night [2]. Patients suffering from osteoarthritis are reported to have less pain in the morning than night, while patients suffering from rheumatoid arthritis feel more pain in the morning hours [2]. The release of some drugs is preferred in pulses. A single dosage form provides an initial dose of drug followed by one release- free interval, after which second dose of drug is released, which is followed by additional release-free interval and pulse of drug release.
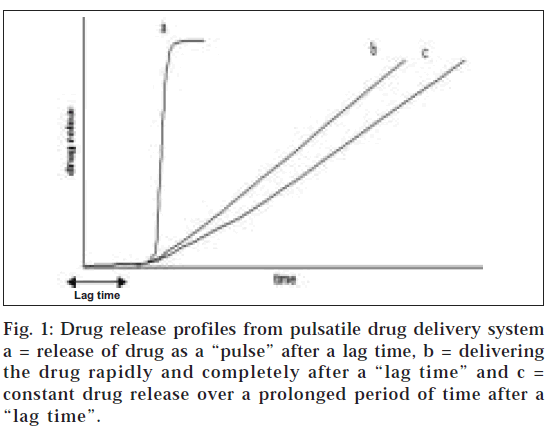
The pulsatile effect, i.e., the release of drug as a “pulse” after a lag time has to be designed in such a way that a complete and rapid drug release should follow the lag time (fig. 1). Such systems are also called time-controlled as the drug released is independent of the environment. Pulsatile drug delivery systems are gaining a lot of interest and attention these days. These systems have a peculiar mechanism of delivering the drug rapidly and completely after a “lag time,” i.e., a period of “no drug release.” Though most delivery systems are designed for constant drug release over a prolonged period of time, pulsatile delivery systems are characterized by a programmed drug release, as constant blood levels of a drug may not always be desirable (fig. 1). Pulsatile systems are designed in a manner that the drug is available at the site of action at the right time in the right amount. These systems are beneficial for drugs having high first-pass effect; drugs administered for diseases that follow chronopharmacological behaviour; drugs having specific absorption site in GIT, targeting to colon; and cases where night time dosing is required [3].
Classification Of Pulsatile Systems
Pulsatile systems can be classified into single- and multiple-unit systems. Single-unit systems are formulated either as capsule-based or osmosis-based systems. Single-unit systems are designed by coating the system either with eroding/soluble or rupturable coating. In multiple-unit systems, however, the pulsatile release is induced by changing membrane permeability or by coating with a rupturable membrane.
Single unit pulsatile systems
These are sub-classified as capsule-based systems, osmotic systems, delivery systems with soluble or erodible membranes, and delivery systems with rupturable coating.
Capsule based systems
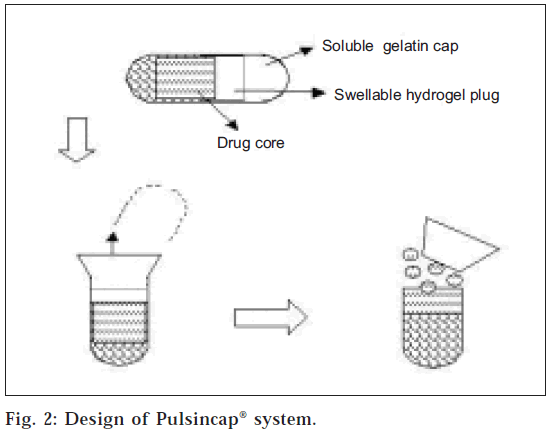
Single-unit systems are mostly developed in capsule form. The lag time is controlled by a plug, which gets pushed away by swelling or erosion, and the drug is released as a “Pulse” from the insoluble capsule body. Pulsincap® was developed by R. P. Scherer International Corporation, Michigan, US, and is one such system that comprises of a water-insoluble capsule enclosing the drug reservoir. A swellable hydrogel plug was used to seal the drug contents into the capsule body [4]. When this capsule came in contact with the dissolution fluid, it swelled; and after a lag time, the plug pushed itself outside the capsule and rapidly released the drug. Polymers used for designing of the hydro gel plug were various viscosity grades of hydroxyl propyl methyl cellulose, poly methyl methacrylates, poly vinyl acetate and poly ethylene oxide. The length of the plug and its point of insertion into the capsule controlled the lag time. Pulsincap® was studied in human volunteers and was reported to be well tolerated [5-7]. A low-volume diagnostic test kit was marketed in 1997 under the trade name of ‘Sprintsalmonella’ by Oxoid Ltd., Basingstoke, U.K. Steven et al. developed a Pulsincap® system with erodible compressed tablet8. As the swelling hydrogel polymer plug replaced the erodible tablet, the dependence of the dimensional accuracy between the plug and the capsule for the pulling mechanism of the plug from the capsule was also overcome (fig. 2). Ross et al. used low substituted hydroxypropylcellulose for the expulsion system for the release of propranolol over a time period of 2-10 h. This could be controlled using compressed erodible tablets made of lactose and HPMC [9].
Krogel and Bodmeier [10] studied the release of chlorpheniramine utilizing the erodible plugs fitted in the capsules. Altering the composition and the weight of the erodible plug could control release of drug. Stevens et al. designed a hydrophilic sandwich capsule that was based on a system where a capsule was enclosed within a capsule and the space in between was a gel barrier layer composed of HPMC. When the outer capsule dissolved, the delay in the second pulse was provided by the barrier gel layer [11]. Soutar et al. studied the delivery of 500 mg paracetamol with a gastroresistant hydrophilic sandwich capsule targeted to ileocaecal junction / proximal colon. Analysis of salivary samples gave a mean Tmax of 7.9 h (SD±0.96) [12].
Systems based on osmosis
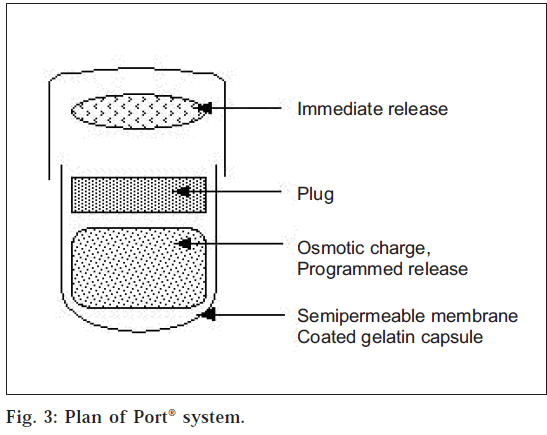
The Port® system was developed by Therapeutic system research laboratory Ann Arbor, Michigan, USA, and consists of a capsule coated with a semipermeable membrane. Inside the capsule was an insoluble plug consisting of osmotically active agent and the drug formulation [13]. When this capsule came in contact with the dissolution fluid, the semipermeable membrane allowed the entry of water, which caused the pressure to develop and the insoluble plug expelled after a lag time (fig. 3). Such a system was utilized to deliver methylphenidate used in the treatment of attention deficit hyperactivity disorder as the pulsatile port system. This system avoided second time dosing, which was beneficial for school children during daytime.
Linkwitz et al. invented an osmotic drug (single-unit) delivery capsule from which the delivery of the drug was driven by the osmotic infusion of the moisture by the capsule from a physiological environment. It was provided with a delivery orifice that opened intermittently to achieve a pulsatile delivery effect [14]. The technology involves a movable position that divides the capsule interior into two compartments – one for the beneficial agent and the other for the osmotically active agent. The orifice is located on the capsule wall surrounding the beneficial agent side. The whole capsule is surrounded by an elastic wall in the osmotically active compartment due to inward diffusion of water, which is transmitted through the partition. When the pressure in the drug compartment exceeds the threshold, it results in opening of the orifice and as a result, an amount of beneficial agent is released and it relieves the pressure in the drug compartment. This release in pressure causes the elastic material to relax and results in closure of the orifice. There are various factors affecting the degree and manner in which the pulsatile effect can be controlled, viz., the choice of elastic material, the thickness of the wall section, the configuration and location of the orifice and the viscosity and surface tension of the beneficial agent formulation. The choice of elastic material is done on the ability to stretch at least twice their original length and to retract very rapidly when released. Examples include styrene butadiene copolymer polychlorophene, nibile rubbers, butyl rubber and polycrylatis. The thickness of the elastic material may vary, but best results are generally obtained with thickness of at least 0.06 cm.
The pulsatile delivery provided by the aforementioned devices in this invention may be for therapeutic purpose, nutritional purpose, preventive purpose, and a wide variety of situations in general.
Drug delivery system with eroding or soluble barrier coating
These systems are based upon a drug reservoir surrounded with a soluble barrier layer that dissolves with time, and the drug releases at once after this lag time. Chronotropic® system consists of a core containing drug reservoir coated by a hydrophilic polymer HPMC [15-17]. An additional enteric-coated film is given outside this layer to overcome intra-subject variability in gastric emptying rates [18]. The lag time and the onset of action are controlled by the thickness and the viscosity grade of HPMC.
The time clock system is a delivery device based on solid dosage form that is coated by an aqueous dispersion [19]. This coating is a hydrophobic-surfactant layer to which a water-soluble polymer is added to improve adhesion to the core. Once in contact with the dissolution fluid, the dispersion rehydrates and redisperses. The lag time could be controlled by varying the thickness of the film. After the lag time, i.e., the time required for rehydration, the core immediately releases the drug. This system has shown reproducible results in vitro and in vivo. The effect of low calorie and high calorie meal on the lag time was studied using gamma scintigraphy. The mean lag time of drug release was 345 and 333 min, respectively.
Midha et al. invented a pulsatile delivery system for dthreomethyl phenidate, an additional CNS stimulant in a dosage form comprising at least two individual drugs containing dosage limit housed in a closed capsule. The dosage units are designed in the form of compressed tablets [20]. The dosage form is designed in such a manner that upon ingestion, the first drug release pulse occurs within 1-2 h, followed by period during which no release occurs. Second dose is released in 3-5 h of ingestion. This is again followed by a second no-release interval. Release of third dose occurs within 7-9 h of ingestion. To provide such delayed-release dosage units, coating is done without bioerodible gradually hydrolysable polymers. The release amount of coating material per dosage unit decides the time interval between ingestion and drug release.
Dittigen et al. planned a multiple unit dosage form comprising of compressed compositions having different amounts of ingredients and combinations. Hormones, viz., progesterone, testosterone, dehydro-epi-amdrosteron, estriol, and estradiol follow circadian rhythm and hence their concentration in blood varies over 24 h. Melatonin is also reported to be secreted mainly in the night. Some of the analogues and inhibitory substances for these hormones also follow a circadian rhythm. Examples of such classes include antidiabetics, glucocorticoids, mineralocorticoids and antihistamines. The formulation comprises of four compressed compositions in a capsule. The first composition is formed to provide a rapid release in which at least 75% of the effective ingredients are delivered within 45 min. The second compressed combination provides a uniformly maintained release profile in which 100% of effective ingredient is released within 3 h of ingestion. Third compressed combination delivers at least 75% of effective ingredient within 45 min of reaching duodenum and intestine at a pH of 6 to 7.5. Coating is given by gastric-resistant agents (PMMA or shellac). Fourth compressed composition releases 100% of the effective ingredient 3 h after reaching a pH of 6-7.5 [21].
Pope et al. developed a drug delivery device with readily adjustable intervals between drug delivery pulses. This could be accomplished by providing for a constant driving force against multiple layers contained in an impervious compartment having an opening away from the constant driving force. The design of the multiple layers was such that the drug layer was adjacent to an expansion layer with an inert and impervious spacer layer alternating with the adjacent drug layer and so on. Two factors affected the duration between pulses, viz., the rate of constant driving force and the thickness of the spacer layer and the multiple layers (drug or combined drug/expansion layer). A thicker layer exhibited a longer duration between pulses of drug since it took a long time for thicker spacer layer to completely traverse the opening [22].
Drug delivery system with rupturable layers/ membranes
These systems are based upon a reservoir system coated with a rupturable membrane. The outer membrane ruptures due to the pressure developed by effervescent agents or swelling agents. Sungthongjeen et al. designed a pulsatile drug delivery system where the tablets of buflomedil HCl prepared by direct compression with varying amounts of spray-dried lactose and microcrystalline cellulose were coated with an inner swelling layer using croscarmellose sodium and an outer rupturable layer using ethyl cellulose. It was observed that by increasing the amount of ethyl cellulose coating, the lag time could be prolonged. Ethyl cellulose, being water insoluble, retarded the water uptake. Similar results were obtained with croscarmellose sodium. Increasing the amount of microcrystalline cellulose decreased the lag time substantially [23].
Bussemer et al. worked on a pulsatile system with rupturable coating on drug present in hard gelatin capsules. These capsules were first coated with a swelling layer and then with an insoluble but water-permeable outer coating. These coated capsules when immersed in the release media could take up the media at a constant rate up to a point when the outer coating would rupture because of the pressure caused by the swelling layer. It could be concluded that by increasing the swelling layer, the lag time could be shortened. However, by increasing the outer coating, the lag time could be prolonged. It was also observed that addition of HPMC to the outer coating shortens the lag time [24].
In another similar work, Bussemer et al. studied the effect of various swelling agents and the outer polymeric coating on the lag time and the drug release. They developed a pulsatile system based on soft gelatin capsules with an inner swelling and outer polymeric coating. It could be concluded from this study that croscarmellose sodium was a better swelling agent as compared to HPMC (E5 and K100 M). Also, cellulose acetate propionate and ethyl cellulose gave better rupturing by virtue of their brittle nature when compared to Eudragit RS, which gave a flexible polymer coating. Cellulose acetate propionate coated capsules gave better drug release as compared to those coated with ethyl cellulose [25].
Multiple unit pulsatile systems
More reliable gastric emptying patterns are observed for multiparticulate formulations as compared to single-unit formulations, which suffer from ‘all or none’ concept. As the units of multiparticulate systems are distributed freely throughout the gastrointestinal tract, their transport is affected to a lesser extent than single-unit formulations by the transit time of food [26]. Multiparticulate systems are further classified as systems based upon change in membrane permeability and systems based upon rupturable coating.
Pulsatile system based on change in membrane permeability
A Sigmoidal release system (SRS) is reported which is based upon the interaction of acrylic polymers with quaternary ammonium groups in the presence of different counter ions. SRS system consists of pellet cores having drug and succinic acid coated with ammonio-methacrylate copolymer USP/NF type (B). The water in the medium dissolves succinic acid. The drug inside and the acid solution increase the permeability of the polymer film. This system was used to design an acid-containing core. The system was tested in beagle dogs. Good in vitro/in vivo correlation of lag time was observed [27].
Chen et al. developed an osmotic multiparticulate drug delivery system for diltiazem. It was designed in such a way that the drug released in divided doses over timed intervals throughout the day to produce a pulsatile blood concentration curve with time. The dosage form comprised of a gelatin capsule containing three types of pellets. Each pellet contained a core that comprised of drug and water-soluble modulating agent (NaCl in this case). The core was held in place with the help of binding agent, viz., PVP. Enclosing each core was a water-insoluble and water-permeable film-forming agent and a hydrophilic agent. The thickness of this coating varied in each kind of pellet. In one kind of pellets, the thickness was the least; in the second kind of pellets, a thicker coating was given; and in the third kind of pellets, the thickest coating was given. When the dosage form was exposed to the physiological environment, the capsule dissolved and the pellets were exposed to the gastric environment. The rate of release was controlled by the relative thickness of the coating on the respective kind of pellets, the proportion of hydrophobic agent in the coating, and the proportion of osmotic agent in the pellet. To ensure that pH doesn’t disturb the preset release time intervals, the coating given was of pH-independent material [28].
Pulsatile systems with rupturable coating
Similar to single-unit system, the rupturing effect is achieved by coating the individual units with effervescent or swelling agents. Bai et al. invented a pulsatile drug delivery system comprising of a plurality of particles that are divided into several individual delivery units, each having its own distinct composition. Drug delivery was controlled by the rupture of the membrane. The timing of release was controlled by the thickness of coating and the amount of water-soluble polymer to achieve the pulsed release. The individual particles had the same composition of internal core, but the thickness of the external coating layer varied [29].
Commercial products
A lot of work is being done to achieve pulsatile release so that the drug release can be delivered according to circadian rhythms of our body. Advancis Pharmaceutical Corp., German town, Maryland, USA has developed once-a-day pulsatile delivery system called Pulsys®, which enables the delivery of antibiotic amoxicillin in regular concomitant pulses. The rationale behind designing such a system is that it has been reported that antibiotics are more effective against fast-growing bacteria. When an immediate release antibiotic is administered, bacteria respond to it by going into a dormant stage, while the administration of a pulsatile system in such a case is more effective because the regular release of increased pulses of antibiotic does not let defence system of the bacteria to go into a dormant stage. The preclinical studies have shown that pulsatile approach of delivering antibiotic is more effective. Advancis is developing Pulsysâ versions of three of the top five most prescribed antibiotics in the United States. Asthmatic patients suffer from lung discomfort more in early morning due to circadian changes [30]. Therefore, it is desirable to get maximum bronchodilating effect in the morning hours. One such example is of a bronchodilator “Uniphyl” (theophylline) [31], which was developed by Purdue Pharmaceuticals Products L. P., Stamford, USA, and approved by FDA in 1989. It’s a once-a-day formulation. When taken in the evening, it reaches to peak blood levels in the morning hours, resulting in improved lung functioning and relief to the patient.
There are examples where varying plasma levels are required during the day time. Elan applied this technology to a product of Novartis, Ritalinâ, containing methylphenidate to get a pulsatile once-daily dosage form that replaces the twice-a-day regimen.
Conclusions
There is a constant need for new delivery systems that can provide increased therapeutic benefits to the patients. Pulsatile drug delivery is one such system that, by delivering drug at the right time, right place, and in right amounts, holds good promises of benefit to the patients suffering from chronic problems like arthritis, asthma, hypertension, etc.
References
- Gurny, R., Junginger, H.E. and Peppas, N., Eds., In; Pulsatile DrugDelivery: Current Application and Future Trends, WissenscheflicheVerlagsgesellschaft, Stuttgart, Germany, 1993, 36.
- Lemmer, B., J. Pharm. Pharmacol., 1999, 51, 887.
- Chourasia, M.K. and Jain, S.K., J. Pharm. Pharm. Sci., 2003, 6, 33.
- Mc Neill, M.E., Rashid, A. and Stevens, H.N.E., GBPatent No., GB2230442, 1993.
- Bakshee, M., Burns, J.S., Stevens, H.N.E. and Miller, C.J., PharmRes.,1992, 9 (Suppl), F230.
- Hebden, J.M., Wilson, C.G., Spiller, R.C., GilChrist P.J., BlackShaw P.E., Frier, M. and Perkins, A.C., Pharm. Res., 1999, 16, 1087.
- Burns, J.S., Stevens, H.N.E., McEwen, J., Pritchard, G., Brewer, F.M., Clarke, A., Johnsons, E.S. and McMillan, I., J. Control.Release, 1996, 38, 151.
- Stevens, H.N.E., Rashid, A. and Bakshee, M., US Patent No., US5474784, 1995.
- Ross, A.C., Macrae, R.J., Walther, M., Stevens, H.N.E. J. Pharm.Pharmcol., 2000, 52, 903.
- Krogel, I. and Bodmeier, R., Pharm. Res., 1998, 15, 474.
- Stevens, H.N.E., Ross, A.C. and Johnson, J.R., J. Pharm.Pharmcol., 2000, 52, S41.
- Soutar, S., Stevens, H.N.E., Mahony, B.O., Bakshee, M., Perkins, A.C., Grattan, T. and Wilson, C.G., Proc. Int. Symp. Control ReleaseBioact. Mater.,2001, 28, 790.
- Crison, J.R., Siersna, P.R., Taylor, M.D. and Amidon, G.L., Proc. Int.Symp. Control Release Bioact. Mater.,1995, 22, 278.
- Linkwitz, A., Magruder, J.A., Merrill, S., US Patent No., US5318558, 1994.
- Gazzaniga, A., Ianartino, P., Maffione, G. and Sangalli, M.E., Int. J.Pharm., 1994, 2, 77.
- Gazzaniga, A., Sangalli, M.E., Giordano, F., Eur J. Biopharm.Pharm., 1994, 40, 246.
- Gazzaniga, A., Busetti, C., Moro, L., Crimella, T., Sangalli, M.E. and Giordano, F., Proc. Int. Symp. Control Release Bioact.Mater.,1995, 22, 242.
- Polli, S., Busetti, C. and Moro, L., EP Patent No., EP0572942, 1993.
- Sangalli, M.E., Maroni, A., Zema, L., Busetti, C., Gazzaniga, A. and Giordano, F., J. Control. Release, 2001, 73, 103.
- Midha, K.K., Teicher, M.H. US Patent No., US6217904, 2001.
- Dittigen, M., Fricke, S., Timpe, C., Gercke, H., Eichardt, A. USPatent No., US 6117450, 2000.
- Pope, D.G., Royce, A.E. US Patent No., US473958, 1988
- Sungthongjeen, S., Puttipipakhachorn, S., Paeratakul, O., Dashevsky, A. and Bodmeier, R., J. Control. Release, 2004, 95, 147.
- Bussemer, T., Dashevsky, A. and Bodimeier, R., J. Control.Release, 2003, 93, 331.
- Bussemer, T., Bodmeier, R., Int. J. Pharm., 2003, 267, 59.
- Bechgaard, H., Ladefoged, K. J. Pharm. Pharmacol, 1978, 30, 690.
- Guo, X. PhD thesis, The University of Texas, Austin, 1996.
- Chen ,C.M., US Patent No., US5260068, 1993.
- Bai. US Patent No., US 5840329, 1998.
- Dethlefsen, U. and Repgas, R., Med Klinik, 1985, 80, 44.
- Urwitz, H., Karim, A. and Burns, H.S., J. of ClinicalPharmocol.,1987, 27, 855.