- *Corresponding Author:
- Kotagale N.R
Smt. Kishoritai Bhoyar College of Pharmacy, Behind Railway Station, New Kamptee, Dist. Nagpur–441 002, India
Email: nandukotagale@gmail.com
| Date of Submission | 05 February 2011 |
| Date of Revision | 11 November 2011 |
| Date of Acceptance | 24 November 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 626-633 |
Abstract
A floating type of dosage form of ranitidine hydrochloride in the form of microspheres capable of floating on simulated gastric fluid was prepared by solvent evaporation technique. Microspheres prepared with ethyl cellulose, Eudragit® RS100 alone or in combination were evaluated for percent yield, drug entrapment, percent buoyancy and drug release and the results demonstrated satisfactory performance. Microspheres exhibited ranitidine hydrochloride release influenced by changing ranitidine hydrochloride-polymer and ranitidine hydrochloride-polymer-polymer ratio. Incorporation of a pH modifier has been the usual strategy employed to enhance the dissolution rate of weakly basic drug from floating microspheres. Further citric acid, fumaric acid, tartaric acid were employed as pH modifiers. Microspheres prepared with ethyl cellulose, Eudragit® RS100 and their combination that showed highest release were utilized to study the effect of pH modifiers on ranitidine hydrochloride release from microspheres which is mainly affected due to modulation of microenvironmental pH. In vitro release of ranitidine hydrochloride from microspheres into simulated gastric fluid at 37º showed no significant burst effect. However the amount of release increased with time and significantly enhanced by pH modifiers. 15% w/w concentration of fumaric acid provide significant drug release from ranitidine hydrochloride microspheres prepared with ranitidine hydrochloride:ethyl cellulose (1:3), ranitidine hydrochloride:Eudragit® RS100 (1:2) and ranitidine hydrochloride:ethyl cellulose:Eudragit® RS100 (1:2:1) whereas citric acid, tartaric acid showed significant cumulative release at 20% w/w. In all this study suggest that ethyl celluose, Eudragit® RS100 alone or in combination with added pH modifiers can be useful in floating microspheres which can be proved beneficial to enhance the bioavailability of ranitidine hydrochloride.
Keywords
Ethyl cellulose, Eudrgit® RS100, floating microspheres, microenvironmental pH, pH modifiers, ranitidine hydrochloride.
For oral solid drug delivery systems, drug absorption is unsatisfactory and highly variables between individuals. The major problem is the physiological variability in addition to gastric retention time which plays a dominating role in overall transit of the dosage form. Even though the slow release can be achieved in controlled release system, drug release after 12 h is undesired as the formulation passes out from absorption site which accelerated the research towards retention of the drug delivery system in the stomach for prolonged and predictable time. Such prolonged gastric retention not only controls the time but also the space in the stomach by maintaining the delivery system positioned at the steady state and thereby properly delivering the drug [1-3]. Floating drug delivery system prepared using the polymer increase the gastric retention time which in turn may result in increased bioavailability of drugs, which shows high gastric absorption and poorly absorbed in lower GI tract. Hydroxypropylmethyl cellulose (HPMC) and ethyl cellulose (EC) prepared microspheres have prolonged cimetidine release (~8 h) which remained buoyant for >10 h [4]. Eudragit RS100® (Eu RS100) has also been exploited for hollow microspheres of riboflavin, aspirin, salicylic acid, ethoxybenzamide and indomethacin. Gamma scintigraphy of riboflavin microballoons prepared with Eu RS100 exhibited the gastro-retention in human volunteers [5].
Polymeric microspheres and microcapsules have received much attention as drug delivery systems in recent years to modify and retard drug release [6]. Earlier studies have investigated the influence on In vitro ranitidine release from microspheres prepared using cellulose acetate and chitosan polymers [7].
Moreover hollow microspheres developed using ethyl cellulose demonstrated sustained ranitidine delivery [8]. Microspheres preparation involves coating of individual drug particles by inert polymeric material, through which the drug diffuse at a controlled and predictable rate in the surrounding medium [9]. Variability and bioavailability for weakly basic drug substances can be overcome through increased residence time at absorption site and releasing the drug at a controlled rate. Incorporation of pH modifiers such as citric, fumaric or sorbic acid to alter drug release and hence bioavailability is a common approach employed with matrix and coated systems [10-13] but have not been investigated for their influence in the floating delivery system. The enhancement in the release of weakly basic drugs by incorporated pH modifiers occurs mainly through modulation of microenvironmental pH (pHM) [14]. However, the selection of appropriate pH modifier for a specific drug is not as straightforward as it may appear. The majority of the frequently used pH modifiers are more soluble at higher pH as compared to most basic drug compounds. Since we assume that the pH modifiers diffuse out more rapidly as compared to the drug, the pH-modifying effect within and in the interface of the dosage form may influence the diffusion of drug molecules.
Ranitidine hydrochloride (RHCl), H2 receptor antagonist, is widely prescribed in Zollinger-Ellison syndrome, gastroesophageal reflux disease, erosive esophagitis, gastric and duodenal ulceration. It is absorbed from upper GIT and has only 50% bioavailability. Colonic metabolism of RHCl is partly responsible for poor bioavailability of drug. Hence it was thought that formulation residing at absorption window for prolonged period may be a useful approach to enhance bioavailability of RHCl. Therefore this study deals with the formulation and evaluation of RHCl floating microspheres prepared with EC and Eu RS100 alone or in combination. Additionally this report also investigated effect of incorporation of various pH modifiers on RHCl release from microspheres.
Materials and Methods
RHCl, EC and Eu RS100 were obtained as a gift sample from Zydus Cadila Healthcare, India, Glenmark Pharmaceuticals, India and Colorcon Asia Ltd., India, respectively. All other chemicals/reagents used were of analytical pharmaceutical grade.
Preparation of floating microspheres
The microspheres were prepared by non-aqueous solvent evaporation technique. Briefly, drug and polymer i.e. RHCl, EC and/or Eu RS100 were mixed in acetone at various ratio by using methanol as blending solvent with added Tween 80 (2% w/v) as stabilizer. Prepared solution was introduced into 200 ml of liquid paraffin while being stirred at 2000 rpm by mechanical stirrer (REMI- RQT-124A) for 2 h at 35±2º to allow the solvent to evaporate and microspheres were collected by filtration. The microspheres were washed repeatedly with petroleum ether until freed from oil, dried for 24 h at room temperature and subsequently stored in desiccators over fused calcium [15]. Percentage yield of microspheres was calculated by dividing the total weight of microspheres by total mass of nonvolatile compounds used. Morphological and surface characteristic were studied by scanning electron microscopy (SEM).
Morphology of floating microspheres
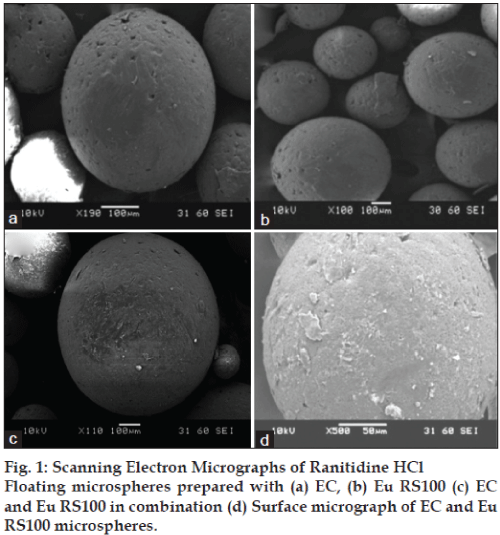
The shape and surface morphology of RHCl floating microspheres with polymer EC and/ Eu RS100 alone and in combination were investigated using Scanning Electron Microscopy (SEM) (Joel, JSM-6380, USA). The samples for SEM study were prepared by lightly sprinkling the formulation on a double-adhesive tape stuck to an aluminium stub. The stuck were then coated with gold to a thickness of ~300 A° under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. The coated samples were then randomly scanned and photomicrographs were taken with SEM.
Particle size determination
Size distribution analysis of microspheres was done by calibrated optical microscopy using Motic Microscope since the size of microspheres is known to affect drug release. A small quantity of microspheres was spread on the glass slide and the diameters (average particle size) were sized using a suitable objective (10X and 40X).
Bulk and tapped density
The bulk and tapped density were determined by Digital Automatic Tap/Bulk Density Test Apparatus (Veego Instruments: VTAP/MATIC-II). Accurately weighed microspheres (100±0.1 g) placed it in the graduated cylinder provided and unsettled volume was noted. The cylinder was then tapped for 100 times to determine the tapped volume. Each experiment was carried out in triplicate. The bulk density and tapped density was determined as per formulae [16]. Tapped Density (g/ml) = Mass of microspheres/ Volume of microspheres after tapping; % Compressibility Index (C.I.) = (ρt - ρo)/ ρt × 100; Hausner’s ratio = ρt/ ρo. Where, ρt =Tapped density, ρo =Bulk density
The angle of repose (θ)
Angle of repose of prepared microspheres (n=3) was determined by fixed funnel standing method [17]. The granules were allowed to flow through funnel orifice on a plane paper kept on the horizontal surface to form a pile of granules. The angle of repose was calculated by substituting the values radius ‘r’ and height ‘h’ in the following equation. tan θ=r/h. Where, θ=angle of repose, r=radius, and h=height.
Buoyancy of microspheres
Buoyancy studies were carried out to ascertain the floating behavior of microspheres prepared with EC or Eu RS100 or their combinations. Microspheres (300 mg) were spread over the surface of 900 ml of 0.1 N HCl containing 0.2% w/v tween 80 in USP-II dissolution apparatus and agitated using paddle (100 rpm) for 12 h. The floated and settled portions of microspheres were separated, dried and weighed. Buoyancy was calculated as percentage of the mass of microspheres that remained floated to the total mass of microspheres [4]. % buoyant microspheres = Weight of floating microspheres/Total mass of microspheres×100
Determination of drug entrapment
Accurately weighed 100 mg of microspheres was triturated with 50 ml 0.1 N HCl, sonicated for 2 h and filtered to remove the debris. Volume was made to 100 ml with 0.1 N HCl and diluted suitably before the recording of absorbance at 315 nm using UV spectrophotometer (UV-1700, Shimadzu, Japan). % Drug entrapment = Calculated drug concentration/ Theoretical drug concentration×100
In vitro drug release
In vitro drug release was studied using USP basket apparatus [18-20] in 900 ml of 0.1 N HCl (pH 1.2) filled in a dissolution vessel at a temperature 37±0.5º and paddle speed of 100 rpm. The 10 ml of dissolution medium was withdrawn at time interval of 1 h for 10 h and replaced with same volume of fresh dissolution medium for simulation. The withdrawn samples were filtered through whatmann filter paper and analyzed for the drug content at 315 nm using UV spectrophotometer (UV-1700, Shimadzu, Japan).
Effect of pH modifiers
Following the above mentioned studies, formulations showing higher drug release were selected for evaluation of influence of pH modifiers on RHCl release. Citric acid (5-20%) (CA), fumaric acid (5-20%) (FA) and tartaric acid (5-20%) (TA) were added for the drug-polymer/s solution and prepared microspheres were evaluated for drug release as mentioned earlier.
Results and Discussion
SEM revealed that the prepared microspheres were spherical with smooth surface; although distinct pores were evident on the surface of microspheres, which may be subsequently responsible for drug release (fig. 1). The surface porosity was found crucial for the drug that released by dissolution and diffusion from EC containing formulation that allow water to permeate through surface without dissolving in it [21]. The microphotographs also showed presence of loose crystals of drug on the surface of few microspheres.
Particle size was studied keeping drug ratio constant and varying polymer ratio. As the polymer concentration increases viscosity increases which influence the interaction between disperse phase and dispersion medium that affects the size distribution of particle (Table 1). The increase in polymer concentration in a fixed volume of solvent increases the viscosity of the medium resulting in enhanced interfacial tension. Shearing efficiency is also diminished at higher viscosities thus leading to an increase of the emulsion droplet size and finally a higher microsphere size [22,23].
Floating microspheres prepared by the solvent evaporation method using EC, Eu RS100 and their combination exhibited buoyant behaviour for prolonged period without any apparent gelation over the surface of the dissolution medium. Microspheres prepared at stirring speed of 2000 rpm yielded spherical microspheres with good entrapment efficiency (>60%), yield (>63%) and percent buoyancy (>61%) (Table 1).
Increasing EC and Eu RS100 in prepared microspheres, the microsphere size ranged from 200 μm to 350 μm with Carr’s compressibility index between 9–16 %, varied angle of repose from 30.23±1.87 to 39.23±1.11 and increased Hausner ratio from 1.031±0.04 to 2.56±0.095 demonstrating good flow properties. Percent buoyancy of prepared microspheres was found satisfactory in the range of 61.07±8.40 to 79.25±3.18 at 10 h. The percent buoyancy of EC prepared microspheres was higher than Eu RS100 and their combination (Table 1).
| Formulations | Mean | True | Tapped | Hausener’s | Carr’s | Angle of | Drug | Buoyancy | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| partical | Density | Density | Ratio | Index | Repose | Entrapment | (%) | ||
| size (µm) | (g/cc) | (g/cc) | (%) | ||||||
| RHCl: EC(1:1) | 205±8.1 | 0.748±0.02 | 0.772±0.01 | 1.032± 0.03 | 3.11± 1.97 | 36.37± 0.56 | 60.34±0.13 | 75.14±3.47 | 63.33±0.29 |
| RHCl: EC(1:2) | 219.0±2.7 | 0.488±0.01 | 0.510±0.016 | 1.045±0.01 | 4.38±1.63 35.76±0.47 61.39±0.48 76.54±2.01 66.24±0.36 | ||||
| RHCl: EC(1:3) | 242.0±3.6 | 0.296±0.02 | 0.316±0.01 | 1.066±0.04 | 5.26±0.82 35.13±0.74 64.05±0.69 79.25±3.18 68.37±0.37 | ||||
| RHCl: Eu RS100 | 237.2±1.02 | 0.435±0.023 | 0.451±0.17 | 1.031±0.00 | 3.02±0.73 | 38.88±1.02 | 69.50±0.18 | 71.26±2.30 | 67.90±0.50 |
| (1:1) | |||||||||
| RHCl: Eu RS100 | 273.5±9.6 | 0.593±0.019 | 0.624±0.04 | 1.052±0.04 | 4.97±3.49 | 40.27±1.11 | 75.71±0.49 | 76.35±3.70 | 71.18±0.37 |
| (1:2) | |||||||||
| RHCl: Eu RS100 | 258.0±6.7 | 0.723±0.019 | 0.772±0.012 | 1.066±0.03 | 6.23±2.99 | 39.01±1.01 | 73.41±0.24 | 72.35±2.92 | 74.11±0.28 |
| (1:3) | |||||||||
| RHCl: EC: Eu | 253.1±12.8 | 0.623±0.043 | 0.763±0.012 | 1.23±1.022 | 4.23±1.34 | 34.26±1.65 | 64.23±3.71 | 61.07±8.40 | 64.31±0.59 |
| RS100 (1:1:1) | |||||||||
| RHCl: EC: Eu | 264.2±7.95 | 0.446±0.017 | 0.532±0.018 | 1.56±1.023 | 5.23±1.65 | 37.12±3.12 | 66.70±2.70 | 68.66±1.59 | 67.99±1.06 |
| RS100 (1:2:1) | |||||||||
| RHCl: EC: Eu | 264.4±8.5 | 0.589±0.072 | 0.644±0.063 | 1.56±0.034 | 6.23±2.34 | 32.54±1.22 | 66.45±2.28 | 63.87±3.94 | 72.15±0.92 |
| RS100 (1:3:1) | |||||||||
| RHCl: EC: Eu | 252.1±9.3 | 0.715±0.012 | 0.876±0.045 | 2.56±0.095 | 5.23±1.44 | 30.23±1.87 | 66.50±3.64 | 62.63±2.06 | 65.57±0.47 |
| RS100 (1:1:2) | |||||||||
| RHCl: EC: Eu | 258.1±13.86 | 0.617±0.056 | 0.788±0.034 | 2.48±1.002 | 8.23±1.34 | 37.23±1.23 | 65.37±2.97 | 64.87±3.33 | 68.22±0.61 |
| RS100 (1:1:3) | |||||||||
Microspheres RHCl and EC and/or Eu RS100 in different drug:polymer/s ratio were prepared and evaluated for particle size, density, flow property, drug entrapment and buoyancy.
Table 1: Evaluation Of Rhci Microspheres Prepared With Ec, Eu Rs100 Alone Or In Combination
EC being insoluble and unswellable remained floated, whereas Eu RS100 microspheres swelled and eroded with time. The microspheres prepared with combination of EC and Eu RS100 showed lower buoyancy than the microspheres prepared individually which might be due to higher density and internal voids as compared to individual polymer.
The maximum percentage yield for formulations RHCl:EC (1:3), RHCl:Eu RS100 (1:3) and RHCl:EC:Eu RS100 (1:3:1) was found to be 68.32±0.37, 74.11±0.28 and 72.15±0.92, respectively (average percentage yield was greater than 55%) as compared to other polymeric microspheres. Buoyancy determinations of microspheres from RHCl:EC, 1:3 (79.25±3.18), RHCl:Eu RS100, 1:2 (76.35±3.70) and RHCl:EC:Eu RS100, 1:2:1 (68.66±1.59) indicated that most of the microspheres remained floated after 10 h because of low density and internal voids. The percent RHCl entrapment of 64.05±0.69, 75.71±0.49 and 66.70±2.70, respectively. from RHCl:EC, 1:3; RHCl:Eu RS100, 1:2 and RHCl:EC:Eu RS100, 1:2:1 when compared to other polymeric formulations showed lesser entrapment. This can be attributed to the permeation characteristics of each polymer used that may have facilitated the diffusion of entrapped drug to the surrounding medium during the preparation of floating microspheres (Table 1).
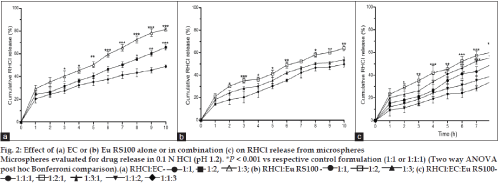
Microspheres prepared with EC (RHCl:EC, 1:3) exhibited higher release (81%) and found to increase significantly with its level [Two way ANOVA: F(2, 66) = 126.05, P<0.001 vs RHCl:EC, 1:1] (fig. 2a) while RHCl:Eu RS100, 1:2 formulation showed higher RHCl release than RHCl:Eu RS100, 1:1 and RHCl:Eu RS100, 1:3) [Two way ANOVA: F(2, 66) = 52.93, P<0.001 vs Eu RS100, 1:1] (fig. 2b).
Fig. 2: Effect of (a) EC or (b) Eu RS100 alone or in combination (c) on RHCl release from microspheres
Microspheres evaluated for drug release in 0.1 N HCl (pH 1.2). *P < 0.001 vs respective control formulation (1:1 or 1:1:1) (Two way ANOVA post hoc Bonferroni comparison).(a) RHCl:EC- 1:1, 1:2, 1:3; (b) RHCl:Eu RS100 - 1:1, 1:2, 1:3; (c) RHCl:EC:Eu RS100-1:1:1, 1:2:1, 1:3:1, 1:1:2, 1:1:3
The microspheres prepared using combination of EC and Eu RS100 significantly influenced the release rate, while highest release observed from the microspheres prepared with RHCl:EC:Eu RS100, 1:2:1 [Two way ANOVA: F(4, 110) = 47.59, P<0.001 vs RHCl:EC:Eu RS100, 1:1:1] (fig. 2c). Microspheres prepared with higher proportion of EC influenced RHCl release than Eu RS100.
Organic acids have been reported to delay or sustain drug release in formulations where enteric polymers were used as matrix and film forming agents as a result of lowered pHM [24-26]. This effect gives synergistic effect with polymer to retard the drug release by maintaining acidic environment in polymeric microspheres. Hence, further to influence the release rate from the prepared microspheres, formulations exhibiting higher release (RHCl:EC, 1:3; RHCl:Eu RS100, 1:2) when used alone or in combination (RHCl:EC:Eu RS100, 1:2:1) were used for studying the effect of pH modifiers viz. FA, CA and TA on drug release. The addition of CA (5-20-% w/w), FA (5-20-% w/w) or TA (5-20-% w/w) in the drug-polymer/s solution did not exhibit any significant effect on drug entrapment, size, shape, flow properties when compared against the microspheres prepared with same polymer without pH modifiers, whereas release of drug from floating microspheres evaluated at pH 1.2 was found almost linear with time for 10 h (figs. 3 to 5).
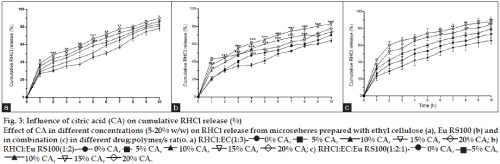
Fig. 3: Influence of citric acid (CA) on cumulative RHCl release (%)
Effect of CA in different concentrations (5-20% w/w) on RHCl release from microspheres prepared with ethyl cellulose (a), Eu RS100 (b) and in combination (c) in different drug:polymer/s ratio. a) RHCl:EC(1:3)- 0% CA, 5% CA, 10% CA, 15% CA, 20% CA; b)
RHCl:Eu RS100(1:2)- 0% CA, 5% CA, 10% CA, 15% CA, 20% CA; c) RHCl:EC:Eu RS100(1:2:1)- 0% CA, 5% CA,
10% CA, 15% CA, 20% CA.
Two-way ANOVA indicated the significant effect of CA on release rate from RHCl:EC, 1:3 formulations [F(4, 110)= 70.31, P<0.001] (fig. 2a) and RHCl:EC:Eu RS100, 1:2:1 [F(4, 110) = 55.01, P<0.001] (fig. 2c).
The release of RHCl from formulation RHCl:EC, 1:3 (38.77±3.04) and RHCl:EC:Eu RS100, 1:2:1 (42.88±0.91) microspheres was found increased at the end of 10 h (89.96±2.69 and 87.89±5.55 respectively) at 20% w/w CA. RHCl:Eu RS100, 1:2 microspheres showed release of 83.03±2.06% at 15% w/w CA and decreased to 77.87±0.94% with increased CA to 20% w/w [F(4, 110) = 146.90, P<0.001] (fig. 2b). The release of ranitidine from microspheres of RHCl:EC, 1:3; RHCl:Eu RS100, 1:2 and RHCl:EC:Eu RS100, 1:2:1 formulations was significantly enhanced by CA at 15% w/w, 15% w/w and 20% w/w respectively. It is noteworthy that CA (15 and 20% w/w) addition in Eu RS100 microspheres (RHCl:Eu RS100, 1:2) exerted significant effect on drug release throughout the dissolution studies (1-10 h). However significant influence of CA remained only for 6 h in EC (RHCl:EC, 1:3) formulations whereas delayed (7-10 h) effect on ranitidine release was observed for EC:Eu RS100 (RHCl:EC:Eu RS100, 1:2:1) microspheres.
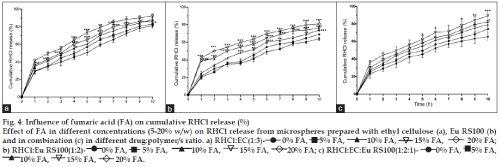
As depicted in (fig. 4), addition of FA in the polymeric microspheres (15-20%) significantly enhanced the ranitidine release. Two way ANOVA indicated the significant effect on release rate from formulation RHCl:EC, 1:3 (85.89±2.43) [F(4,110)= 57.47, P<0.001] (fig. 4a) and RHCl:EC:Eu RS100, 1:2:1 (89.34±5.34) [F(4,110)= 24.01, P<0.001] (fig. 4c) with 15% w/w of FA and found to decrease the microspheres with 20% FA whereas addition of FA in 15 and 20% both exerted release enhancement in RHCl:Eu RS100, 1:2 microspheres (89.34±5.34) [F(4, 110) = 182.24, P<0.001] (fig. 4b). The release rate from microspheres with 10–20% FA was more significant from EC (RHCl:EC, 1:3) and Eu RS100 (RHCl:Eu RS100, 1:2) microspheres than formulation prepared with their combination (RHCl:EC:Eu RS100, 1:2:1).
Fig. 4: Influence of fumaric acid (FA) on cumulative RHCl release (%)
Effect of FA in different concentrations (5-20% w/w) on RHCl release from microspheres prepared with ethyl cellulose (a), Eu RS100 (b) and in combination (c) in different drug:polymer/s ratio. a) RHCl:EC(1:3)- 0% FA, 5% FA, 10% FA, 15% FA, 20% FA;
b) RHCl:Eu RS100(1:2)- 0% FA, 5% FA, 10% FA, 15% FA, 20% FA; c) RHCl:EC:Eu RS100(1:2:1)- 0% FA, 5% FA,
10% FA, 15% FA, 20% FA.
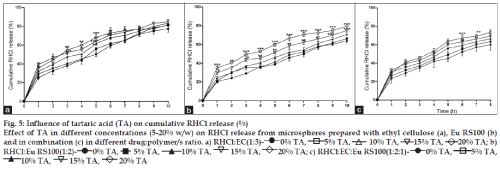
Addition of TA (15-20%) produced significant effect on RHCl release from the prepared microspheres. Two way ANOVA demonstrated significant effect on release rate with 20% w/w TA in microspheres prepared with EC (RHCl:EC-81.66±1.31) [F(4,110)=29.50, P<0.001] (fig. 5a) and its combination with Eu RS100 (RHCl:EC:Eu RS100-80.14±1.54) [F(4,110)=25.80, P<0.001] (fig. 5c). TA (20% w/w but not 15% w/w) showed significant effect on drug release from the floating microspheres prepared with Eu RS100 (RHCl:Eu RS100-79.58±2.64) [F(4,110)=128.99, P<0.001]. TA (20%) produced significant influence on the RHCl release throughout the dissolution period of 10 h in the microspheres prepared with Eu RS100 whereas delayed and early effect was seen in the EC and combination polymeric microspheres.
Fig. 5: Influence of tartaric acid (TA) on cumulative RHCl release (%)
Effect of TA in different concentrations (5-20% w/w) on RHCl release from microspheres prepared with ethyl cellulose (a), Eu RS100 (b) and in combination (c) in different drug:polymer/s ratio. a) RHCl:EC(1:3)- 0% TA, 5% TA, 10% TA, 15% TA, 20% TA; b)
RHCl:Eu RS100(1:2)- 0% TA, 5% TA, 10% TA, 15% TA, 20% TA; c) RHCl:EC:Eu RS100(1:2:1)- 0% TA, 5% TA,
10% TA, 15% TA, 20% TA
Several researchers have successfully enhanced the release of weakly basic drug compounds from swellable tablets using hydrophilic polymers by incorporating pH modifiers, such as succinic, fumaric or adipic acid [12,15,27,28]. They act principally by reducing the pHM and thereby enhancing the drug solubility and dissolution. The difference in the extent and duration of pH modulation and therefore drug release depended on the acidic strength and the aqueous solubility of the included pH modifiers. Included pH modifiers are assumed to modulate the pHM independently from the bulk pH thus enhancing drug solubility and drug dissolution. As illustrated in figs. 3-5 incorporation of pH modifiers significantly enhanced the drug release rate at pH 1.2, but the extent of release enhancement was dependent on the type and levels of pH modifier. The addition of FA (>15%) led to the highest drug release (86.8%), followed by CA (>15%) (65.6%) and TA (20%) (51.7%) while lower levels of pH modifiers failed to produce any significant effect on release rate which may be due their ability to influence significant change in pHM. However, even though the difference in solubility between CA and TA is five-fold, their release profiles were almost identical. pH modifiers, showing good aqueous solubility, dissolved immediately in the infiltrated medium and afterwards rapidly diffused out. Due to low saturation solubility, a large amount of the initially incorporated FA remained undissolved and might have replenished the released acid over time. Espinoza et al. [12] proposed that CA acts more as a hydrosoluble excipient than an acidic one. That means, the rapid release of CA from the microspheres increases the porosity and thereby the drug release. This phenomenon is illustrated by the respective release. The low pHa1 (3.03) values and poor water solubility (10 mg/ ml) of FA led to a significant and extended effect on pHM modification. As demonstrated previously, the addition of higher FA amounts (>15% w/w) resulted in a constant increase in drug release over the entire dissolution period [14]. FA with a high acidic strength and the lowest aqueous solubility resulted in the most pronounced enhancement of the drug release. The less soluble FA acidified and might have maintained the microenvironment throughout the entire dissolution period of 10 h. Earlier studies have found a close relationship between the pHM and release enhancement by pH modifiers [14,29,30]. The maintenance of a low pHM is the principal explanation for the higher drug release in the presence of FA. Although not determined the addition of modifiers viz FA, CA, TA to RHCl-EC and/or Eu RS100 might have decreased the pHM in the floating microspheres, enhanced dissolution and therefore release of weakly basic drug. The maintenance of constant and low acidic microenvironment by FA is therefore enhanced RHCl release from all the polymeric microspheres to the greatest extent, followed by CA (pHa1: 3.12) and TA (pHa2: 4.2) being the weak (compared to FA) exhibited the least pronounced effect on drug release. pH modifiers besides the effect on pHM additionally influence drug release by altering the gel layer properties, osmotic pressure, swelling dynamics and contribute for the enhanced drug release [31,32]. In conclusion, incorporation of pH modifiers viz CA, FA and TA have successfully enhanced drug release probably through modulation of pHM affecting dissolution behaviour and therefore can be a useful tool to improve the bioavailability of the drug like RHCl which degrades in lower intestine or may show poor absorption towards the lower end of GIT [33].
Since RHCl has poor bioavailability (30-50%) and has absorption window in upper part of gastrointestinal tract, therefore it can be proposed that floating microspheres may enhance the absorption and bioavailability of RHCl by increasing the gastric residence. However the bioavailability studies are needed to be carried to confirm. Present study also demonstrates the suitability of EC and/or Eu RS100 polymer/polymeric system for formulating buoyant microspheres which may prove useful in controlled drug delivery of weakly basic drug like ranitidine with or without pH modifier.
References
- Chawla G, Gupta P, Koradia V, Bansal AK. Gastroretention a Means to Address Regional Variability in Intestinal Drug Absorption. Pharm Tech 2003;50-68.
- Yeole PG, Khan S, Patel VF. Floating drug delivery systems: Need and development. Indian J Pharm Sci 2005;67:265-72.
- Singh BN, Kim KH. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Control Rel 2000;63:235-59.4. Srivastava AK, Ridhurkar DN, Wadhwa S. Floating microspheres of cimetidine: formulation, characterization and In vitro evaluation. Acta Pharm 2005;55:277-85.
- Satoa Y, Kawashimab Y, Takeuchib H, Yamamoto H. Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm 2003;55:297-304.
- Zhang L, Wan M, Wei Y. Hollow Polyaniline Microspheres with Conductive and Fluorescent Function. Macro Rapid Comm 2006;27:888-93.
- Zhou HY, Chen XG, Liu CS, Meng XH, Liu CG, He Jun, et al. Celluose Acetate/Chitosan Multiiospheres Preparation and Ranitidine Hydrochloide Release In vitro. Drug Delivery 2006;13:261-7.
- Wei Y, Zhao L. In vitro and in Vivo Evaluation of Ranitidine Hydrochloride Loaded Hollow Microspheres in Rabbits. Arch Pharm Res 2008;31:1369-77.
- Hosny EA, Abd Al-Raheem M Al-Helw. Effect of coating of aluminum carboxymethylcellulose beads on the release and bioavailability of diclofenac sodium. Pharm ActaHelv 1998;72:255-61.
- Thoma K, Ziegler I. Investigations on the influence of the type of extruder for pelletization by extrusion-spheronization. I. Extrusion behavior of formulations. Drug Develop Ind Pharm 1998;25:413-22.
- Streubel A, Siepmann S, Dashevsky A, Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J Control Release 2000;67:101-10.
- Espinoza R, Hong E, Villafuerte L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int J Pharm 2000;201:165-73.
- Nie S, Pan W, Li X, Wu X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Develop Ind Pharm 2004;30:627-35.
- Siepe S, Herrmann W, Borchert H, Lueckel B, Kramer A, Ries A, et al. Microenvironmental pH and microviscosity inside pH-controlled matrix tablets: An EPR imaging study. J Control Release 2006;112:72-8.
- Siepmann SJ, Bodmeier R. Multiple unit gastroretentive drug delivery systems: A new preparation method for low density microparticles. J Microencap 2003;20:329-47.
- Trivedi P, Verma AM, Garud N. Preparation and characterization of aceclofenac microspheres. Asian J Pharm 2008;2:110-5.
- Rahman Z, Kohli K, Khar RK, Ali M, Charoo NA, Shamsher AA. Characterization of 5-Fluorouracil Microspheres for Colonic Delivery. AAPS Pharm Sci Tech 2006;7:47-53.
- Singh BN, Kim KH. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Control Release 2000;63:235-59.
- Dinarvand R, Mirfattahi S, Atyabi F. Preparation, characterization and In vitro drug release of isosorbidedinitrate microspheres. J Microencap 2002;19:73-81.
- Abrol S, Trehan A, Katare OP. Formulation, characterization, and In vitro evaluation of silymarin-loaded lipid microspheres. Drug Delivery 2004;11:185-91.
- Lee JH, Park G, Choi HK. Effect of formulation and processing variables for water soluble drugs prepared by w/o/o double emulsion solvent diffusion method. Int J Pharm 2000;196:75-83.
- Behera BC, Sahoo SK, Dhal S, Barik BB, Gupta BK. Characterization of glipizide-loaded polymethacrylate microspheres prepared by an emulsion solvent evaporation method. Trop J Pharm Res 2008;7: 879-85.
- Pachuau L, Sarkar S, Mazumder B. Formulation and evaluation of matrix microspheres for simultaneous delivery of salbutamol sulphate and theophylline. Trop J Pharm Res 2008;7:995-1002.
- Ishibashi T, Ikegami K, Kubo H, Kobayashi M, Mizobe M, Yoshino H. Evaluation of colonic absorbability of drugs in dogs using a novel colon-targeted delivery capsule (CTDC). J Control Release 1999;59: 361-76.
- Ethyl Cellulose. In: Rowe RC, Sheskey PJ, Owen CS, editors. Handbook of Pharmaceutical Excipient, 4th edn., London: Pharmaceutical Press
- 2000. p.737-52.
- Nykanen P, Lempaa S, Aaltonen ML, Jurjenson H, Veski P, Marvola M. Citric acid as excipient in multiple-unit enteric-coated tablets for targeting drugs on the colon. Int J Pharm 2001;229:155-62.
- Gabr KE. Effect of organic acids on the release patterns of weakly basic drugs from inert sustained release matrix tablets. Eur J Pharm Biopharm 1992;38:199-202.
- Burnside BA, Chang RK, Guo X. Sustained release pharmaceutical dosage forms with minimized pH dependent dissolution profiles. 2001; US Patent 6287599.
- Tatavarti S, Hoag SW. Microenvironmental pH modulation based release enhancement of a weakly basic drug from hydrophilic matrices. J Pharm Sci 2006;95:1459-68.
- Badawy SI, Hussain MA. Microenvironmental pH modulation in solid dosage forms. J Pharm Sci 2007;96:948-59.
- Pillay V, Fassihi R. Electrolyte-induced compositional heterogeneity: a novel approach for rate-controlled oral drug delivery. J Pharm Sci 1999;88:1140-8.
- Durig T, Fassihi R. Guar-based monolithic matrix systems: effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J Control Release 2002;80:45-56.
- Kaza R, Usharani E, Nagaraju R, Haribabu R, Siva Reddy PV. Design and Evaluation of Sustained release Floating tablets for the treatment of Gastric Ulcers. J Pharm Sci Res 2009;1:81-7.