- *Corresponding Author:
- R. Sahu
Department of Pharmaceutical Chemistry, Institute of Pharmaceutical Research, GLA University, Mathura, Uttar Pradesh 281406, India
E-mail: rakeshsahu1100@gmail.com
| Date of Received | 04 June 2022 |
| Date of Revision | 16 February 2023 |
| Date of Acceptance | 04 December 2023 |
| Indian J Pharm Sci 2023;85(6):1539-1550 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Alzheimer's disease is a neurodegenerative disease that impairs motor and cognitive function it is spreading very quickly in the current scenario with a male:female ratio between 1:2 to 1:5 due to various causes associated with it which include education, diet and nutrition, sleep and cardiac rhythm and head trauma etc. Previously available drugs like tacrine failed to prove their efficacy and later were withdrawn from the market due to excess toxicity, lower solubility, and slow bioavailability. Among them, benzofuran-based drugs make a significant contribution because they are one of the important pharmacophores found both synthetically and naturally in heterocyclic compounds, with a wide range of therapeutic applications in the field of drug discovery that offers many opportunities for further improvement in anti-Alzheimer agents by acting on numerous prominent receptors. So, this article focuses on various benzofuran derivatives and their advancement in the treatment of Alzheimer’s disease with a summary of the synthesis of benzofuran and benzofuran-based derivatives which assist scientists in developing successful medications with the necessary pharmacological action.
Keywords
Alzheimer’s disease, benzofuran, derivatives, biomarkers, synthetic scheme
Alzheimer's Disease (AD) is a progressive neurological disease that causes permanent cognitive decline, linguistic degradation and severe behavioural abnormalities before death[1]. It is also reported as one of the primary causes associated with dementia in old age, with 14 to 130 million people expected to be affected in Europe and globally by 2050[2,3]. The pathophysiology of AD is difficult and poorly understood. However, several variables are considered bioindicators such as low Acetylcholine (ACh) value[4,5], abnormal-amyloid peptide deposition, oxidative stress, tau protein hyperphosphorylation[6,7] and biometal dyshomeostasis. In 2013, approximately 85 000 people died from AD which is the sixth biggest cause of mortality in the United States[8,9]. It is reported as the most common cause of continued dementia in the aging population, contributing to 65 %-70 % of all cases[10]. Furthermore, women have a higher frequency of AD, with men-to-women ratios found between 1:2 to 1:5[11,12]. The various study revealed that the expected lifetime risk for alzheimer's at 45 age was found to be around one in five which is almost (20 %) for women and shows one in ten (10 %) for men at 65 age. This has been illustrated graphically in which the chances increased slightly for both genders and can be seen as a gender bias[13].
Acetylcholinesterase (AChE) inhibitor therapy has dominated the management of AD, consequently, memory and cognitive performance have improved slightly. By restricting ACh turnover and restoring synaptic levels of this neurotransmitter, these medications make up for the loss of cholinergic neurons and provide symptomatic relief[14,15]. Current AD therapy options memory and cognitive performance have improved slightly with cholinesterase potent inhibitor[14] and an N-Methyl-D-Aspartate receptor antagonist (memantine). However, they do not prevent or stop the progressive neurodegeneration. Nonetheless, experimental investigations revealed that when an AChE inhibitor is associated with the therapy, it modifies its enzymatic and pharmacological characteristics and preventing such connection may be advantageous in the treatment of AD patients[16,17].
Benzofuran is an important family of heterocyclic chemicals that can be understood as benzene fused with furan ring and has formula C8H6, its chemical structure is shown in fig. 1[18]. It may be found in various bioactive natural items, medicines, and polymers[19]. Because of its broad biological profile, it is regarded as one of the most significant heterocyclic rings[20]. Benzofuran has several medicinal applications, including antiarrhythmic, antitussive, antitubercular[21], antidepressant[22], anti-gout, anti-fungal and antihypertensive agents[23]. According to an amyloid-β peptide model, fomannoxin is a natural benzofurancontenting compound, showing exceptional neuroprotective capabilities. The significance of this discovery stems from the fact that antiamyloid treatments are seen as a viable alternative to present Alzheimer's drugs[24]. As a result, in this article, we will look into benzofuran-based derivatives as a viable alternative therapeutic option for AD. Some important physicochemical properties are tabulated in Table 1[25,26].
| S No. | Molecular weight | Solubility | Boiling point | Melting point | Refractive index | pKa |
|---|---|---|---|---|---|---|
| 1 | 118.1326 g/mol | Soluble in organic solvent and insoluble in water and | 173° (343°F; 446 K) | -18° (0°F; 255 K) | 34.9 m3.mol-1 | -2.9 |
Table 1: Physical Property of Benzofuran
Classification of Antibiotics
According to a recent study, the causes of AD are hereditary, since a family history of Alzheimer’s dementia has been linked to the late onset of AD[27]. Education, higher levels of education are linked to having a lower risk accounting for AD[28]. Physical, mental and a variety of activities including cognitive, social and recreational activities have been linked to a reduced risk of dementia[29-32]. Additionally, some important factors also associated with AD like sleep and the 24 h cycle there is evidence that sleep is crucial for amyloid clearance and less sleep is therefore associated with amyloid build-up in the brain. However, the relationship appears bidirectional since amyloid disease can disturb sleep patterns[33]. Trauma to the head, traumatic brain injury has been associated with a higher risk for dementia[34]. However, whether AD is more likely to lead to pathologic outcomes than other neurodegenerative disease processes such as the recently discovered chronic traumatic encephalopathy[35-44].
The patent of benzofuran-based derivative which has the potential to become an alternative drug is tabulated in Table 2. Also, a list of benzofuranbased drugs which has recently been marketed is in Table 3.
| S no. | Patent no | Patent date | Inventors | Description |
|---|---|---|---|---|
| 1 | JP6330011B2 | May 23, 2018 | Steven Martin, Steven Martin Courtney, Michael Prime, Michael Prime, William Mitchell, William Mitchell, Christopher John Brown, K Wristfer John Brown, Agia Pena, Paula Se. Duagia Pena, Paula Se. Dopeter Johnson, Peter Johnson, Celia Dominguez, Celia Dominguez, Leticia Em. Toledo Sherman, Leticia Em. Toledo Sherman, Ignacio Munos, Ignacio Munos | Kynurenin-3-monooxygenase inhibitor, pharmaceutical composition thereof, and method of use thereof. |
| 2 | CN105294662B | April 20, 2018 | Li Xingshu, Wang Zhiren, Huang Ling and Chen Xinzi | Benzofuran quinolone derivative and application of benzofuran quinolone derivative in preparation of medicine for treating alzheimer's disease. |
| 3 | KR101662853B1 | May 10, 2016 | Didier RochegisleMutanno Ingo KoberphrancesComtarsejuChristman-FrancisaumitraCentaguptarameshSistlaoGumadiBenkateshawar | Benzofurane, benzothiophene, and benzothiazol derivatives as fxr modulators. |
| 4 | JP5767211B2 | August 19, 2015 | Johann Andershon, Helena Yübeckanf, Johansson Christian, Erik Lindejonas, MalmströmgunnarNordwalgitteTelputachanaWeigelt | 2-Carboxamide-7-piperazinyl-benzofuran derivative 774 |
| 5 | USRE44354E1 | July 09, 2013 | HankF.Kung, Mei PingKung, Zhi-Ping Zhuang,VirginiaM.Y.Lee, JohnQ.Trojanowski,Daniel M. Skovronsky | Amyloid plaque aggregation inhibitors and diagnostic imaging agents |
| 6 | EP1945622B1 | December 28, 2011 | William E. Klunk, Jr. Chester A. Mathis | Isotopically-labeled benzofuran compounds as imaging agents for amyloidogenic proteins. |
| 7 | DE60310753T2 | November 10, 2007 | Ana Martinez Gil, Isabel Dorronsoro Diaz, Laura Rubio Arrieta, Diana Alonso Gordillo, Ana Fuertes Huerta, Susana Morales-Alcelay, Maria Del Monte Millan, Esther Garcia Palomero, Paola Usan Egea, Celia De Austria, Miguel Medina Padilla | Dual binding acetylcholinesterase inhibitors for the treatment of alzheimer. |
| 8 | AU2005280921B2 | May 19, 2011 | Makoto Jitsuoka, Norikazu Ohtake, NagaakiSatoShigeruTokita, Daisuke Tsukahara | Carbamoyl-substituted spiro derivative. |
| 9 | EP1497279B1 | March 09, 2011 | Oliver Schadt, Henning Böttcher, Joachim Leibrock, Kai Schiemann, Timo Heinrich, Günter Hölzemann, Christoph Van Amsterdam, Gerd Bartoszyk, Christoph Seyfried | Substituted indoles and their use as 5ht-reuptake inhibitors and as 5ht ligands |
| 10 | US7741354B2 | June 22, 2010 | Michael Thormann, Michael Altmstetter, Andreas Treml, Ulrich Heiser, Mirko Buchholz | Novel inhibitors of glutaminyl cyclase |
| 11 | US20090082434A1 | March 26, 2009 | Jonathan Laird Gross, Marla Jean Williams, Gary Paul Stack, Hong Gao, Dahul Zhou | Dihydro benzofuranyl Alkanamine Derivatives and Methods for Using Same. |
Table 2: List of Patented Drugs having Benzofuran Moiety
| S No. | Name of drug | Primary target | Chemical structure | Reference |
|---|---|---|---|---|
| 1 | 99mTc]BAT-bp-1 | β-amyloid plaques | 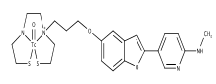 |
[35] |
| 2 | 5-bromo-2-(4 hydroxybenzyl)benzofuran | Butyrylcholinesterase Inhibitor | 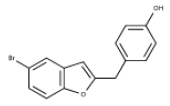 |
[36] |
| 3 | Benzofuran-tetrazole | Inhibit Aβ aggregation | 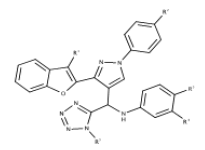 |
[37] |
| 4 | Benzofuran–Chalcone Hybrids | inhibit Ab fibril formation, directly scavenge (ROS). | 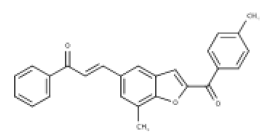 |
[38] |
| 5 | Tacrine−Benzofuran Hybrids | AChE and BChE Inhibitor | 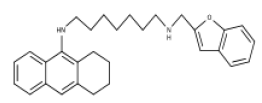 |
[39] |
| 6 | Coumarin-Benzofuran Hybrids | Inhibit Aβ aggregation and AChE | 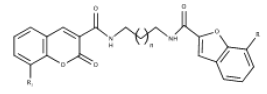 |
[40] |
| 7 | 2-benzylidene-benzofuran-3 | anti-acetylcholinesterase (AChE)/butyrylcholinesterase (BChE) | 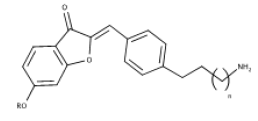 |
[41] |
| 8 | Benzofuran-Based Hybrid based on SKF-64346 | Inhibition of Cholinesterase Activity, β-Amyloid Aggregation. | 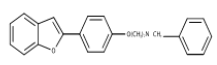 |
[42] |
| 9 | MMBO | Inhibit neurofibrillarytangles, tau phosphorylation. | 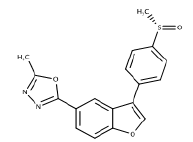 |
[43] |
| 10 | 2-aryl Benzofuran | Anti-acetylcholinesterase | 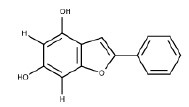 |
[44] |
Table 3: List of Recently Marketed Drugs having Benzofuran Moiety
Biomarkers
Even at specialized centres, around 20 % of people are misdiagnosed with AD when the diagnosis is entirely clinical. As a result, the role of biomarkers in the diagnostic process is growing in importance, as positive biomarkers enhance diagnosis accuracy[45-47]. Structural imaging; Functional Brain Imaging (FBI); Cerebrospinal Fluid (CSF); amyloid beta Positron Emission Tomography (PET) were used.
General synthetic scheme for benzofuran analogues:
Since the medicinal significance of benzofurans is very broad, major efforts have been made to find novel synthetic techniques for their synthesis[48]. Some important syntheses of benzofurans are listed below.
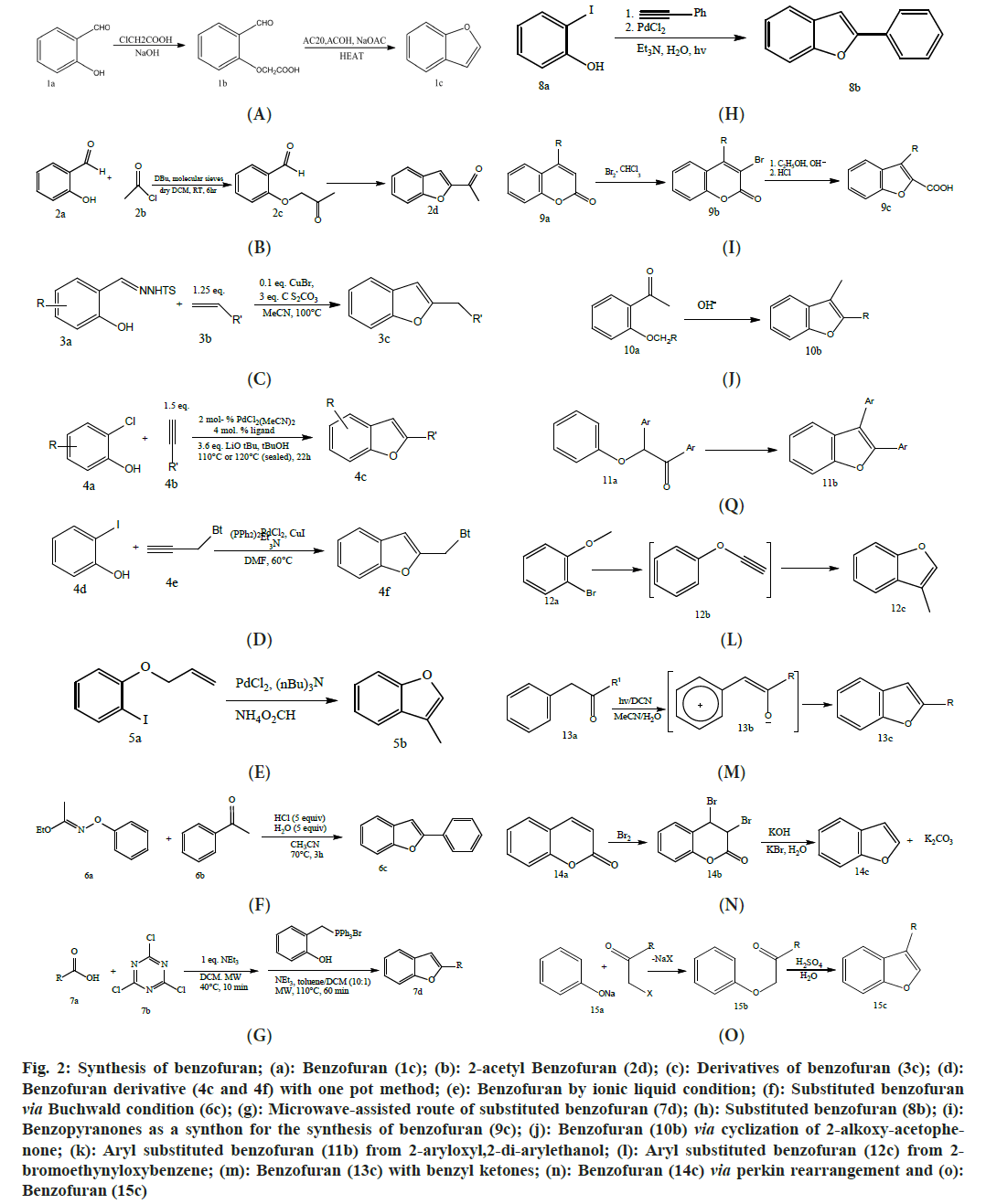
Abu-Hashem et al.[49] reported salicylaldehyde(1a) interacts with chloroacetic acid, to form o-formylphenoxyacetic acid(1b). It yields benzofuran(1c) when glacial acetic acid is refluxed with acetic anhydride as shown in fig. 2a. Rangaswamy et al.[50] reported Salicylaldehyde(2a) was treated with 1chloropropan-2one(2b) in the occurrence of 1,8diazabicyclo-undec- 7ene which work as an activator to produce 2-acetylbenzofuran(2d) (fig. 2b). Zhou et al.[51] reported the synthesis of benzofurans derivatives is made possible by the ligand-free cyclization of closing alkynes2(3b) in the presence of CuBr catalyst with the N-tosylhydrazones produced from ortho hydroxybenzaldehyde(3a). The reaction conditions are tolerated by a wide spectrum of functional groups (fig. 2c).
Fig. 2: Synthesis of benzofuran; (a): Benzofuran (1c); (b): 2-acetyl Benzofuran (2d); (c): Derivatives of benzofuran (3c); (d): Benzofuran derivative (4c and 4f) with one pot method; (e): Benzofuran by ionic liquid condition; (f): Substituted benzofuran via Buchwald condition (6c); (g): Microwave-assisted route of substituted benzofuran (7d); (h): Substituted benzofuran (8b); (i): Benzopyranones as a synthon for the synthesis of benzofuran (9c); (j): Benzofuran (10b) via cyclization of 2-alkoxy-acetophenone; (k): Aryl substituted benzofuran (11b) from 2-aryloxyl,2-di-arylethanol; (l): Aryl substituted benzofuran (12c) from 2-bromoethynyloxybenzene; (m): Benzofuran (13c) with benzyl ketones; (n): Benzofuran (14c) via perkin rearrangement and (o): Benzofuran (15c)
Wang et al.[52] reported an efficient ligand for Pd catalyzation with one-pot formation of benzofuran derivatives (4c and 4f), 2-chlorophenols(4a) combined with alkynes(4b) in the availability of hydroxyterphenylphosphine (fig. 2d). Xie et al.[53] reported the heck reaction with palladium (Pd) catalyzation is well-known in the formation of the benzofuran analogs (5b). The benefit of intramolecular heck reaction-Pd-catalyzed through ionic liquid catalysis is obvious (fig. 2e). Sheradsky et al.[54] reported a one-pot technique by treating acetophenone (6a) under modified Buchwald conditions, employing acetonitrile instead of dioxane (fig. 2f). This resulted in an excellent yield of benzofuran (6c) via hydrolysis and rearrangement[55].
de Luca et al.[56] reported an efficient and gentle microwave-assisted method for producing ortho-substituted benzofurans(7d) straight from acid derivatives (7a). Enables the synthesis of alkyl 2-benzofuran-methane amines from the 2,4,6-trichloro(1,3,5)triazine (7b), non-racemized N-protected amino acids (fig. 2g). Ghosh et al.[57] reported One-pot intermolecular attachment and cyclization which is driven by visible light was performed for 5-endodig in the water of orthohalophenol derivative(8a) with closing alkynes generated by Pd have already been established to provide elevated quantities of 2aryl or alkyl benzofurans (8b) with no as such accomplice of Ru or Ir complexes or any other possible component (fig. 2h). Zykov et al.[58], Kadieva et al.[59], and Brent et al.[60] reported benzopyranones(9a) or coumarins (LI) can be utilized as synthons for the Fittig-Ebert-Perkin reaction or pyrolysis to produce benzofurans(9c) as shown in fig. 2i.
A group of researchers reported several kinds of intramolecular cyclization of 2-alkoxyacetophenones (10a) have been described, resulting in 2-R-3-methylbenzofurans (10b). As condensing agents, Na and K alcoholates[61-63] or alkalis[64-68] in the polar solvent/environment are being utilized[69,70] as shown in fig. 2j. Montfort et al.[71] and Grinev et al.[72] reported by providing heat in the environment of a catalytic amount of poly-phosphoric acid, the cyclization of the 2-aryloxyl,2di-arylethanol(11a) or any possible available alkoxybenzenes may be exploited to produce benzofurans derivative(11b) with the aromatic substituents at ortho or meta position as shown in fig. 2k.
Inanaga et al.[73] and Tsukazaki et al.[74] reported benzofuran synthesis from the cyclization of 2bromoethynyloxybenzene(12a), 2bromovinyloxybenzene(12b), or benzoylmethyl- benzenes, a radical reaction which connects the alkyl and aryl carbon atoms as shown in fig. 2l. Pandey et al.[75] reported benzyl ketones(13a) when exposed to a mercury lamp in the presence of 1,4dicyanonaphthalene (DCN), undergo a transformation into the intermediate compound(13b), which is then later converted to the equivalent related benzofuran analog (13c). The carbonyl oxygen attacks the furan ring intramolecularly before it closes as shown in fig. 2m.
Blennow et al.[76] reported coumarone(14a) was used to create benzofuran from coumarin. By perkin rearrangement, the available intermediate 3,4-dibromo-3,4-dihydrocoumarin(14b) with the presence of KOH leads to benzofuran synthesis(14c) as shown in fig. 2n. Speicher et al.[77] reported benzofurans(15c) which are produced by reacting phenolates with halo ketones (15a) and then cyclo dehydrating them with H2SO4, polyphosphoric acid, or zeolites as shown in fig. 2o.
Anti-Alzheimer Activity of Benzofuran-Based Derivative and Their Synthetic Scheme
Ab fibril formation inhibitor:
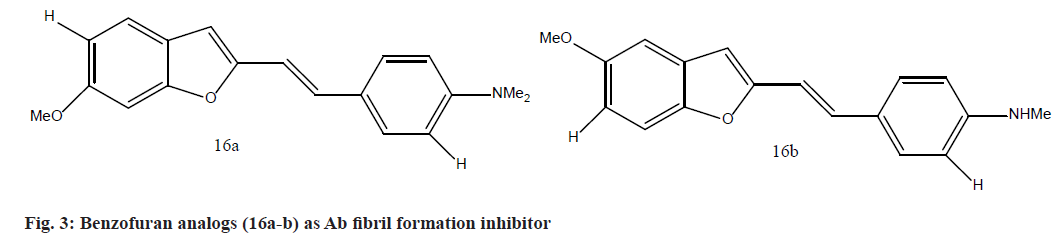
Byun et al.[78], and Cheng et al.[79,80] offered many potential ways for developing ligands with specialized high binding affinity to Ab fibrils. They are designing via the novel series of amino-styryl benzofuran analogs and detailed their inhibitory effects for Ab fibril production. Thioflavin T (ThT) test is used to assess the activity of synthesized compounds against the fibrillization of Ab. Compounds (16a)as well as (16b) (fig. 3) showed better inhibitory actions (IC50 14 0.07 and 0.08 mM, respectively) than the curcumin (IC50 14 0.80 mM) and IMSB (IC50 14 8.00 mM) as reference compounds.
Cholinesterase inhibitor:
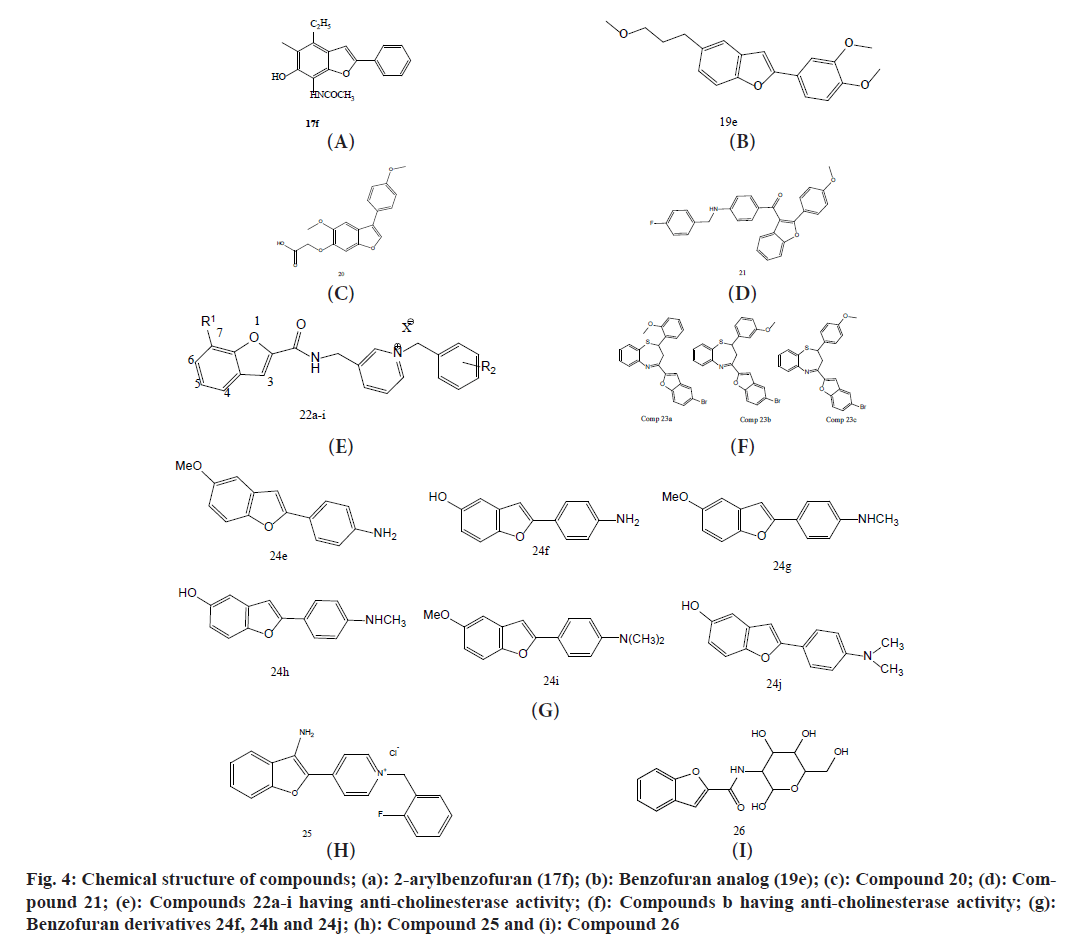
Yun et al.[45] reported to study its anti-AD efficacy, 2-arylbenzofurans were synthesized. Benzofuran compounds have received a lot of attention due to their numerous biological and as well as therapeutical activities, such as anti-inflammatory[81], anti- bacterial[82], hypoglycaemic[83], anti-oxidant[84], anti-tumor[85], anti-cholinesterase[86], anti- fungal[87], anti-monoamine oxidase[88] and others. AD etiology is multifaceted, and multi-targeted therapies outperform single-targeted treatments in this illness[89]. Recently, many benzofuran-based compounds have been found as potent AChE inhibitors. the production and activity of 2aryl-benzofuran (17f) analogs as BACE1 and ChE inhibitors. 2-arylbenzofuran (17f) was synthesized from substituted 2hydroxy-benzaldehyde in 3 steps. The routes for the production of 2aryl- benzofuran analogs are represented in fig. 4a. It was synthesized by utilizing a slightly modified drozdzik approach.
Fig. 4: Chemical structure of compounds; (a): 2-arylbenzofuran (17f); (b): Benzofuran analog (19e); (c): Compound 20; (d): Compound 21; (e): Compounds 22a-i having anti-cholinesterase activity; (f): Compounds b having anti-cholinesterase activity; (g): Benzofuran derivatives 24f, 24h and 24j; (h): Compound 25 and (i): Compound 26
Benzofuran analogue with anti-aggregation activity:
Ha et al.[90] produced an orally active, Blood- Brain-Barrier (BBB) permeable benzofuran derivative with significant anti-aggregation action of compound 19e (MDR-1339). 2-(3,4-dimethoxyphenyl)-5-(3-methoxypropyl) benzofuran (fig. 4b) not only restored cellular survival after amyloid beta-induced cytotoxicity but also enhances adaptive learning ability and memory brain function in AD of the model mice by decreasing amyloid beta accumulation in the brains.
Kim et al.[91] studied by using thioflavin T tests, gel electrophoresis, and a variety of Aβ aggregation inhibition and aggregates' disaggregation assayed. Out of all of them, 20 (fig. 4c) were chosen as the final Aβ-disaggregator candidate, demonstrating that the Aβ-oligomers and plaques in the hippocampus had been lowered. Montanari et al.[92] reported 2-arylbenzofuran based derivatives and evaluated their anticholinesterase and neuroprotective activity. Among them, compound 21 (fig. 4d) showed multiple anti-AD effects, including cholinergic system restoration by inhibiting BChE, neuroprotective activity against Aβ oligomers, potent and selective cannabinoid receptor-2 ligand activity, and immunomodulatory effects that changed microglia from the pro- inflammatory M1 to the neuroprotective M2 phenotype.
Anticholinesterase inhibitory activity:
Abedinifar et al.[93] reported in 2018 that AChE and BChE architectures are remarkably similar, with 65 % amino acid sequence similarity. By restoring ACh levels, the inhibition of AChE and butyrylcholinesterase enzymes alleviated symptoms related to Alzheimer’s and including cognitive level and also includes short-term memory. Table 4 summarises the findings, which include target compounds with their IC50 values. None of the drugs inhibited AChE better than donepezil, however except 22j (fig. 4e), all of them had superior inhibitory activity of BChE and inhibited AchE the best of the target drugs, but 22h inhibited BChE more efficiently than donepezil, with an IC50 value of 0.054 M, which is about 100 times greater than the positive control. Mostofi et al.[94] reported 1,5-benzothiazepine-based derivatives and evaluated their anticholinesterase activity. Among them, compounds 23a, 23b, and 23c (fig. 4f) displayed the uppermost inhibition of BChE with IC50 values of 1.0, 1.0, and 1.8 µM respectively.
| Compound | R1 | R2 | X | AChE Inhibitor IC50 value (μM) | BChE Inhibitor IC50 value (μM) |
|---|---|---|---|---|---|
| 22a | H | H | Br | 5.50±0.3 | 0.29±0.01 |
| 22b | H | 3-Me | Br | 13.8±0.5 | 0.11±0.01 |
| 22c | H | 4-Me | Br | 19.8±0.8 | 0.65±0.04 |
| 22d | H | 2-NO2 | Br | 3.5±0.3 | 0.15±0.01 |
| 22e | H | 4-NO2 | Br | 40.0±4.0 | 0.76+0.05 |
| 22f | H | 4-F | Br | 12.3±0.3 | 0.37±0.01 |
| 22g | H | 2,4-Cl2 | Cl | 4.4±0.3 | 0.37±0.01 |
| 22h | H | 2-F-6-NO2 | Br | 33.8±1.2 | 0.054±0.002 |
| 22i | OCH3 | H | Cl | 4.7±0.4 | 0.87±0.07 |
| Donepezil | - | - | - | 0.031±0.005 | 5.4±0.1 |
Table 4: Inhibitory Activity of Synthesized Compounds 1A-N Cholinesterase Inhibitor
PET Imaging of beta-amyloid plaques:
Ono et al.[95] reported the synthesis of benzofuran derivatives for PET imaging of beta-amyloid plaques. A reaction called the intramolecular Wittig reaction between the compound triphenylphosphonium salt and 4-nitrobenzoyl chloride was used to efficiently synthesize the required Wittig reagent (fig. 4g). By treating derivatives 24e, 24g, and 24i with BBr3, the O-methyl groups were removed, yielded 24f, 24h, and 24j with yields of 47, 39, and 7%, respectively. Among all, the derivative 24h was found with the lowest Ki value 0.7 nM and highest brain penetration activity.
AChE/BuChE and β-/γ- secretase inhibitors:
The cholinergic neurotransmitter system’s significant variability, chronic cholinergic deficiency and synaptic alterations all play essential roles in the development of AD. AChE is the most important enzyme in the catalytic breakdown of ACh[95], as AD advances, the concentration of ACh in the brain decreases. It also binds to A, increasing A peptide aggregation[96].
Hasanvand et al.[96] reported 3-aminobenzofuran based derivatives and evaluated their potency against AChE and BuChE. Among them, compound 25 which has a 2-fluorobenzyl moiety (fig. 4h) displayed the excellent inhibitory activity. Furthermore, the kinetic study also revealed that it showed mixed-type inhibition on AChE.
AChE inhibitory activity:
Wu et al. reported N-glycosyl benzofuran based derivatives and evaluated their potency against AChE. Among them, compound 26 (fig. 4i) displayed the excellent inhibitory activity about 84 %[97].
Conclusion
AD is a neurodegenerative disease that impairs motor and cognitive function and is rapidly spreading in the current scenario with a male:female ratio of 1.2:1.5 due to various causes associated with it such as education, diet and nutrition, sleep, and cardiac rhythm, and head trauma, among others. It is the third greatest cause of mortality, and treating AD is a huge issue due to the lack of an effective medicine. However, data suggests that the benzofuran derivative has strong bioactivity against AD.
Acknowledgments:
The authors would like to thank the Dean (Prof.) SK Abdul Rahaman, School of Medical and Allied Sciences, Galgotias University, Greater Noida-201310 for providing technical support and guidance.
Conflict of interests:
The authors confirm that this article's content has no conflict of interest.
References
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Sci 2006;314(5800):777-81.
[Crossref] [Google Scholar] [PubMed]
- Alzheimer's Association. 2017 Alzheimer's disease facts and figures. Alzheimers Dement 2017;13(4):325-73.
- Lillo-Crespo M, Riquelme J, Macrae R, de Abreu W, Hanson E, Holmerova I, et al. Experiences of advanced dementia care in seven European countries: Implications for educating the workforce. Glob Health Action 2018;11(1):1478686.
[Crossref] [Google Scholar] [PubMed]
- Talesa VN. Acetylcholinesterase in Alzheimer's disease. Mech Ageing Dev 2001;122(16):1961-9.
[Crossref] [Google Scholar] [PubMed]
- Selkoe DJ. Folding proteins in fatal ways. Nature 2003;426(6968):900-4.
[Crossref] [Google Scholar] [PubMed]
- Maccioni RB, Farías G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer's disease. Arch Med Res 2010;41(3):226-31.
[Crossref] [Google Scholar] [PubMed]
- Greenough MA, Camakaris J, Bush AI. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem Int 2013;62(5):540-55.
[Crossref] [Google Scholar] [PubMed]
- Wimo A, Winblad B, Aguero-Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord 2003;17(2):63-7.
[Crossref] [Google Scholar] [PubMed]
- Qiu C, Kivipelto M, Von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 2009;11(2):111-28.
[Crossref] [Google Scholar] [PubMed]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet 2006;368(9533):387-403.
[Crossref] [Google Scholar] [PubMed]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, et al. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology 1999;53(9):1992-7.
[Crossref] [Google Scholar] [PubMed]
- Copeland JR, McCracken C, Dewey ME, Wilson KC, Doran M, Gilmore C, et al. Undifferentiated dementia, Alzheimer's disease and vascular dementia: Age-and gender-related incidence in Liverpool: The MRC–ALPHA Study. Br J Psychiatry 1999;175(5):433-8.
[Crossref] [Google Scholar] [PubMed]
- Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11(3):310-20.
[Crossref] [Google Scholar] [PubMed]
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and beyond. Curr Alzheimer Res 2009;6(2):86-96.
[Crossref] [Google Scholar] [PubMed]
- Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer's disease. Drugs Today 2003;39(1):75-83.
[Crossref] [Google Scholar] [PubMed]
- Rampa A, Bartolini M, Bisi A, Belluti F, Gobbi S, Andrisano V, et al. The first dual ChE/FAAH inhibitors: new perspectives for Alzheimer's disease? ACS Med Chem Lett 2012;3(3):182-6.
[Crossref] [Google Scholar] [PubMed]
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and memory. Chem Biol Interact 2010;187(1-3):403-8.
[Crossref] [Google Scholar] [PubMed]
- Carvajal FJ, Inestrosa NC. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: therapeutic relevance of IDN 5706. Front Mol Neurosci 2011;4:19.
[Crossref] [Google Scholar] [PubMed]
- Benzofuran. PubChem. 2022
- Yeung KS. Furans and benzofurans. Metalation of azoles and related five-membered ring heterocycles. In: Topics in Heterocyclic Chemistry 2012;29:47-76.
- Kamal M, Shakya AK, Jawaid T. Benzofurans: A new profile of biological activities. Int J Med Pharm Sci 2011;1(3):1-5.
- Xu Z, Zhao S, Lv Z, Feng L, Wang Y, Zhang F, et al. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur J Med Chem 2019;162:266-76.
[Crossref] [Google Scholar] [PubMed]
- Nevagi RJ, Dighe SN, Dighe SN. Biological and medicinal significance of benzofuran. Eur J Med Chem 2015;97:561-81.
- Asif M. Mini review on important biological properties of benzofuran derivatives. J Anal Pharm Res 2016;3(2):00050.
- Lal R, Lin H, Quist AP. Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim Biophys Acta 2007;1768(8):1966-75.
[Crossref] [Google Scholar] [PubMed]
- Benzofuran. DrugBank Online; 2022.
- Favre HA, Powell WH. Nomenclature of organic chemistry: IUPAC recommendations and preferred names 2013. Royal Society of Chemistry; 2013.
- Alzheimer's Association. 2018 Alzheimer's disease facts and figures. Alzheimers Dement 2018;14(3):367-429.
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271(13):1004-10.
[Crossref] [Google Scholar] [PubMed]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004;3(6):343-53.
[Crossref] [Google Scholar] [PubMed]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 2001;57(12):2236-42.
[Crossref] [Google Scholar] [PubMed]
- Wang HX, Xu W, Pei JJ. Leisure activities, cognition and dementia. Biochim Biophys Acta 2012;1822(3):482-91.
[Crossref] [Google Scholar] [PubMed]
- Wilson RS, de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Jama 2002;287(6):742-8.
[Crossref] [Google Scholar] [PubMed]
- Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014;10(2):115-9.
[Crossref] [Google Scholar] [PubMed]
- Jellinger KA. Head injury and dementia. Curr Opin Neurol 2004;17(6):719-23.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Ono M, Kimura H, Ueda M, Saji H. Technetium-99m labeled pyridyl benzofuran derivatives as single photon emission computed tomography imaging probes for β-amyloid plaques in Alzheimer’s brains. J Med Chem 2012;55(5):2279-86.
[Crossref] [Google Scholar] [PubMed]
- Delogu GL, Fais A, Pintus F, Goyal C, Matos MJ, Era B, et al. Structural insight of new butyrylcholinesterase inhibitors based on benzylbenzofuran scaffold. Pharmaceuticals 2022;15(3):304.
[Crossref] [Google Scholar] [PubMed]
- Kushwaha P, Fatima S, Upadhyay A, Gupta S, Bhagwati S, Baghel T, et al. Synthesis, biological evaluation and molecular dynamic simulations of novel Benzofuran-tetrazole derivatives as potential agents against Alzheimer’s disease. Bioorg Med Chem Lett 2019;29(1):66-72.
[Crossref] [Google Scholar] [PubMed]
- Sashidhara KV, Modukuri RK, Jadiya P, Dodda RP, Kumar M, Sridhar B, et al. Benzofuran–chalcone hybrids as potential multifunctional agents against Alzheimer’s disease: Synthesis and in vivo studies with transgenic Caenorhabditis elegans. ChemMedChem 2014;9(12):2671-84.
[Crossref] [Google Scholar] [PubMed]
- Zha X, Lamba D, Zhang L, Lou Y, Xu C, Kang D, et al. Novel tacrine–benzofuran hybrids as potent multitarget-directed ligands for the treatment of Alzheimer’s disease: Design, synthesis, biological evaluation, and X-ray crystallography. J Med Chem 2016;59(1):114-31.
[Crossref] [Google Scholar] [PubMed]
- Hiremathad A, Chand K, Keri RS. Development of coumarin–benzofuran hybrids as versatile multitargeted compounds for the treatment of Alzheimer’s Disease. Chem Biol Drug Des 2018;92(2):1497-503.
[Crossref] [Google Scholar] [PubMed]
- Mehrabi F, Pourshojaei Y, Moradi A, Sharifzadeh M, Khosravani L, Sabourian R, et al. Design, synthesis, molecular modeling and anticholinesterase activity of benzylidene-benzofuran-3-ones containing cyclic amine side chain. Future Med Chem 2017;9(7):659-71.
[Crossref] [Google Scholar] [PubMed]
- Rizzo S, Rivière C, Piazzi L, Bisi A, Gobbi S, Bartolini M, et al. Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, β amyloid aggregation, and Aβ neurotoxicity. J Med Chem 2008;51(10):2883-6.
[Crossref] [Google Scholar] [PubMed]
- Onishi T, Iwashita H, Uno Y, Kunitomo J, Saitoh M, Kimura E, et al. A novel glycogen synthase kinase‐3 inhibitor 2‐methyl‐5‐(3‐{4‐[(S)‐methylsulfinyl] phenyl}‐1‐benzofuran‐5‐yl)‐1, 3, 4‐oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer’s disease. J Neurochem 2011;119(6):1330-40.
[Crossref] [Google Scholar] [PubMed]
- Yun Y, Miao Y, Sun X, Sun J, Wang X. Synthesis and biological evaluation of 2-arylbenzofuran derivatives as potential anti-Alzheimer’s disease agents. J Enzyme Inhib Med Chem 2021;36(1):1345-55.
[Crossref] [Google Scholar] [PubMed]
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers 2015;1(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack Jr CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7(3):263-9.
[Crossref] [Google Scholar] [PubMed]
- Apostolova LG. Alzheimer disease. Continuum 2016;22(2):419-34.
[Crossref] [Google Scholar] [PubMed]
- Godoi B, Schumacher RF, Zeni G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem Rev 2011;111(4):2937-80.
[Crossref] [Google Scholar] [PubMed]
- Abu-Hashem AA, Hussein HA, Aly AS, Gouda MA. Synthesis of benzofuran derivatives via different methods. Synthetic Commun 2014;44(16):2285-312.
- Rangaswamy J, Kumar HV, Harini ST, Naik N. Synthesis of benzofuran based 1, 3, 5-substituted pyrazole derivatives: As a new class of potent antioxidants and antimicrobials-A novel accost to amend biocompatibility. Bioorg Med Chem Lett 2012;22(14):4773-7.
[Crossref] [Google Scholar] [PubMed]
- Zhou L, Shi Y, Xiao Q, Liu Y, Ye F, Zhang Y, et al. CuBr-catalyzed coupling of N-tosylhydrazones and terminal alkynes: Synthesis of benzofurans and indoles. Org Lett 2011;13(5):968-71.
[Crossref] [Google Scholar] [PubMed]
- Wang JR, Manabe K. Hydroxyterphenylphoshine− palladium catalyst for benzo [b] furan synthesis from 2-chlorophenols. Bifunctional ligand strategy for cross-coupling of chloroarenes. J Org Chem 2010;75(15):5340-2.
[Crossref] [Google Scholar] [PubMed]
- Xie X, Chen B, Lu J, Han J, She X, Pan X. Synthesis of benzofurans in ionic liquid by a PdCl2-catalyzed intramolecular Heck reaction. Tetrahedron Lett 2004;45(33):6235-7.
- Sheradsky T. Application of the Fischer indole synthesis to the preparation of benzofurans. Tetrahedr Lett 1966;7(43):5225-7.
- De Luca L, Giacomelli G, Nieddu G. A facile approach to the synthesis of chiral 2-substituted benzofurans. J Org Chem 2007;72(10):3955-7.
[Crossref] [Google Scholar] [PubMed]
- Ghosh S, Das J, Saikh F. A new synthesis of 2-aryl/alkylbenzofurans by visible light stimulated intermolecular Sonogashira coupling and cyclization reaction in water. Tetrahedr Lett 2012;53(44):5883-6.
- Zykov DA, Markova TA, Kirsanova ZD, Shestaeva MM, Kaverina NV, Zagorevskii VA. Khim Farm Zh 1971;5(9):23.
- Kadieva MG, Oganesyan ET. Methods for the synthesis of benzofuran derivatives. Chem Heterocycl Compd 1997;33(11):1245-58.
- Brent DA, Hribar JD, DeJongh DC. Pyrolysis of 2-pyrone, coumarin, and 2-pyridone. J Org Chem 1970;35(1):135-7.
- Belanger PC, Scheigerz J, Rokach J. US Patent No. 5087638; 1993.
- Belanger PC, Scheigerz J, Rokach J. US Patent No. 4863958; 1990.
- Johnson 62, US Patent No. 4455427; 1985.
- Akiyama S, Akimoto H, Nakatsuji SI, Nakashima K. Development and Application of Organic Reagents for Analysis. VI. Synthesis and Fluorescence Spectral Properties of 2-(4-Substituted phenyl) benzofurans. Bull Chem Soc Japan 1985;58(8):2192-7.
- Belanger PC, Dufresne C, Lau CK, Scheigetz J. An improved synthesis of ethyl 4-hydroxy-3-methylbenzofuran-2-carboxylate. Org Preparation Proced Int 1988;20(3):299-302.
- Kadieva MG, Oganesyan ET. Methods for the synthesis of benzofuran derivatives. Chem Heterocycl Compd 1997;33(11):1245-58.
- Horaguchi T, Matsuda S, Tanemura K, Suzuki T. Benzofuran derivatives. Part 3 On the reactivities of the intermediates in benzofuran synthesis. J Heterocycl Chem 1987;24(4):965-9.
- Sastry CR, Rao KS, Rastogi K, Jain ML, Reddi Gs. Antimicrobial Agents. 5. Synthesis And Antifungal Activity Of 1-[(Benzofuran-2-Yl)(3-Oxo-1, 4-Benzoxazin-6-Yl) Methyl]-1h-Imidazoles. Indian J Chem Section B 1989;28(12):1096-8.
- Aikinson JG, Guindon Y, Lau CK. US Patent No. 4663347; 1988.
- Lamotte G, Demerseman P, Royer R. A one-step synthesis of ethyl (2-benzofuroyl)-acetates. Synthesis 1984;1984(12):1068-70.
- Montfort B, Laude B, Vebrel J, Cerutti E. Cyclodehydration of 2-aryloxy-1,2-diaryl ethanones to 2-diaryl,3-benzofurans. Influence of substitution on the competition between two reaction paths. Bull Chem Soc France 1987(5):848-54.
- Grinev AN, Zotova SA, Mikhailova IN, Stolyarchuk AA, Gaevoi VP, Matsak VV. Synthesis and study of the pharmacological activity of aminomethyl derivatives of halogen-substituted benzofurans. Pharm Chem J 1978;12(12):1560-5.
[Crossref ] [Google Scholar]
- Inanaga J, Ujikawa O, Yamaguchi M. Sml2-promoted aryl radical cyclization. A new synthetic entry into heterocycles. Tetrahedr Lett 1991;32(14):1737-40.
- Tsukazaki M, Snieckus V. Directed ortho metalation–radical-induced cyclization synthetic connections. A route to highly substituted benzofurans. Canadian J Chem 1992;70(5):1486-91.
- Pandey G, Krishna A, Bhalerao UT. A one step synthesis of 2-substituted benzofurans from 2-aryl-1-substituted ethane-1-ones by photoinduced set reactions. Tetrahedr Lett 1989;30(14):1867-70.
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet 2006;368(9533):387-403.
[Crossref] [Google Scholar] [PubMed]
- Eicher T, Hauptmann S, Speicher A. The chemistry of heterocycles: Structures, reactions, synthesis, and applications. John Wiley & Sons;2013.
- Byun JH, Kim H, Kim Y, Mook-Jung I, Kim DJ, Lee WK, et al. Aminostyrylbenzofuran derivatives as potent inhibitors for Aβ fibril formation. Bioorg Med Chem Lett 2008;18(20):5591-3.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Ono M, Kimura H, Kagawa S, Nishii R, Kawashima H, et al. Fluorinated benzofuran derivatives for PET imaging of β-amyloid plaques in Alzheimer's disease brains. ACS Med Chem Lett 2010;1(7):321-5.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Ono M, Kimura H, Kagawa S, Nishii R, Saji H. A novel 18F-labeled pyridyl benzofuran derivative for imaging of β-amyloid plaques in Alzheimer’s brains. Bioorg Med Chem Lett 2010;20(20):6141-4.
[Crossref] [Google Scholar] [PubMed]
- Tan YX, Wang HQ, Chen RY. Anti-inflammatory and cytotoxic 2-arylbenzofurans from Morus wittiorum. Fitoterapia 2012;83(4):750-3.
[Crossref] [Google Scholar] [PubMed]
- Fukai T, Oku Y, Hano Y, Terada S. Antimicrobial activities of hydrophobic 2-arylbenzofurans and an isoflavone against vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Planta Med 2004;70(7):685-7.
[Crossref] [Google Scholar] [PubMed]
- Basnet P, Kadota S, Terashima S, SHIMIZU M, Namba T. Two new 2-arylbenzofuran derivatives from hypoglycemic activity-bearing fractions of Morus insignis. Chem Pharm Bull 1993;41(7):1238-43.
[Crossref] [Google Scholar] [PubMed]
- Han SJ, Ryu SN, Kang SS. A new 2-arylbenzofuran with antioxidant activity from the black colored rice (Oryza sativa L.) bran. Chem Pharm Bull 2004;52(11):1365-6.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Zeng X, Gao C, Ming P, Zhang J, Guo C, et al. A new arylbenzofuran derivative functions as an anti-tumour agent by inducing DNA damage and inhibiting PARP activity. Sci Rep 2015;5(1):10893.
[Crossref] [Google Scholar] [PubMed]
- Pouramiri B, Mahdavi M, Moghimi S, Firoozpour L, Nadri H, Moradi A, et al. Synthesis and antiacetylcholinesterase activity evaluation of new 2-aryl benzofuran derivatives. Lett Drug Des Discov 2016;13(9):897-902.
- Aslam SN, Stevenson PC, Kokubun T, Hall DR. Antibacterial and antifungal activity of cicerfuran and related 2-arylbenzofurans and stilbenes. Microbiol Res 2009;164(2):191-5.
[Crossref] [Google Scholar] [PubMed]
- Ferino G, Cadoni E, Matos MJ, Quezada E, Uriarte E, Santana L, et al. MAO inhibitory activity of 2‐arylbenzofurans versus 3‐arylcoumarins: Synthesis, in vitro study, and docking calculations. Chem Med Chem 2013;8(6):956-66.
[Crossref] [Google Scholar] [PubMed]
- Rizzo S, Tarozzi A, Bartolini M, da Costa G, Bisi A, Gobbi S, et al. 2-Arylbenzofuran-based molecules as multipotent Alzheimer's disease modifying agents. Eur J Med Chem 2012;58:519-32.
[Crossref] [Google Scholar] [PubMed]
- Ha HJ, Kang DW, Kim HM, Kang JM, Ann J, Hyun HJ, et al. Discovery of an orally bioavailable benzofuran analogue that serves as a β-amyloid aggregation inhibitor for the potential treatment of Alzheimer’s disease. J Med Chem 2018;61(1):396-402.
[Crossref] [Google Scholar] [PubMed]
- Kim D, Bae GH, Kim HY, Jeon H, Kim K, Shin J, et al. Orally administered benzofuran derivative disaggregated Aβ plaques and oligomers in the brain of 5XFAD Alzheimer transgenic mouse. ACS Chem Neurosci 2020;12(1):99-108.
- Montanari S, Mahmoud AM, Pruccoli L, Rabbito A, Naldi M, Petralla S, et al. Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer's disease treatment. Eur J Med Chem 2019;178:243-58.
[Crossref] [Google Scholar] [PubMed]
- Abedinifar F, Farnia SM, Mahdavi M, Nadri H, Moradi A, Ghasemi JB, et al. Synthesis and cholinesterase inhibitory activity of new 2-benzofuran carboxamide-benzylpyridinum salts. Bioorg Chem 2018;80:180-8.
[Crossref] [Google Scholar] [PubMed]
- Mostofi M, Ziarani GM, Lashgari N. Design, synthesis and biological evaluation of benzofuran appended benzothiazepine derivatives as inhibitors of butyrylcholinesterase and antimicrobial agents. Bioorg Med Chem 2018;26(12):3076-95.
[Crossref] [Google Scholar] [PubMed]
- Ono M, Kawashima H, Nonaka A, Kawai T, Haratake M, Mori H, et al. Novel benzofuran derivatives for PET imaging of β-amyloid plaques in Alzheimer's disease brains. J Med Chem 2006;49(9):2725-30.
[Crossref] [Google Scholar] [PubMed]
- Hasanvand Z, Motahari R, Nadri H, Moghimi S, Foroumadi R, Ayati A, et al. Novel 3-aminobenzofuran derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Front Chem 2022;10:882191.
[Crossref] [Google Scholar] [PubMed]
- Wu YR, Ren ST, Wang L, Liu XJ, Wang YX, Liu SH, et al. Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives. Heterocycl Commun 2019;25(1):162-6.