- *Corresponding Author:
- Qian Tan
Department of Burns and Plastic Surgery, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing 210008
Department of Burns and Plastic Surgery, Anqing Shihua Hospital,Nanjing Drum Tower Hospital Group, Anqing 246002, China
E-mail: smmutanqian@sina.com
| Date of Received | 23 July 2022 |
| Date of Revision | 08 October 2021 |
| Date of Acceptance | 23 July 2022 |
| Indian J Pharm Sci 2022;84(4):1-7 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Diabetic ulcers, a type of chronic and refractory wound, are the most common diabetic complication and their incidence is increasing. However, the specific mechanisms underlying the formation of diabetic ulcers have not been clarified and the current therapeutic methods are unsatisfactory. In this study, we found that asiaticoside facilitated the recovery of skin wounds in diabetic rats. Intensive studies demonstrated that asiaticoside promoted fibroblast proliferation and migration in a high-glycemic environment. Mechanistically, asiaticoside upregulated Wnt1 at the protein and immunohistochemistry levels and activated the Wnt/beta-catenin signaling cascade, thereby promoting fibroblast functions. Thus, asiaticoside promotes skin cell activities relevant to diabetic ulcers healing and thus may have therapeutic value.
Keywords
Asiaticoside, diabetic ulcers, wound healing, Wnt/beta-catenin signaling cascade
Diabetic Ulcers (DUs), a type of chronic refractory wound, represent the most common diabetic complication[1]. Vascular damage in patients with diabetes leads to ischemia, hypoxia and nerve damage in the distal limbs, and a variety of factors lead to the formation of diabetic wounds and damage to the skin barrier[2,3]. Woundrelated damage is associated with changes in the systemic or local immune status caused by a diet high in sugar and fat. Such wounds are difficult to heal and prone to repeated infections and tissue necrosis[4]. The canonical Wnt-beta (β)/catenin signaling cascade, a substantially conserved pathway, involves the interaction of Wnt with its receptors and the subsequent association of Axin with phosphorylated Lipoprotein Receptor-Related Protein (LRP). The destruction complex consists of Axin, the Adenomatous Polyposis Coli (APC) gene product and Glycogen Synthase Kinase 3 (GSK3), and its breakdown leads to the reversal of Axin-mediated β-catenin phosphorylation[5-8]. Subsequently, the unphosphorylated β-catenin shuttle binds to the T-Cell Factor/Lymphocyte Enhancer Factor (TCF/LEF) of the nucleus to adjust the expression of target genes, such as cellular Myc (c-Myc). Previous research by our group showed that the messenger Ribonucleic Acid (mRNA) levels of Wnt7a and Wnt7b were considerably downregulated under high glucose conditions and the activation of the Wnt signaling cascade by the exogenous application of Wnt7a protein promoted the healing of DUs[9]. Thus, novel drugs against the Wnt/β-catenin signaling cascade should be developed to treat DUs effectively.
Centella asiatica is a traditional Chinese medicinal plant from the family Apiaceae. Asiaticoside (AC) (fig. 1A) is a triterpenoid compound extracted from Centella asiatica and its biological activity is vigorous[10]. AC exhibits various pharmacological effects, such as antiinflammation, anti-hyperlipidemia, anti-apoptosis, antifibrosis and anti-hyperglycemia. Recent investigation has documented that AC can trigger type I collagen biosynthesis by activating the Type I Transforming Growth Factor Beta Receptor Kinase (TβRI)-independent SMAD cascade[11]. Moreover, it can accelerate the cell cycle by inducing fibroblast collagen tissue synthesis in the dermis by regulating the cell cycle; thereby increasing wound healing speed and improving the tension in local tissues. In addition, AC presents antioxidant and angiogenesis activity and thus is suitable for wound, burn and chronic wound treatments[12-14]. However, the mechanisms underlying the effects of AC should be further investigated to exploit its unique advantages fully. Therefore, we investigated the potentiality of AC in the treatment of DU, assessed its healing effects and evaluated its relevant molecular mechanisms both in vivo and in vitro.
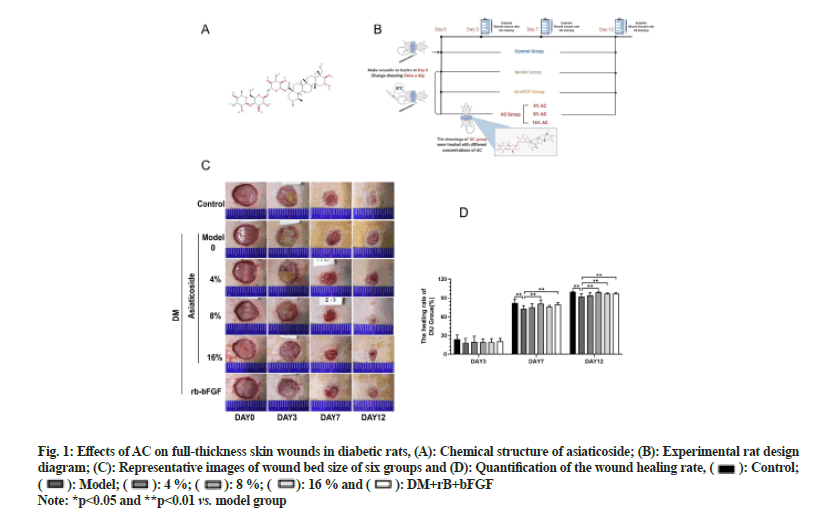
Fig. 1: Effects of AC on full-thickness skin wounds in diabetic rats, (A): Chemical structure of asiaticoside; (B): Experimental rat design diagram; (C): Representative images of wound bed size of six groups and (D): Quantification of the wound healing rate,  Control;
Control;  Model;
Model; 4 %;
4 %;  8 %;
8 %;  16 % and
16 % and  DM+rB+bFGF
DM+rB+bFGF
Note: *p<0.05 and **p<0.01 vs. model group
Materials and Methods
Materials:
AC (95 %, 16830-15-2) was purchased from Nanjing Jingzhu Biotechnology Co. Ltd. Streptozotocin (STZ), S0130 was obtained from Sigma (United States of America (USA)) and recombinant bovine-basic Fibroblast Growth Factor (rb-bFGF) was purchased from Zhuhai Essex Bio-Technology Company. Antibodies for Western blotting were purchased from Abcam.
Animal experiments:
Healthy male Specific-Pathogen Free (SPF) Sprague- Dawley (SD) rats weighing (190±10) g were provided by SPF Beijing Biotechnology Co., Ltd. All rats were maintained under a 12:12 h cycle of dark/light under pathogen-free conditions at the Animal Center of Nanjing Drum Tower Hospital The animal experiments were authorized by the Animal Use and Care Committee of Nanjing Drum Tower Hospital and conducted based on the guidelines of the National Institutes of Health (NIH), USA.
To establish the diabetic rat model, a portion of the SD rats were fasted overnight and then intraperitoneally inoculated with 65 mg/kg STZ, which was dissolved in 0.1 mol/l citrate buffer. In this study, rats with blood glucose levels >16.7 mM steady (for >3 w) were regarded diabetic (performed by Yuwell, China). The rats were randomly stratified into six treatment groups and each group had 9 rats; blank control group (control group), diabetes model group (model group), 4 % AC low-dose group (4 % AC group), 8 % AC middle-dose group (8 % AC group), 16 % AC high-dose group (16 % AC group) and positive control rb-bFGF group (rbbFGF group). Rats were allowed to acclimate on d 1 and d 2 was regarded as the 1st d of the experiment. The dressings in each group were changed once a day.
Wound-closure rate:
The wound-closure rate was assessed using ImageJ software (v1.37; NIH, Bethesda, MD, USA) and computed based on the following formula (W0-Wd)/W0×100 %, where W0 represents the wound area on d 0 and Wd represents the wound area on a particular day.
Hematoxylin and Eosin (HE) and Masson staining and immunohistochemistry:
On 3rd, 7th and 12th d postoperatively, formaldehyde-fixed, paraffin-embedded wound-edge tissue was subjected to HE or Masson staining through standard procedures. An Olympus BX43 microscope was used to observe the pathological morphology of the wounds.
Cell culture:
Human Foreskin Fibroblasts (HFF)-1 from normal human skin fibroblasts were acquired from the National Collection of Authenticated Cell Cultures (SCSP-109). The HFF-1 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) enriched with streptomycin (final level 100 μg/ml), penicillin (final level 100 U/ml) and 10 % Fetal Bovine Serum (FBS) (Gibco).
Cell proliferation analysis:
A Cell Counting Kit-8 (CCK-8) assay of HFF-1 cells under the AC treatment was measured by CCK-8 Kit (Beyotime).
Cell migration analysis:
For the scratch wound migration assay, after the cells proliferated to 80 %–90 % of the 6-well plate, cell wounds were made using a 10 μl pipette. Cell migration images were recorded at 0 h and 24 h via an inverted phase-contrast microscope (Leica, Germany).
For the transwell assay, HFF-1 cells in the logarithmic growth phase from each group were incubated with 5 μg/ml mitomycin solution at 37° for 1 h, followed by digestion with trypsin and then centrifugation. Subsequently, the supernatant was discarded and 2.5×104 cells/ml were inoculated with 200 μl DMEM containing 0.1 % FBS in the upper compartment of 24-well plates. The lower compartment was filled with DMEM augmented with 10 % FBS. After incubating for 24 h, the cells attached to the lower surface were fixed with 70 % methanol for 30 min and stained with 0.1 % crystal violet for 30 min. Finally, images of the migrated cells were photographed under a microscope. The migrated cell number was counted using ImageJ software.
Cell cycle analysis:
The cell cycle was detected using flow cytometry.
Western blotting:
The Wnt/β-catenin signaling cascade were measured by Western blotting as previously described[15]. The related antibodies were anti-Wnt1 (1:1000 ab15251), anti-Wnt3A (1:1000 ab219412), anti-Wnt4 (1:1000 ab262696), anti-Wnt5A (1:1000 ab179824), anti-Wnt7A (1:1000 ab274321), anti-GSK3β (1:5000 ab32391), antiphos- GSK3β (Ser9) (1:1000 ab75814), anti-β-catenin (1:500 ab68183), anti-Axin-1 (1:500 ab96856) and antic- Myc (1:1000 ab32072) that purchased from Abcam.
Statistical analysis:
Statistical tests were performed using GraphPad Prism software (La Jolla, California, USA). Data are presented as the mean±Standard Deviation (SD) and analyzed using one-way Analysis of Variance (ANOVA) or Tukey’s test. For all analyses, the significance level was set at p<0.05.
Results and Discussion
To evaluate whether AC could accelerate DU healing, we used AC to treat diabetic rats having full-thickness wounds and pictures were taken on 3rd, 7th and 12th d. After AC inoculation, the healing rate of the 8 % AC group was higher than that of the model group on 7th d (p<0.01). Furthermore, the 8 % AC, 16 % AC and rbbFGF groups exhibited substantial differences in the healing rate compared with the model group on 12th d (p<0.01) as shown in fig. 1. These results implied that AC could efficiently accelerate the repair of DUs.
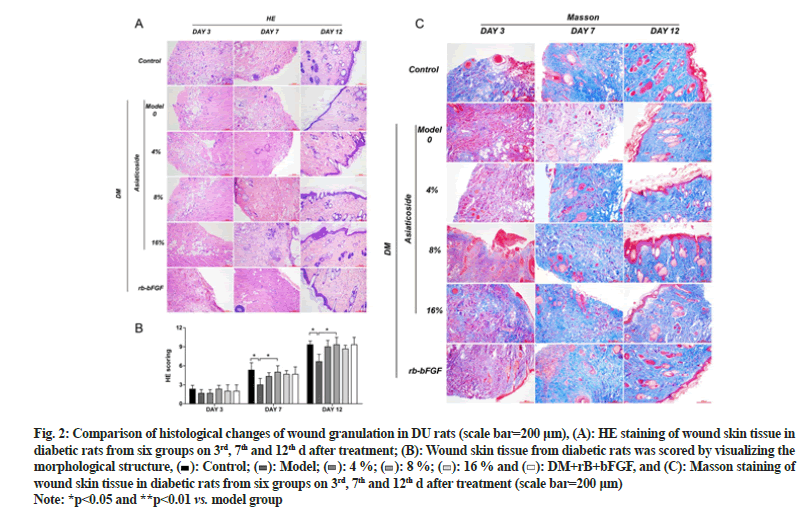
The HE staining results illustrated that compared with the model group, the AC groups show less inflammation, increased distribution of fibroblasts and a greater abundance of new capillaries on 7th d. On 12th d, fibroblast aggregation and granulation tissue were seen in each AC group, with the 8 % AC group showing thicker granulation tissue and abundant new capillaries. Masson staining, on 7th d, more collagen fibers, thinner shapes and irregular arrangements were observed in the AC group. On 12th d, collagen fibers of in the AC group were formed in large numbers and presented thicker shapes and more regular arrangements than in the model group. Moreover, the 8 % AC group had the most collagen fiber deposition among the AC groups as shown in fig. 2.
Fig. 2: Comparison of histological changes of wound granulation in DU rats (scale bar=200 μm), (A): HE staining of wound skin tissue in diabetic rats from six groups on 3rd, 7th and 12th d after treatment; (B): Wound skin tissue from diabetic rats was scored by visualizing the morphological structure,  Control;
Control;  Model;
Model;  4 %;
4 %;  8 %;
8 %;  16 % and
16 % and  DM+rB+bFGF, and (C): Masson staining of wound skin tissue in diabetic rats from six groups on 3rd, 7th and 12th d after treatment (scale bar=200 μm)
DM+rB+bFGF, and (C): Masson staining of wound skin tissue in diabetic rats from six groups on 3rd, 7th and 12th d after treatment (scale bar=200 μm)
Note: *p<0.05 and **p<0.01 vs. model group
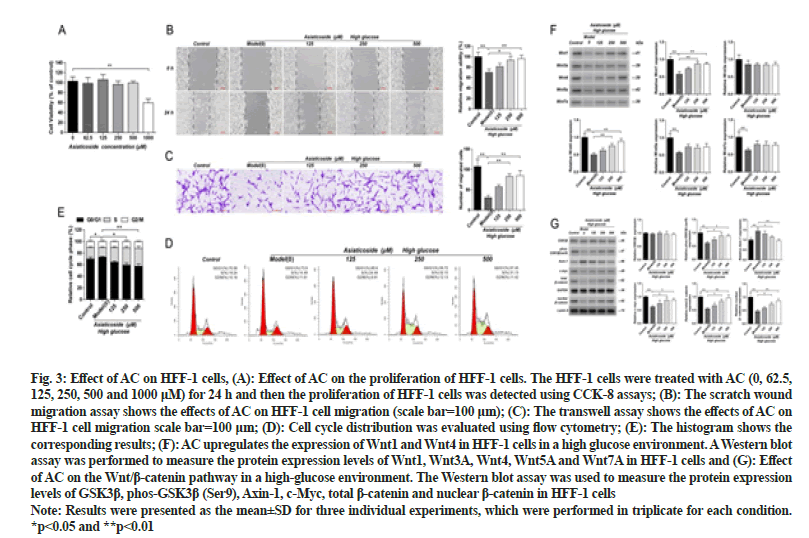
HFF-1 cells were treated with different concentrations of AC (0, 62.5, 125, 250, 500 and 1000 μM) for 24 h. Based on the results of this experiment, AC doses of 125 μM, 250 μM and 500 μM were chosen for follow-up experiments. The administration of different concentrations of AC could reverse the effect of high glucose on HFF-1 to varying degrees. Significant changes were not observed in the migration of HFF-1 cells in the 125 μM ACtreated group, whereas those of the HFF-1 cells in the 250 and 500 μM AC-treated groups were significantly increased (p<0.05). The cell-cycle analysis revealed that the number of HFF-1 cells in the S phase under highglucose conditions was significantly lower than that of the control group (p<0.05). Significant changes were not observed in the proliferation of HFF-1 cells at various stages after the administration of 125 μM AC. However, the number of HFF-1 cells in the S phase increased significantly after the administration of 250 and 500 μM AC (p<0.05) as shown in fig. 3. Overall, these results suggest that AC promotes the migration and proliferation of HFF-1 cells in a high-glucose environment.
Fig. 3: Effect of AC on HFF-1 cells, (A): Effect of AC on the proliferation of HFF-1 cells. The HFF-1 cells were treated with AC (0, 62.5, 125, 250, 500 and 1000 μM) for 24 h and then the proliferation of HFF-1 cells was detected using CCK-8 assays; (B): The scratch wound migration assay shows the effects of AC on HFF-1 cell migration (scale bar=100 μm); (C): The transwell assay shows the effects of AC on HFF-1 cell migration scale bar=100 μm; (D): Cell cycle distribution was evaluated using flow cytometry; (E): The histogram shows the corresponding results; (F): AC upregulates the expression of Wnt1 and Wnt4 in HFF-1 cells in a high glucose environment. A Western blot assay was performed to measure the protein expression levels of Wnt1, Wnt3A, Wnt4, Wnt5A and Wnt7A in HFF-1 cells and (G): Effect of AC on the Wnt/β-catenin pathway in a high-glucose environment. The Western blot assay was used to measure the protein expression levels of GSK3β, phos-GSK3β (Ser9), Axin-1, c-Myc, total β-catenin and nuclear β-catenin in HFF-1 cells
Note: Results were presented as the mean±SD for three individual experiments, which were performed in triplicate for each condition. *p<0.05 and **p<0.01
Western blot results showed that the expression levels of Wnt1, Wnt4, Wnt5A and Wnt7A in HFF-1 cells of the high-glucose model group were significantly decreased (p<0.01) than those in the control group. The expression levels of Wnt1 and Wnt4 in HFF-1 cells of the 250 and 500 μM AC-treated groups were significantly increased (p<0.01) as shown in fig. 3. Significant differences in the Wnt3A expression were not observed in HFF-1 cells in all treatment groups. Hence, these results indicate that AC can upregulate the expression of the Wnt/β-catenin cascade-linked proteins Wnt1 and Wnt4 in a highglucose environment.
The Western blot results showed that the GSK3β phosphorylation level and the expression of c-Myc, total β-catenin and nuclear β-catenin in the HFF-1 cells of the high-glucose model group were significantly reduced while the Axin-1 expression level was significantly higher (p<0.05) than those of the control group. The GSK3β phosphorylation level and the expression of c-Myc, total β-catenin and nuclear β-catenin in HFF-1 cells of the 250 μM and 500 μM AC-treated groups were significantly increased (p<0.05) while the Axin-1 level was significantly decreased (p<0.05) as shown in fig. 3. Significant differences were not observed in the GSK3β expression in all groups.
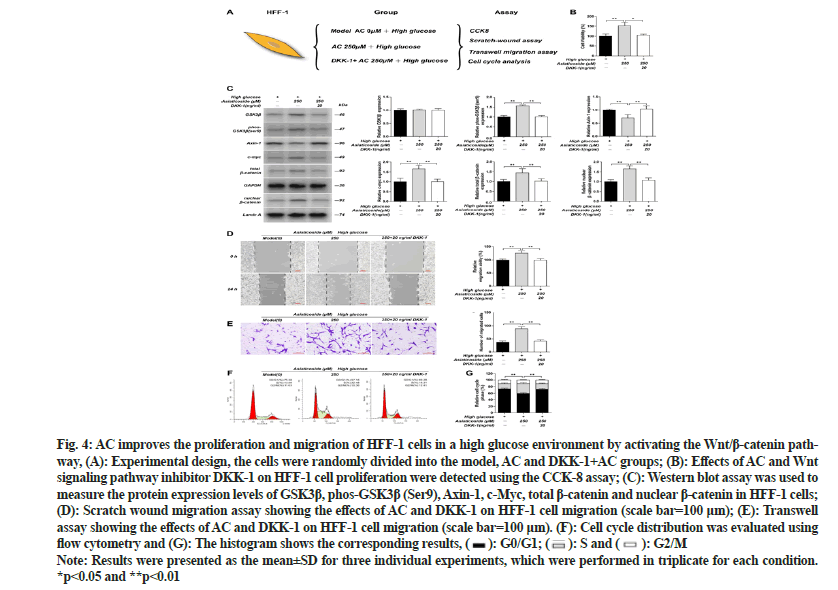
To explore whether AC influences the proliferation and migration of fibroblasts via the Wnt cascade, we applied inhibitors of the Wnt signaling cascade. The Western blot results revealed that the GSK3β phosphorylation level and the expression of c-Myc, total β-catenin and nuclear β-catenin in HFF-1 cells were drastically increased after the administration of 250 μM AC in a high-glucose environment; however, the Axin-1 expression level was substantially reduced (p<0.01). After adding the Wnt signaling cascade inhibitor Dickkopf-1 (DKK-1), the AC effect was reversed, with the GSK3β phosphorylation level and the expression of c-Myc, total β-catenin and nuclear β-catenin being substantially reduced and the Axin-1 expression substantially increased (p<0.01). However, noticeable differences in the GSK3β expression were not observed in all groups. The CCK- 8, scratch and transwell assay results showed that the viability and migration of HFF-1 cells in the 250 μM AC-treated group were substantially increased than those in the high-glucose model group (p<0.01). However, after the inhibitor DKK-1 was applied, the viability and migration of HFF-1 cells were substantially reduced (p<0.01). Furthermore, the cell cycle analysis revealed that in a high-glucose environment, the substantially elevated number of HFF-1 cells in the S phase after administering 250 μM AC was considerably reduced under the inhibitory effect of DKK-1 (p<0.01) as shown in fig. 4. These findings indicate that AC promotes the proliferation and migration of HFF-1 cells via the Wnt/β- catenin cascade.
Fig. 4: AC improves the proliferation and migration of HFF-1 cells in a high glucose environment by activating the Wnt/β-catenin pathway, (A): Experimental design, the cells were randomly divided into the model, AC and DKK-1+AC groups; (B): Effects of AC and Wnt signaling pathway inhibitor DKK-1 on HFF-1 cell proliferation were detected using the CCK-8 assay; (C): Western blot assay was used to measure the protein expression levels of GSK3β, phos-GSK3β (Ser9), Axin-1, c-Myc, total β-catenin and nuclear β-catenin in HFF-1 cells; (D): Scratch wound migration assay showing the effects of AC and DKK-1 on HFF-1 cell migration (scale bar=100 μm); (E): Transwell assay showing the effects of AC and DKK-1 on HFF-1 cell migration (scale bar=100 μm). (F): Cell cycle distribution was evaluated using
flow cytometry and (G): The histogram shows the corresponding results, G0/G1;
G0/G1;  S and
S and  G2/M
G2/M
Note: Results were presented as the mean±SD for three individual experiments, which were performed in triplicate for each condition. *p<0.05 and **p<0.01
In our experiments, we observed that after the administration of AC in vivo, inflammation was reduced, the distribution of fibroblasts was increased, new capillaries were abundant and collagen deposition was increased. Compared with the model group, different concentrations of AC exhibited positive healing effects on DUs in terms of the wound healing rate, as indicated by HE and Masson staining. In addition, the results of the CCK-8, scratch and transwell experiments together showed that AC improved the proliferation and wound healing ability of HFF-1 cells in a high glucose environment. Furthermore, the Western blotting assay showed that the AC treatment of HFF-1 cells upregulated the expression of Wnt1, Wnt4, total β-catenin and nuclear β-catenin and the phosphorylation of GSK3β. The Wnt/β-catenin signaling cascade plays a critical role in modulating the migration, proliferation and differentiation of cells that are functionally relevant to skin tissue repair[16,17]. The key switch in the canonical Wnt cascade is the cytoplasmic protein β-catenin and its stability is controlled by the destruction complex[5]. Previous investigations have shown that AC activates the canonical Wnt/β-catenin signaling cascade[18], which plays a crucial role in modulating the proliferation and migration of fibroblasts[19].
To further verify that AC activated downstream proteins via the classical Wnt/β-catenin cascade, we used the inhibitor DKK-1, which can dock to Wnt coreceptors LRP 5/6 and prevent crosstalk between Wnt proteins and their receptors[20]. After adding DKK-1, the effect of AC was reversed, with substantial reductions in the GSK3β phosphorylation level and the expression of c-Myc, total β-catenin and nuclear β-catenin. Recent investigations revealed that when Wnt and β-catenin are elevated, epidermal cell proliferation, differentiation and migration are enhanced, and wound healing is accelerated[21]. In addition, activation of Wnt signaling can affect the differentiation and migration of keratinocytes and regeneration of follicles by targeting c-Myc, ultimately resulting in enhanced wound healing[16]. In the present study, we identified that Wnt1 was upregulated by AC and functioned positively in DU healing. We further demonstrated that AC could promote the proliferation and migration of HFF-1 cells by activating the Wnt/β-catenin signaling cascade. Overall, our study revealed that Wnt1 represents a novel therapeutic target for accelerating DU healing. Thus, our study provides mechanistic evidence for the treatment of DUs with AC.
Admittedly, some limitations were observed in this research. AC can also promote angiogenesis and antioxidation, which were not evaluated. In future research, we will further study the mechanism underlying its ability to promote DU healing.
In conclusion, this study demonstrates that AC can promote the proliferation and migration of HFF-1 cells in a high-glucose environment. Furthermore, AC concentration of 8 % was the most effective in treating DUs. AC can also upregulate the expression of Wnt1, which is relevant for activating the Wnt/β-catenin cascade and facilitating the healing of DUs, thus providing a convincing rationale for the therapeutic usage of AC in DUs. Moreover, these findings reveal a new target and strategy for DU prevention and treatment based on TCM.
Funding:
The National Natural Science Foundation of China funded this research (No. 81974288 and 81671922).
Ethics approval:
The animal experimentation was permitted by the Medical School for Animal Use and Care Committee of Nanjing Drum Tower.
Conflict of interests:
The authors declared no conflict of interests.
References
- Aldana PC, Khachemoune A. Diabetic foot ulcers: Appraising standard of care and reviewing new trends in management. Am J Clin Dermatol 2020;21(2):255-64.
[Crossref] [Google Scholar] [PubMed]
- Ferreira L, Carvalho A, Carvalho R. Short-term predictors of amputation in patients with diabetic foot ulcers. Diabetes Metab Syndr 2018;12(6):875-9.
[Crossref] [Google Scholar] [PubMed]
- Boutoille D, Féraille A, Maulaz D, Krempf M. Quality of life with diabetes-associated foot complications: Comparison between lower-limb amputation and chronic foot ulceration. Foot Ankle Int 2008;29(11):1074-8.
[Crossref] [Google Scholar] [PubMed]
- Weigelt C, Rose B, Poschen U, Ziegler D, Friese G, Kempf K, et al. Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care 2009;32(8):1491-6.
[Crossref] [Google Scholar] [PubMed]
- Nusse R, Clevers H. Wnt/β-catenin signaling, disease and emerging therapeutic modalities. Cell 2017;169(6):985-99.
[Crossref] [Google Scholar] [PubMed]
- Yue Y, Xue Q, Yang J, Li X, Mi Z, Zhao G, et al. Wnt-activated olfactory ensheathing cells stimulate neural stem cell proliferation and neuronal differentiation. Brain Res 2020;1735:146726.
[Crossref] [Google Scholar] [PubMed]
- Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 2018;138(4):811-25.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Tian XJ, Xing J. Signal transduction pathways of EMT induced by TGF-β, SHH and WNT and their crosstalks. J Clin Med 2016;5(4):41.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Yan X, Lin Y, Ge H, Tan Q. Wnt7a promotes wound healing by regulation of angiogenesis and inflammation: Issues on diabetes and obesity. J Dermatol Sci 2018;91(2):124-33.
[Crossref] [Google Scholar] [PubMed]
- Sun B, Wu L, Wu Y, Zhang C, Qin L, Hayashi M, et al. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front Pharmacol 2020;11:568032.
- Lee J, Jung E, Kim Y, Park J, Park J, Hong S, et al. Asiaticoside induces human collagen I synthesis through TGFβ receptor I kinase (TβRI kinase)-independent Smad signaling. Planta Med 2006;72(4):324-8.
[Crossref] [Google Scholar] [PubMed]
- Shukla A, Rasik AM, Dhawan BN. Asiaticoside?induced elevation of antioxidant levels in healing wounds. Phytother Res 1999;13(1):50-4.
[Crossref] [Google Scholar] [PubMed]
- Kimura Y, Sumiyoshi M, Samukawa KI, Satake N, Sakanaka M. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur J Pharmacol 2008;584(2-3):415-23.
[Crossref] [Google Scholar] [PubMed]
- Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch Dermatol Res 2014;306(7):601-17.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Zhang F, Yan X, Tan Q. Wnt7a regulates high autophagic and inflammatory response of epidermis in high-glucose environment. Burns 2020;46(1):121-7.
[Crossref] [Google Scholar] [PubMed]
- Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J, et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther 2015;6(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Huang P, Yan R, Zhang X, Wang L, Ke X, Qu Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol Ther 2019;196:79-90.
[Crossref] [Google Scholar] [PubMed]
- Fitri AR, Pavasant P, Chamni S, Sumrejkanchanakij P. Asiaticoside induces osteogenic differentiation of human periodontal ligament cells through the Wnt pathway. J Periodontol 2018;89(5):596-605.
[Crossref] [Google Scholar] [PubMed]
- Seo SH, Lee SH, Cha PH, Kim MY, Min DS, Choi KY. Polygonum aviculare L. and its active compounds, quercitrin hydrate, caffeic acid and rutin, activate the Wnt/β?catenin pathway and induce cutaneous wound healing. Phytother Res 2016;30(5):848-54.
[Crossref] [Google Scholar] [PubMed]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature 2002;417(6889):664-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Nie X, Shi X, Zhao J, Chen Y, Yao Q, et al. Regulatory mechanisms of the Wnt/β-catenin pathway in diabetic cutaneous ulcers. Front Pharmacol 2018;9:1114.
[Crossref] [Google Scholar] [PubMed]