- *Corresponding Author:
- P. Sun
Department of Cardiovascular Medicine, Hanyang Hospital Affiliated to Wuhan University of Science and Technology, Wuhan 430050, Hubei Province,China
E-mail: gxdnpqq46631@163.com
| Date of Received | 30 September 2020 |
| Date of Revision | 15 January 2021 |
| Date of Acceptance | 31 March 2021 |
| Indian J Pharm Sci 2021;83(2):378-383 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze the effect of resveratrol on myocardial remodeling and cardiac function after myocardial infarction by inhibiting myocardial cell apoptosis through regulating silent information regulator 1/protein kinase R-like endoplasmic reticulum kinase pathway. 32 healthy male sprague dawley rats were divided into control group (without any treatment), model group (ligation of left anterior descending branch to establish a model), sham operation group (ligation of left anterior descending branch without ligation) and resveratrol group (ligation of left anterior descending branch+resveratrol 8 mg/kg/d), 8 rats each. The changes of cardiac function, myocardial remodeling, myocardial apoptosis and silent information regulator 1/protein kinase R-like endoplasmic reticulum kinase pathway related proteins were observed 4 w after modeling. Left ventricular end-diastolic diameter and left ventricular end-systolic dimension levels in the model group were significantly higher than those in the control group, left ventricular ejection fraction and left ventricular short axis shortening rate levels were significantly lower than those in the control group (p<0.01), left ventricular end-diastolic diameter and left ventricular end-systolic dimension levels in the resveratrol group were significantly lower than those in the model group and left ventricular ejection fraction and left ventricular short axis shortening rate levels were significantly higher than those in the model group (p<0.01). In the model group, the myocardial cells were arranged in a disordered manner and fibrogenic hyperplasia occurred, while the pathological changes of the myocardial cells in the resveratrol group were reduced compared with the model group. The collagen volume fraction of myocardial tissue in the model group was significantly higher than that in the control group (p<0.05) and the collagen volume fraction in the resveratrol group was significantly lower than that in the model group (p<0.05). The apoptosis rate of myocardial cells in the model group was significantly higher than that in the control group (p<0.01) and the apoptosis rate of myocardial cells in the resveratrol group was significantly lower than that in the model group (p<0.01). The expression levels of Phosphate-protein kinase R like endoplasmic reticulum kinase, Phosphate eukaryotic initiation factor 2 and Activating transcription factor 4 in the model group were significantly higher than those of the control group and the expression levels of Silent Information Regulator 1 were significantly lower than those of the control group (p<0.01). The expression levels of Phosphate-protein kinase R like endoplasmic reticulum kinase, Phosphate eukaryotic initiation factor 2 and Activating transcription factor 4 in the resveratrol group were significantly lower than those of the model group and the expression levels of silent information regulator 1 were significantly higher than those of the model group (p<0.01). Resveratrol can reduce apoptosis of myocardial cells after myocardial infarction by regulating the silent information regulator 1/protein kinase R-like endoplasmic reticulum kinase pathway endoplasmic reticulum stress pathway and improve myocardial remodeling and cardiac function in rats, providing a new target for clinical treatment of myocardial infarction.
Keywords
Resveratrol, silent information regulator 1/protein kinase R-like endoplasmic reticulum kinase pathway pathways, myocardial infarction, myocardial remodeling, heart function
Myocardial infarction is caused by acute and persistent coronary ischemia and hypoxia, which can be the normal population. In recent years, the incidence in my country has been obvious. The upward trend has brought a heavy burden to medical and health care[1]. Myocardial infarction has become a common cause of heart failure at home and abroad, and the remodeling of myocardial structure after myocardial infarction is an important basis for the development of heart failure. Myocardial cell necrosis, apoptosis and fibrosis in the infarcted area, local denervation followed by sympathetic sprouting, increased the density of sympathetic nerves in the non-infarcted area, thereby increasing the heart’s susceptibility to ventricular arrhythmias and sudden cardiac death[2]. Therefore, exploring the relevant mechanisms of cardiac remodeling after myocardial infarction is very important to prevent the occurrence of heart failure. The endoplasmic reticulum is the site of protein synthesis, folding and quality control. Infection, inflammation, nutritional deficiencies and excessive lipids will destroy the homeostasis of the endoplasmic reticulum. It causes the protein to be folded or misfolded, which damages the normal physiological functions of the endoplasmic reticulum, which in turn triggers endoplasmic reticulum stress[3]. Resveratrol is a kind of plant antibiotic produced by plants of the genus Vitis, mainly from grapes, knotweed, mulberry and other plants. It has a variety of physiological and pharmacological effects such as inhibiting platelet aggregation, clearing free radicals, anti-atherosclerosis, anti-oxidation and regulating blood lipids[4]. Relevant data show that resveratrol can improve myocardial remodeling after myocardial infarction by inhibiting oxidative stress and can up regulate Silent Information Regulator 1 (SIRT1) to inhibit endoplasmic reticulum stress[5]. However, whether resveratrol inhibits endoplasmic reticulum stress and protects hypoxic cardiomyocytes through the SIRT1/Protein Kinase R-like Endoplasmic Reticulum Kinase (PERK) pathway is still unknown. The purpose of this study is to analyze that resveratrol can inhibit myocardial cell apoptosis by regulating the SIRT1/ PERK pathway to improve myocardial remodeling and cardiac function after myocardial infarction.
Materials and Methods
Experimental reagents and instruments
Resveratrol (Shanghai Baoman Biological Technology Co., Ltd.); RPMI1640 cell culture medium (Shanghai Guandao Biological Engineering Co., Ltd.); Protein extraction kit (Shanghai Jizhi Biochemical Technology Co., Ltd.); sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gel configuration kit (Shanghai Ruichu Biotechnology Co., Ltd.); PVDF membrane (Beijing Biolab Technology Co., Ltd.); ECL Luminescence Kit (Shanghai Chuanqiu Biotechnology Co., Ltd.); Anti-SIRT1, phosphorylated PERK (p-PERK), Phosphorylated Eukaryotic Initiation Factor 2 α (p-eIF2), Activating transcription factor 4 (ATF4) antibodies (Aimeage Technology Co., Ltd.).
Heating magnetic stirrer (Shanghai Rongyingda Industrial Co., Ltd.); Paraffin Embedding Machine (Wuhan Tianzhirui Medical Technology Co., Ltd.); Flow Cytometer (Shanghai Ranzhe Instrument Equipment Co., Ltd.); Optical microscope (Shanghai Yiji Industrial Co., Ltd.); ultra-high-speed cryogenic centrifuge (Sichuan Shuke Instrument Co., Ltd.); digital gel imaging system (Beijing Biolab Technology Co., Ltd.); Fully automatic incubator (Shanghai Fuze Trading Co., Ltd.); high-speed tissue grinding machine (Shanghai Jingxin Industrial Development Co., Ltd.).
Experimental animals and grouping
32 healthy male SD rats were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., weighing 280-320 g and the rats were raised in the animal experiment center of our hospital. The environment is 21~25° %, the humidity is 50 %~60 % and the white nights are alternately set for 12 h. They are reared in a single cage with free water and food. After 2 w of adaptive feeding, they will be used for follow-up research.
Model establishment: 16 rats were selected by intraperitoneal injection of sodium pentobarbital for anesthesia, the extremities of the rats were clamped with vascular clamps and fixed on the animal dissecting board, the hair on the neck and left front chest of the rats were removed and the skin was prepared by iodophor disinfection. The trachea was intubated and connected to the electrocardiogram (ECG), a 2 cm incision was made between the 3 and 4 ribs of the left chest, the chest cavity was opened to expose the heart, the open heart capsule was torn, the left atrial appendage was located between the pulmonary artery cone and the left anterior descending branch below the pulmonary artery cone and ligated. Observe the ligation site until the apex of the heart turns white and the ECG shows ST-segment elevation, it is judged that the model is successful. The chest is closed and sutured, the rat’s consciousness and spontaneous breathing are restored and the tracheal intubation is pulled out. The successfully modeled rats were divided into model group and resveratrol group and normal rats were selected as control group and sham operation group (threading under the left anterior descending branch without ligation). Rats in the resveratrol group were intraperitoneally injected with resveratrol 8 mg/kg/d and the other three groups were intraperitoneally injected with dimethyl sulfoxide 0.5 ml/kg for 4 w.
Observation indicators
Echocardiographic detection: The rats were anesthetized 4 w after modeling and the left ventricular end diastolic diameter (LVEDD) of the rats in each group was measured by cardiac M-mode ultrasound. Left ventricular end systolic diameter (LVESD), left ventricular ejection fraction (LVEF), and left ventricular short axis shortening rate (LVFS).
Hematoxylin and eosin staining (HE Staining): the myocardial tissues of each group of rats were embedded in paraffin, sectioned and then deparaffinized and dehydrated gradually, stained with hematoxylin for 8 min and washed with water. Differentiate with 1 % hydrochloric acid and ethanol for 1 min, stain with eosin for 8 sec, rinse with running water, dehydrated and transparent and mount with neutral gum. the myocardial tissues of each group of rats were embedded in paraffin, sectioned and then deparaffinized and dehydrated gradually, stained with hematoxylin for 8 min and washed with water. Differentiate with 1 % hydrochloric acid and ethanol for 1 min, stain with eosin for 8 sec, rinse with running water, dehydrated and transparent and mount with neutral gum.
Masson staining: Myocardial tissue sections were stained with hematoxylin for 5-10 min, Masson Ponceau red for 5-10 min and rinsed with tap water. The 1 % phosphomolybdic acid solution was differentiated for 3~5 min, then dyed with aniline blue for 5 min, dehydrated and transparent and mounted with neutral gum.
Cardiomyocyte apoptosis rate: TUNEL staining kit detects cardiomyocyte apoptosis. Cardiomyocytes are fixed in 4 % formaldehyde solution. The negative control is no Terminal deoxynucleotidyl transferase (TDT) enzyme and the nucleus is positive (the nucleus is yellowish brown) as the criterion. Randomly count more than 5 high-power fields per sheet and calculate the number of nuclei and apoptosis-positive nuclei in each field. Apoptosis index (%) =number of apoptosispositive nuclei/total number of cell nuclei×100 %.
Detect protein expression by Western blot: add rat myocardial tissue to lysate to extract total RNA, detect protein concentration by Bicinchoninic acid assay method and take appropriate samples for SDSPAGE. Each group was loaded with the same amount of samples for electrophoresis. After electrophoresis, the protein in the gel was transferred to polyvinylidene fluoride (PVDF) membrane, and 5 % skimmed milk powder was blocked for 1 h. After blocking, SIRT1, p-PERK, p-eIF2α and ATF4 antibodies were added to incubate the PVDF membrane. Incubate overnight in a refrigerator at 4°, add the corresponding secondary antibody and incubate at room temperature for 1 h, use enhanced chemiluminescence (ECL) reagents for exposure and development and store after imaging.
Statistical methods
The data in this study are all analyzed by Statistical Package for the Social Sciences (SPSS) 21.0 software package and the measurement data are all expressed by (x¯±s) and the comparison of data between multiple groups is used by single-factor analysis of variance. Those with statistical significance between the two groups were further subjected to least significant difference (LSD-t) test and p<0.05 was regarded as statistically significant.
Results and Discussion
There was no significant difference in the levels of LVEDD, LVESD, LVEF and LVFS between the control group and the sham operation group after myocardial infarction (p>0.05). The levels of LVEDD and LVESD in the model group were significantly higher than those in the control group and the levels of LVEF and LVFS were significantly lower than those in the control group (p<0.01). The levels of LVEDD and LVESD in the resveratrol group were significantly lower than those in the model group and the levels of LVEF and LVFS were significantly higher than those in the model group (p<0.01) (Table 1).
| Group | cases | LVEDD(mm) | LVESD(mm) | LVEF(mm) | LVFS(mm) |
|---|---|---|---|---|---|
| Control group | 8 | 5.66±0.37 | 2.60±0.35 | 88.53±1.51 | 53.61±5.25 |
| mock surgical group | 8 | 5.41±0.35 | 2.54±0.23 | 88.17±1.25 | 53.02±4.59 |
| Model group | 8 | 7.82±0.59* | 5.39±0.28* | 63.84±2.86* | 30.76±4.27* |
| Resveratrol group | 8 | 5.92±0.45*# | 3.39±0.25*# | 76.89±4.79*# | 40.28±1.02*# |
| F | 47.65 | 179.21 | 124.67 | 56.89 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Compared with the control group *p<0.05; compared with the model group #p<0.05
Table 1: Effects of Resveratrol on Cardiac Function After Myocardial Infarction in Rats
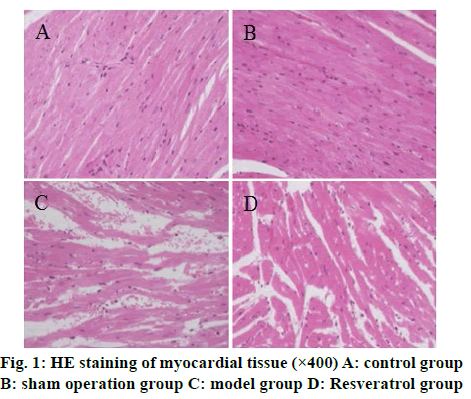
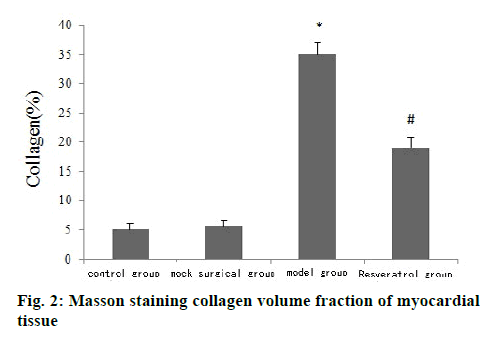
The results of HE staining showed that the cardiomyocytes of the control group and the shamoperated group were arranged tightly and neatly and the capsule was intact. The cardiomyocytes of the model group were arranged disorderly and there was fibrous tissue hyperplasia. The pathological changes of myocardial cells of rats in the resveratrol group were less than that in the model group, as shown in fig. 1. Masson staining results showed that the collagen volume fraction of myocardial tissue in the model group was significantly higher than that of the control group (p<0.05) and the collagen volume fraction of myocardial tissue in the resveratrol group was significantly lower than that of the model group (p<0.05) (fig. 2).
There was no obvious cardiomyocyte apoptosis in the control group and sham operation group. The apoptosis rate of cardiomyocytes in the model group was significantly higher than that in the control group (p<0.01). The apoptosis rate of cardiomyocytes in the resveratrol group was significantly lower than that in the model group (p<0.01) (Table 2).
| Group | cases | Cardiomyocyte apoptosis rate (%) |
|---|---|---|
| Control group | 8 | 3.28±1.02 |
| mock surgical group | 8 | 3.55±1.26 |
| Model group | 8 | 31.52±4.05* |
| Resveratrol group | 8 | 15.44±5.47*# |
| F | 115.91 | |
| p | <0.001 |
Note: Compared with the control group *p<0.05; compared with the model group #p<0.05
Table 2: Effects Of Resveratrol On Cardiomyocyte Apoptosis After Myocardial Infarction In Rats
Western blot detection results showed that the expression of p-PERK, p-eIF2α and ATF4 in the myocardial tissue of the model group was significantly higher than that of the control group and the expression of SIRT1 protein was significantly lower than that of the control group (p<0.01). The expression of p-PERK, p-eIF2α, ATF4 protein in myocardial tissue of resveratrol group was significantly lower than that of model group and the expression of SIRT1 protein was significantly higher than that of model group (p<0.01) (fig. 3 and Table 3).
| Group | cases | SIRT1 | p-PERK | p-eIF2α | ATF4 |
|---|---|---|---|---|---|
| Control group | 8 | 0.75±0.12 | 0.48±0.06 | 0.38±0.08 | 0.50±0.08 |
| mock surgical group | 8 | 0.74±0.13 | 0.49±0.07 | 0.37±0.10 | 0.51±0.12 |
| Model group | 8 | 0.41±0.15* | 1.22±0.36* | 0.85±0.25* | 1.16±0.21* |
| Resveratrol group | 8 | 0.73±0.16*# | 0.68±0.23*# | 0.51±0.17*# | 0.60±0.11*# |
| F | 11.00 | 20.22 | 14.93 | 48.80 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Compared with the control group *p<0.05; compared with the model group #p<0.05
Table 3: Comparison of The Protein Content of Sirt1, P-Perk, P-Eif2α, And Atf4 In Myocardial Tissue Of Rats In Each Group
Resveratrol is a kind of phenolic plant antitoxin. It is widely found in grape skins, seeds, nuts, knotweed and other plants. It is a natural antioxidant and free radical scavenger. It can protect the cardiovascular system in terms of ischemia/reperfusion myocardial injury and atherosclerosis through anti-oxidation, scavenging free radicals, regulating blood lipids, and increasing the body’s oxygen storage. Coupled with its small side effects, it has become a natural medicine with great research value in the current research field[6,7]. A variety of studies have confirmed that resveratrol is beneficial to the treatment of cardiovascular diseases. Some scholars have found through research that resveratrol can significantly improve the left ventricular systolic and diastolic function of rats with heart failure and increase the left ventricular ejection fraction[8]. Some foreign scholars have found through animal model research that the coronary arteries of mice are ligated to establish a myocardial infarction model. After two weeks of treatment with 50 mg/kg/d resveratrol, the LVEF level of mice increased from 39 % to 74 %. It is speculated that resveratrol may reduce the infarct size and improve myocardial remodeling by activating the oxidative stress system[9]. In this study, 4 w after the successful modeling of myocardial infarction rats, echocardiography measured the heart function indicators of the rats in each group and found that the levels of LVEDD and LVESD in the model group were significantly higher than those in the control group. The levels of LVEF and LVFS were significantly lower than those of the control group (p<0.01), suggesting that the ventricular systolic and diastolic functions of rats were reduced after myocardial infarction. The levels of LVEDD and LVESD in the resveratrol group were significantly lower than those in the model group, and the levels of LVEF and LVFS were significantly higher than those in the model group (p<0.01). This suggests that resveratrol can inhibit left ventricular dilation in rats with myocardial infarction and improve heart function.
Pathological stimulation after myocardial infarction will lead to the remodeling process, mainly myocardial cell remodeling and ventricular remodeling. From the cellular point of view, necrosis, apoptosis and autophagy lead to the permanent loss of cardiomyocytes and the proliferation of non-cardiomyocytes such as fibroblasts leads to interstitial fibrosis, which runs through the entire process of myocardial remodeling. The two promote each other to form a vicious circle, which affects myocardial contraction and diastolic function and is an important cause of heart failure. Therefore, reducing myocardial cell apoptosis is of great significance in inhibiting remodeling after myocardial infarction[10,11]. The results of HE staining in this study showed that the pathological changes of myocardial cells in the resveratrol group were less than that in the model group; Masson staining results showed that the myocardial collagen volume fraction in the resveratrol group was significantly lower than that in the model group (p<0.05), and the apoptosis rate of myocardial cells in the resveratrol group was significantly lower than that in the model group (p<0.01). From the above results, it can be seen that resveratrol may improve myocardial remodeling by reducing the apoptosis of myocardial cells.
The main biological function of the endoplasmic reticulum is to participate in the synthesis, modification and transport of proteins, under various stimuli such as ischemia, hypoxia, and oxidative stress. The corresponding transmembrane signaling protein is activated, causing misfolded and unfolded proteins to accumulate in the endoplasmic reticulum and induce endoplasmic reticulum stress in cells[12,13]. If the endoplasmic reticulum stress lasts for too long, it can activate downstream apoptotic signaling molecules to initiate cell apoptosis, causing irreversible damage to the tissue. PERK is a type I transmembrane protein located on the endoplasmic reticulum membrane. After activation, it can further phosphorylate eIF2α outside the endoplasmic reticulum and inhibit protein synthesis; In addition, by activating the downstream ATF4, and transferring to the nucleus to up-regulate the expression of endoplasmic reticulum chaperones, participating in the transcription and expression of amino acid transporters, it helps to restore the homeostasis of the endoplasmic reticulum[14]. Resveratrol is a natural agonist of STRT1. Some scholars have found through research that resveratrol can activate STRT1 to reduce endoplasmic reticulum stress-induced cardiomyocyte apoptosis in rats with diabetic cardiomyopathy[15]. The results of this study showed that the expression of p-PERK, p-eIF2α and ATF4 in myocardial tissue of the resveratrol group was significantly lower than that of the model group, and the expression of SIRT1 protein was significantly higher than that of the model group (p<0.01). This shows that resveratrol can upregulate the expression of STRT1 and inhibit the PERK endoplasmic reticulum stress pathway, thereby reducing cardiomyocyte apoptosis.
In summary, resveratrol can reduce myocardial cell apoptosis after myocardial infarction by regulating the SIRT1/PERK endoplasmic reticulum stress pathway. It can also improve myocardial remodeling and cardiac function in rats, and can provide a new target for clinical treatment of myocardial infarction.
Ethical approval:
This study was reviewed and approved by the Ethics Committee for Experimental Animals of Hubei University of Technology Hospital (ID:HUTU154) and all mice were treated according to the guidelines of principles of laboratory animal care (NIH publication No. 86-23, revised 1985).
Conflict of interests
The authors declared no conflicts of interest.
References
- Wang Z, Liu SH, Bo LJ, Zheng YZ, Shi J, Xu Y, et al. Clinical Features and Laboratory Data Analysis of Glucose-6- Phosphate Dehydrogenase Deficiency. J Exp Hematol 2018;26(5):1437-41.
- Carlsson AC, Bandstein N, Roos A, Hammarsten O, Holzmann MJ. High-sensitivity cardiac troponin T levels in the emergency department in patients with chest pain but no myocardial infarction. Int J Cardiol 2017;228:253-9.
- Belle L, Cayla G, Cottin Y, Coste P, Khalife K, Labèque J-N, et al. French Registry on Acute ST-elevation and nonST-elevation Myocardial Infarction 2015 (FAST-MI 2015). Design and baseline data. Arch Cardiovasc Dis 2017;110(6):366-78.
- Zhongkai H, Yuhan Z, Feng Y. Effects of resveratrol on myocardial protection and Akt/JNK3/caspase-3 signaling pathway in rats with acute myocardial infarction. Chin J Evid Based Cardiovasc Med 2018;10:1310-4.
- Xiaoxiao J, Zuhua Z, Dong C. Effect of resveratrol on cardiac nerve remodeling in rats with acute myocardial infarction and its mechanism. J Chin Med University 2018;47:27-30.
- Chengjun L, Mei D, Ren Faxin. The effect of resveratrol on the levels of myocardial injury markers in AMI rats through the c-fos pathway. Chin J Evid Based Cardiovasc Med 2020;12:436-42.
- Dan L, Fei H, Ke P. The protective effect and mechanism of resveratrol post-treatment on myocardial ischemia/reperfusion injury in rats. Chin Emerg Med 2018;38:910-4.
- Zhongkai H, Yuhan Z, Yao Feng. Resveratrol improves the inflammatory response after acute myocardial infarction in rats by silencing the information regulator 1/nuclear factor kappa B pathway. Chin J Cardiol 2019;24:62-5.
- Tang F, Guo S, Liao H, Yu P, Wang L, Song X, et al. Resveratrol Enhances Neurite Outgrowth and Synaptogenesis Via Sonic Hedgehog Signaling Following Oxygen-Glucose Deprivation/Reoxygenation Injury. Cell Physiol Biochem 2017;43(2):852-69.
- Waterford SD, Eusanio MD, Ehrlich MP, Reece TB, Desai ND, Sundt TM, et al. Postoperative myocardial infarction in acute type A aortic dissection: A report from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg 2017;153(3):521-7.
- Bahit MC, Kochar A, Granger CB. Post-Myocardial Infarction Heart Failure. JACC Heart Fail 2018;6:179-86.
- Xiang C, Wang Y, Zhang H, Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 2017;22(1):1-26.
- Bai X, Geng J, Li X, Wan J, Liu J, Zhou Z, et al. Long Noncoding RNA LINC01619 Regulates MicroRNA-27a/Forkhead Box Protein O1 and Endoplasmic Reticulum Stress-Mediated Podocyte Injury in Diabetic Nephropathy. Antioxid Redox Signal 2018;29(4):355-76.
- Lumley EC, Osborn AR, Scott JE, Scholl AG, Mercado V, McMahan YT, et al. Moderate endoplasmic reticulum stress activates a PERK and p38-dependent apoptosis. Cell Stress Chaperones 2017;22(1):1-12.
- Fan P, Tyagi AK, Agboke FA, Pokharel N, Jordan VC. Abstract 2332: Integral modulation of nuclear factor-kappa B activation by C/EBPß and the endoplasmic reticulum stress sensor PERK to mediate estrogen-induced apoptosis in estrogen-deprived breast cancer cells. Cancer Res 2017;77(13):2332.