- *Corresponding Author:
- A. R. Bhat
Department of Pharmaceutical Analysis, K. L. E. S’s College of Pharmacy, Belgaum-590 010, India
E-mail: anilchandrabhat@yahoo.com
| Date of Submission | 20 May 2005 |
| Date of Revision | 19 August 2005 |
| Date of Acceptance | 22 June 2006 |
| Indian J Pharm Sci,2006, 68 (3): 406-408 |
Abstract
A simple, economical, fast, and precise reverse phase high performance liquid chromatographic method has been developed for the determination of rabiprazole in tablet dosage form. A C 18 Hypersil (5 micron 25 cm×4.6 mm) column from Thermo, in isocratic mode with mobile phase acetonitrile:phosphate buffer:methanol(50:40:10) The flow rate is 1.4 ml/min, and effluent was monitored at 232 nm.
Rabiprazole sodium (RPZ), a benzimidazole PPI, is a new substituted benzimidazole [1] H+/K+-ATPase inhibitor. It acts as an irreversible, noncompetitive inhibitor of the H+/K+-ATPase, and preliminary studies demonstrate that rabiprazole produces a potent and long-lasting inhibition of gastric acid secretion and a low level of hypergastrinemia [2-4]. Literature survey revealed estimation of rabiprazole by HPLC, HPTLC and derivative spectroscopy. The in vitro evaluation study of rabiprazole has been reported [5]. The proposed method describes the determination of rabiprazole sodium by RP-HPLC, which is simple, precise, rapid and selective.
High performance liquid chromatograph (Milton Roy) equipped with CM 4000 spectromonitor 3100 of variable wavelength detector, chromatograph I/F module from indect instrument, injector manual, 20 μl loop and a Shimadzu UV-1201 spectrometer were used.
Standard rabiprazole drug was procured from Cadila Pharma Ltd., (Rabicip) Ahmedabad. Acetonitrile (500 ml), phosphate buffer (400 ml), and methanol (HPLC grade) were mixed and filtered through 4.5 μ filter paper and sonicated. Linearity study was carried out at different concentrations, and it was found to be linear in the range of 10-30 μg/ml. Calibration curves could be represented by the following equation: y=0.0414×(+0.076) (r=0.999); this equation was used for the determination of rabiprazole from tablets
The flow rate was maintained at 1.4 ml/min. Temperature of the column was kept at ambient. The pressure was maintained at 675 psi and the effluent at 232 nm. The mobile phase used was acetonitrile:phosphate buffer:methanol (50:40:10).
Twenty tablets were procured from Cadila Pharmaceuticals Ltd., Ahmedabad, and were powdered and triturated finely. The powder equivalent to 50 mg of rabiprazole was taken and dissolved in the mobile phase and diluted to obtain final concentration of 10 μg/ml. Similar procedure was followed for rabiprazole obtained from FDC (Pepcia).
To study the accuracy, reproducibility, and precision of the proposed method, recovery experiments were carried out. A fixed amount of the preanalysed sample was taken and standard drug was added at three different levels. Each level was repeated at least five times. The summaries of recovery studies are reported in Table 1.
| Label claim amount of std added (mg) | Amount added (mg) | Found (mg*) | Recovery (%) |
|---|---|---|---|
| 10 | 0 | 9.89 | 99.8 |
| 10 | 5 | 15.12 | 100.8 |
| 10 | 10 | 20.19 | 100.95 |
| 10 | 15 | 29.97 | 99.9 |
Table 1: Recovery Of Rabiprazole
The present study comprises a high performance liquid chromatograph method to determine rabiprazole from tablet dosage forms. Experiments were carried out to establish the method. The mobile phase, bearing acetonitrile:phosphate buffer:methanol in proportion of
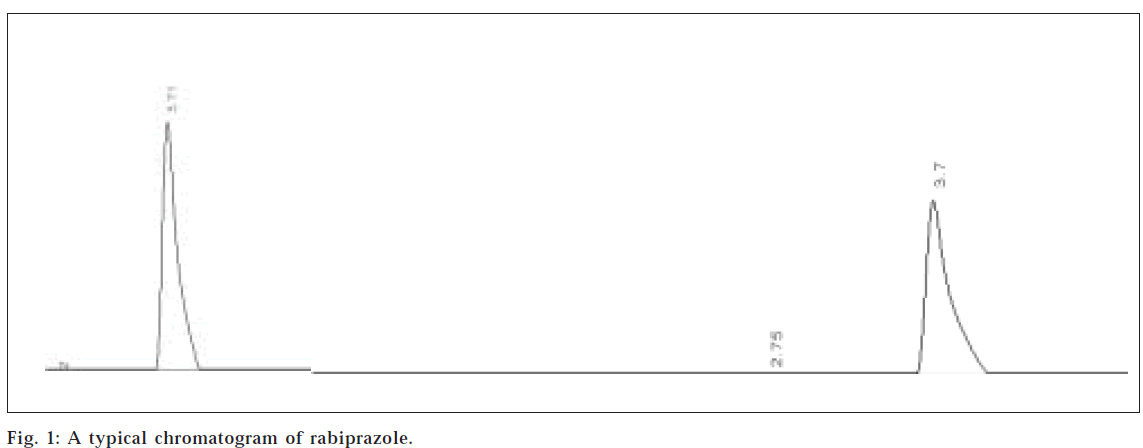
(50:40:10) was found to be ideal. The elution was observed (RT-3.71 min).The values of percent recovery and standard deviation indicate that the method is accurate, reproducible, and precise .The summaries of final results are illustrated in Table 2.
| Brand name | Amount found (mg/tablet) | % RSD | Percentage assay |
|---|---|---|---|
| Rabicip (Cipla Ltd.) | 10.01±0.38 | 0.258 | 100.09 |
| Pepcia (FDC Ltd) | 9.98±0.98 | 0.112 | 99.85 |
Table 2: Analysis Of Rabiprazole Tablets
Acknowledgements
The authors wish to thank M/s Cadila Pharmaceutical Limited for providing the authentic sample of the drug. The authors are grateful to Dr. F. V. Manvi, Principal, K.
L. E. S. ‘s College of Pharmacy, Belgaum; and Prof. A.
D. Taranalli, Vice Principal, for the necessary facilities and encouragement.
References
- Budavari, S., Eds., In: The Merck Index, 13th Edn., Merck and Co., Inc., Whitehouse Station, NJ, 2001, 1450.
- Cloud, M.L., Enas, N., Humphries, J, and Bassion, S., Dig. Dis. Sci., 1998, 43, 993.
- Park, J.B., Imamura, L, and Kobashi, K., Biol. Pharm. Bull., 1996, 19, 182
- Tsuchiya, M., Imamura, L., Park J.B. and Kobashi, K., Biol. Pharm. 2005Bull., 1995, 18, 1053
- Stack, W.A., Knifton, A., Thirlwell, D., Cockayne, A .and Atherton J.C., Amer. J. Gastroenterol., 1998, 93, 1909.