- *Corresponding Author:
- Richa Shri
Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala-147 002, India
| Date of Submission | 01 June 2017 |
| Date of Revision | 15 September 2017 |
| Date of Acceptance | 07 September 2018 |
| Indian J Pharm Sci 2018;80(6):984-995 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The genus Cinnamomum (family Lauraceae) is revered for the pleasant essential oils isolated from various species. Cinnamomum species are extensively employed all around the world not only as a spice but also in traditional and modern systems of medicine. These possess wide range of activities that included neuroprotective properties. Neurodegenerative disorders cause immense mortality and morbidity. Hence search for newer therapeutic targets for the management of such disorders has made herbals an important area for research. The present paper integrates and critically examines the scientific evidence on the role of Cinnamomum species in neuroprotection in in vitro and in vivo models of different neurodegenerative disorders. Literature was reviewed extensively by searching databases such as Google Scholar, Science Direct, PubMed, and Scopus to compile scientific data from 2003 to 2015, on the role of different species of Cinnamomum in various neurodegenerative disorders. Plants/phytoconstituents have demonstrated effects crucial for the management of neurodegenerative disorders. Various extracts, volatile oil and phytoconstituents isolated from Cinnamomum species diminished selective pathological and histological hallmarks in different neurodegenerative disorders such as inhibition of oxidative stress, neuroinflammatory mediators, and ischemic injury. This review summarized different research studies with Cinnamomum species to throw light on the promise held by these species as well as prospects for new drug development to manage neuronal damage. This genus appears to be a valuable source for developing new neuroprotective agents.
Keywords
Cinnamomum species, antioxidant, antineuroinflammatory, antiAlzheimer’s, antiParkinson’s, neuroprotection
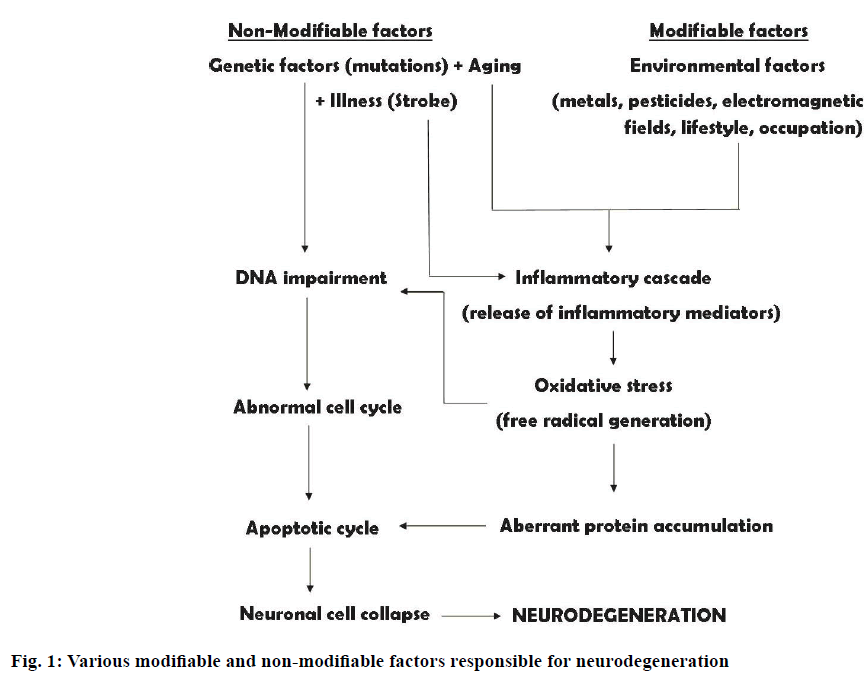
Neurodegenerative disorders (NDD) comprise of disorders marked by pathological events resulting from slow progressive and irreversible deterioration of certain areas of nervous system, ultimately leading to neuronal and synaptic deficit [1]. These disorders include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, amyotrophic lateral sclerosis and frontotemporal dementia [2]. These are multifactorial in nature and they share neuropathological traits such as oxidative stress leading to free radical development, aberrant protein dynamics, which results in faulty protein degradation and accumulation, marred bioenergetics, mitochondrial dysfunctions and neuroinflammatory activities [3]. A brief pathology of all the diseases mentioned above is explained in this article.
AD is a NDD characterized by formation of amyloid plaques due to aberrant cleavage of amyloid precursor protein (APP) by β- and γ-secretases. It causes unevenness in the fabrication and clean-up of amyloid peptide leading to aggregation. Ultimately, the aggregates get deposited in the form of senile plaques. Formation of amyloid peptide stimulates oxidative damage, induces tau hyperphosphorylation, and results in noxious effects on synapses and mitochondria [4]. Loss of cholinergic activity is also one of the main factors contributing to AD [5]. There is reduction in the levels of acetylcholine, a neurotransmitter important in learning and memory [6]. Different studies have also linked insulin and insulin resistance with AD. Enhanced levels of insulin in the brain promote limited clearance of amyloid peptide as they both compete for a common degrading mechanism ‘insulin degrading enzyme’ [7].
PD pathology is characterized by loss of dopaminergic neurons in substantia nigra and deposition of intraneuronal protein aggregates known as Lewy bodies [8]. Relapsed neurons in PD are characterized by the presence of aggregated α-synuclein. It is one of the main constituent of Lewy body fibrils [9]. In some sporadic and autosomal recessive forms of PD there occur mutations in parkin and DJ-1 genes. Parkin is a protein crucial for functioning of mitochondria whereas DJ-1 provides resistance against oxidative stress [10].
Huntington’s disease is a rare NDD, which is caused by a mutation that expands the polymorphic nucleotide (CAG) tract in HTT (Huntington) gene [11]. The disease is exemplified by motor, cognitive and psychiatric symptoms [12]. Motor symptoms include chorea, dystonia, bradykinesia, rigidity, loss of postural reflexes; cognitive symptoms consist of inadequacy due to lack of planning, complexity in coordinating thoughts and activities, problematic communication, absence of insight, obscurity in gathering new skills; anxiety, depression, irritability, apathy and obsessivecompulsive disorder are some of the psychiatric symptoms [13].
Amyotrophic lateral sclerosis is a NDD of human motor system. The disease is characterized by upper and lower motor neuron degeneration. Intracellular inclusion bodies are present in neuronal soma and proximal dendrites. In some cases of the disease, mutations occur in superoxide dismutase 1 gene thus inducing oxidative stress. Other pathogenic mechanisms include excitotoxicity, mitochondrial dysfunction, apoptosis, glial activation and growth factor abnormalities. The consequences of disease include muscle weakness, progressive motor disability, and ultimately loss of life due to respiratory failure or an allied illness [14].
Frontotemporal dementia is illustrated by degeneration of prefrontal and anterior temporal lobes [15]. There is loss of large cortical nerve cells along with microvacuolation of superficial neurophil. The prominent characteristics of the disease include modifications in social behaviour, emotional disturbances which consist of inability in expressing feelings, dietary alterations and disturbances in speech [16]. Various modifiable and nonmodifiable factors lead to neurodegeneration as shown in Figure 1.
Conventional pharmacotherapy includes use of cholinesterase inhibitors for AD [17] and anticholinergics, dopamine precursors, MAO-B inhibitors, catechol- O-methyltransferase inhibitors, dopamine receptor agonists for PD, the two most common NDD [18]. However, so far there is no well-established agent to prevent the onset or progression of NDD. Thus natural sources are being investigated to track down neuroprotective agents. Various plants (Curcuma longa, Ginkgo biloba, Panax ginseng, Scutellaria baicalensis) have shown beneficial results [19-26] in cellular and animal models of NDD.
Spices are reported to prevent or delay neurodegeneration [27,28]. Different species of Cinnamomum are widely used as spice and condiment. Moreover, these possess significant antioxidant and antiinflammatory activities, which may be beneficial in neuroprotection [29,30]. In this context, the present paper summarizes research reports on various species of Cinnamomum that have been explored for the management of NDD.
The objective of this study was to review Cinnamomum species as effective measure for various NDDs. Herbals and their diverse constituents are being established as potent neuroprotective agents against different neuropathologies. In this regard, the present review incorporates the scientific evidence on the role of Cinnamomum species in neuroprotection.
Literature search
Extensive literature review was performed by searching databases that included Google Scholar, ScienceDirect, PubMed, and Scopus; and considering various books so as to gather scientific data from 2003 up to 2015, on the role of different species of Cinnamomum in various NDD.
Genus Cinnamomum, a brief look
The genus Cinnamomum belongs to family Lauraceae and includes more than 250 aromatic evergreen trees and shrubs, chiefly located in Asia and Australia [31,32]. The plant is widely cultivated in tropical areas in various types of soils [33]. Different species of the genus that have been explored for various biological activities relevant to neuroprotection were mentioned in Table 1 [34].
| Species | Common name | Reference |
|---|---|---|
| Cinnamomum burmanii Cinnamomum cassia Cinnamomum philippinensis Cinnamomum zeylanicum |
Indonesian cinnamon Chinese cinnamon Philippine cinnamon Ceylon cinnamon |
[56] [34] [53] [77] |
Table 1: Various species of Cinnamomum explored for neuroprotection
Cinnamomum species are valued greatly for the pleasant, aromatic essential oils present [35,36]. Main source of cinnamon oil is Sri Lanka and the oil has been largely used by both pharmaceutical and food industries [37]. Mucilage, tannin, sugar and resin are also present along with essential oil. Oil from different morphological parts of cinnamon possesses the same type of monoterpene hydrocarbons in varied proportions but with a different characteristic constituent e.g. presence of cinnamaldehyde, eugenol and camphor in the bark oil, leaf oil and root-bark oil, respectively [38]. Cinnamon also contains phenolic compounds mainly flavonoids like quercetin and kaempferol [39,40].
Role of Cinnamomum Species in NDD
Effect on pathways linked to neurodegeneration
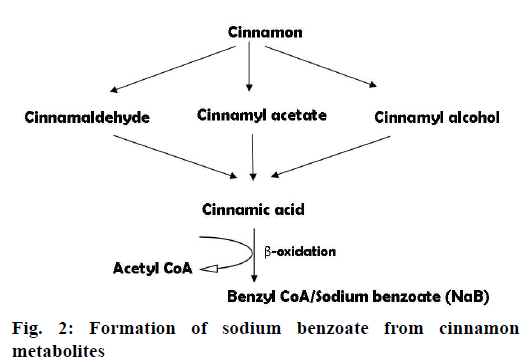
Neuroinflammation is an important pathological cascade in all the NDD. It is mainly mediated by activated microglia in the brain, which are further responsible for the release of numerous proinflammatory and cytotoxic factors, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, nitric oxide (NO) and reactive oxygen species (ROS). All these act bluntly and cause neuronal damage [41]. Various studies showing the role of Cinnamomum species in neuroinflammation are given as follows: antiinflammatory activity of sodium benzoate, a main metabolite of cinnamon (Figure 2), was explored in human astrocytes and mouse microglia using lipopolysaccharide (LPS)-induced inflammation [42]. Table 2 displays the beneficial effects of sodium benzoate; Hwang et al. [43] evaluated two phytoconstituents isolated from C. cassia bark, 2-hydroxycinnamaldehyde (HCA) and its derivative 2′-benzoyloxycinnamaldehyde (BCA), against neuroinflammation in LPS-stimulated microglial cultures and microglia/neuroblastoma co-cultures. Both the compounds, HCA (1.25-20 μM) and BCA (0.3125- 20 μM), exhibited following beneficial effects: reduction in the levels of NO; decrease in the release of TNF-α; reduced expression of NO synthase (iNOS), IL-1β, TNF-α; inhibition of LPS-induced extracellular signal regulated kinase, c-Jun N-terminal kinase, p38 mitogen activated protein kinase and nuclear factor kappa-light chain enhancer of activated B cells pathways; blockade of microglia mediated neuroblastoma B35 death; Ho et al. [29] studied antineuroinflammatory effect of ethanol extract (50 μg/ml) of C. cassia bark and its major constituents i.e. cinnamaldehyde, coumarin, cinnamyl acetate, cinnamic acid, 2-methoxy cinnamaldehyde, α-methoxy cinnamaldehyde, cinnamyl alcohol, eugenol (100 μM for all the compounds) using LPS-activated BV2 microglia culture model. The extract significantly inhibited 65 % of NO production whereas all the constituents exhibited inhibition ability in the sequence cinnamaldehyde>2-methoxy cinnamaldehyde>amethyl cinnamaldehyde>eugenol.
| Affected cell | Pathological event | Beneficial effect of sodium benzoate |
|---|---|---|
| Microglia | Induction of NO production | Inhibition of NO production |
| Microglia | Expression of pro-inflammatory cytokines | Reticence of expression |
| Microglia | Expression of iNOS due to stimulation of cells by reagents of neurological disorders such as fibrillar Aβ peptides (AD), fibrillar PrP peptides (prion diseases), dsRNA in the form of polyinosinic-polycytidylic acid (viral encephalopathy), IL-12 p402 (multiple sclerosis-MS), and 1-(methyl-4-phenylpyridinium) (Parkinsonian toxin) | Impedetion of iNOS expression |
| Microglia | Activation of p21 ras | Suppression of activation |
| Human astrocytes | IL-1β induced expression of iNOS | Obstruction of expression |
| Human astrocytes | Activation of iNOS promoter | Hampered the activation |
Table 2: Beneficial effects of sodium benzoate in human astrocytes and mouse microglia using lipopolysaccharide (LPS)-induced inflammation
Other significant results obtained by the administration of extract and cinnamaldehyde are as follows: decreased levels of iNOS and mRNA; reduction in TNF-α, IL-1β, IL-6; inhibition of NF-κB binding activity; trans-cinnamaldehyde (TCA), one of the main components of young twigs of C. cassia was evaluated for antiinflammatory activity using in vitro and in vivo models. The results are given in the Table 3 [44].
| Type of study | Model | Concentration | Result |
|---|---|---|---|
| In vitro | LPS-induced inflammatory BV2 microglial cells | 1 and 3 µM | Inhibition of NO production |
| In vivo | 6-Oxidopamine | 30 mg/kg | Suppression of iNOS and COX-2 |
Table 3: In vitro and in vivo studies on trans-cinnamaldehyde
Effect on oxidative stress
Brain is more vulnerable to negative effects of ROS than any other organ due to high metabolic rate and diminished ability for cellular regeneration. In case of NDD, specific brain regions express various indices of ROS damage that ultimately enhance the process of neurodegeneration e.g. markers of lipid peroxidation including, 4-hydroxyneonal and malondialdehyde have been identified in the cortex and hippocampus of patients with AD and the substantia nigra of patients with PD [45]. Since antioxidants are reported to be linked to neuroprotection, plants with antioxidant activity can be an excellent source for prevention of neurodegeneration.
Different Cinnamomum species have exhibited efficacious inhibition of oxidative stress though they have not been correlated with neuroprotective effect [46-50]. One of the muscarinic cholinergic antagonists namely scopolamine causes memory deficits which have been linked to Alzheimer [51]. C. zeylanicum bark has displayed significant effect in scopolamine-induced cognitive impairment and oxidative stress model. The bark extract of plant (200 and 400 mg/kg) significantly inhibited the oxidative stress in brain tissue of rats as evidenced by decrease in MDA levels and increase in glutathione levels in rats [30].
Effect on ischemic injury
The neuronal death in NDD involves apoptotic biochemical surges involving mitochondrial modifications and caspase activation. Initiation of such neuronal apoptosis is affected by aging, environmental and genetic factors. At the cellular level, neuronal apoptosis in NDD is prompted by oxidative stress, metabolic compromise and disturbance of calcium homeostasis [52]. Cinnamomum species have shown protective effect in ischemic injuries in brain as given underneath.
Preventive effect of cinnamophilin (CINN), isolated from C. philippinense roots, in oxidative damage and transient cerebral focal ischemia was explored by Lee et al. [53]. Pretreatment with CINN (20-80 mg/kg) caused significant reduction in brain infarction by 33-46 %. Post ischemic treatment also exhibited decrease in brain infarction by 43 %. Animals treated with the compound displayed reduced levels of oxidative damage.
CINN was also evaluated for therapeutic efficacy in oxygen glucose deprivation (OGD) and transient focal cerebral ischemia using in vitro and in vivo studies by Lee et al. [54]. Following results were obtained: in vitro assays revealed that CINN effectively inhibited Fe3+-induced lipid peroxidation with IC50 value of 8.5 μM and DPPH radical scavenging with IC50 value 242.5 μM. Reduction in the generation of proinflammatory cytokines in LPS-stimulated RAW 264.7 and BV2 cells was also shown by CINN at concentration range of 30-100 μM and 10-100 μM, respectively; in vivo results showed reduced levels of malondialdehyde by CINN (80 mg/kg) in rat brain after ischemic onset.
Polyphenolic aqueous extract of C. burmanii was evaluated for astrocyte cell swelling and depolarization of inner mitochondrial membrane followed by OGD. Extract (0.01, 0.05, 0.1 mg/ml) significantly attenuated cell swelling and depolarization of inner mitochondrial membrane potential [55].
Polyphenols isolated from water soluble extract of C. burmanii were evaluated for key features of ischemic injury followed by OGD. Procyanidin type A trimer 1 isolated from the extract completely prevented OGDinduced glial cell swelling at concentration range of (10-2 to 10-7 mg/ml). It also attenuated OGD-induced increase in ROS, RNS (reactive nitrogen species), mitochondrial membrane depolarization, increased intracellular calcium, reduction in glutamate uptake and decline in ATP [56].
Two herbal formulations have also shown protective effect of C. cassia in focal cerebral ischemia as given below: C. cassia is a component of Chinese medical formulation, Guizhi-Fuling capsules, which has shown protective effects in rat in vivo model of focal cerebral ischemia. Administration of formulation (0.3 and 0.9 g/kg, po) after cerebral ischemia exhibited reduced brain infarction and water content in rats following ischemia. The formulation also down regulates proinflammatory cytokines in ischemic brain [57]; the plant is also a component of Joongpoongtang 05, traditional Korean medicine for preventing transient focal cerebral ischemia [58].
Effect on neurotrophic factors
Animal models of various NDD have shown the protective role of neurotrophic factors [59-61]. The following study explained the protective role of C. verum and its metabolite sodium benzoate in the upregulation of neuroptrophic factors in the brain of mice. Sodium benzoate (250 μM) led to increase in the mRNA expression of both brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) in a timedependent and dose-dependent manner in primary human neurons. Oral administration of cinnamon powder (200 mg/kg) to mice caused production of sodium benzoate in the brain of mice and both led to increase in the manifestation of BDNF and NT-3 in mice brain [62].
Role in AD
AD is the most widespread NDD. The disease is characterized by build-up of amyloid plaques, neurofibrillary tangles, cholinergic deficit, neuroinflammation and neuronal diminution along with a progressive waning of cognition and functional aptitudes which eventually leads to dementia [63]. No synthetic medication has been able to cure AD so far. Researchers are exploring herbal remedies to find an effective cure for the disease. Several imbalances in AD have been amended by various Cinnamomum species.
Effect on acetylcholinesterase (AChE) inhibition
Acetylcholine is the neurotransmitter involved in memory and learning. Cholinergic hypothesis of AD suggested that deterioration of cholinergic neurons in basal forebrain and the accompanying loss of cholinergic neurotransmission in the cerebral cortex and other areas interposed appreciably to the depreciation in cognitive function in AD patients [64]. Cinnamon has shown significant beneficial effect by efficient inhibition of AChE.
Ethanol extract of C. cassia bark at 200 μg/ml displayed 63 % inhibition of enzyme in the Ellman’s method and came out to be a good anticholinesterase agent [65]. Anticholinesterase potential of aqueous and ethanol extracts of C. zeylanicum bark, at 100 μg/ml was evaluated using Ellman’s method. Percent inhibition displayed by the extracts was 46.84±0.003 and 40.83±0.005, respectively [66]. Methanol extract and cinnamon oil from C. zeylanicum leaves were evaluated for anticholinesterase potential using Ellman assay. Results revealed that oil showed better activity with IC50 value 45.88±1.94 μg/ml as compared to 77.78±0.3 μg/ml for the extract [67].
Methanol extract, cinnamon oil and the chief biomarker of the oil i.e. linalool from C. tamala leaves were explored for anticholinesterase potential employing Ellman’s method. Cinnamon oil showed better inhibition than the methanol extract. Linalool also possessed good inhibition against the enzyme with IC50 value of 55.10±0.191 μg/ml. Inhibition was in the order linalool>cinnamon oil>methanol extract [68].
Effect on experimentally-induced dementia
Malik et al. [69] evaluated the effect of standardized lyophilized aqueous extract (LCZE) of C. zeylanicum bark (50, 100, 200 mg/kg, po) on learning and memory against streptozotocin-induced memory impairment. Morris water maze test (MWM) and object recognition test (ORT) were used as behavioural models for effect on learning and memory. The effect of LCZE on the AChE activity and oxidative stress parameters in the cerebral cortex and hippocampus of rat brain were also evaluated. Results displayed by LCZE are given as follows: LCZE considerably decreased the transfer latency and increased the time spent by the animals in target quadrant in MWM along with improvement in the discrimination between a familiar object and a novel object in ORT, thus indicating the reversal of STZinduced memory impairment; STZ-induced alterations in AChE activity and oxidative stress parameters in brain parts were also restored by LCZE.
Effect on amyloid-β
Formation of amyloid-β peptide is the most crucial step in AD. It is formed by proteolytic cleavage of a large transmembrane protein i.e. APP by the action of two proteases, β-secretase and γ-secretase. With time, stacks of amyloid peptide lead to neuronal loss and dementia by instigating a pathogenic gush [70]. Cinnamon has shown efficacious results against amyloid peptide formation.
Methanol and chloroform extracts of C. cassia exhibited marginal protection from beta amyloid insult in PC12 cells and primary neuronal cells. EC50 i.e. sample concentration that is required to achieve 50 % cell viability was found out to be 104 μg/ml in primary neurons and 110 μg/ml in PC12 cells for chloroform extract whereas it was found to be >150 μg/ml in both the cells for methanol extract [71].
Frydman-Marom et al. [72] evaluated aqueous cinnamon extract precipitation (CEP) in AD using both in vitro and in vivo models. The study exhibited following results: extract inhibited the formation of amyloid beta oligomer in a dose-dependent manner in in vitro study. It also eliminated the process of fibrillization of amyloid beta; two models were used for in vivo study i.e. AD fly model and aggressive AD mouse model.
In AD fly model, CEP (0.75 mg/ml) repaired the diminished longevity, one of the characteristic symptoms of the AD flies. It also ameliorated the locomotive defects of the AD flies. AD affected flies fed with CEP exhibited climbing capability nearly similar to the control flies. In aggressive AD mouse model, CEP (100 μg/ml) improved cognition in the animals.
Effect of aqueous extract of C. verum on amyloid formation was explored using hen egg-white lysozyme (HEWL) as model protein by Ramshini et al. [73]. Reduced pH and high temperature led to the conversion of HEWL to amyloid. Following results were exhibited by the extract: dose-dependent inhibition of HEWL aggregation by the extract (0.1-1 mg/ml); significantly prevented the cytotoxicity of lysozyme oligomers and improved cell viability at 1 mg/ml dose.
In another study, Ramshini and Ayoubi [74] have compared the inhibitory effect of C. zeylanicum and Camellia sinensis in the dose range of 0.1-1 mg/ml on HEWL fibrillization. Formation of fibrillar assemblies was dose-dependently inhibited by cinnamon but less efficiently than C. sinensis.
Effect on tau aggregation
Another hallmark of AD is accumulation of tau to form abnormal fibers i.e. paired helical filaments that form higher-order aggregates namely neurofibrillary tangles or neuropil threads in neurons and other brain cells [75]. Taupathies are also present in other brain disorders e.g. fronto-temporal dementia, Parkinsonism, pick’s disease, progressive supranuclear palsy [76].
Aqueous extract of C. zeylanicum was evaluated for tau aggregation and filament formation using in vitro tau aggregation assay. The extract at 0.22 mg/ml concentration inhibited the aggregation of tau along with formation of filaments. It also caused disassembly of pre formed tau filaments. Proanthocyanidin trimer (100 μΜ) from the extract also exhibited tau aggregation inhibition but efficacy was one-fourth that of the extract [77].
Polyamines also play a vital role in number of diseased conditions. Studies have linked tau pathology with arginase 1. Overexpression of this enzyme has reduced phospho-tau species and tangle pathology in transgenic mice. It also reduces capability of various kinases causing tau phosphorylation and eliminates hippocampal atrophy in tau transgenic mice. On the other hand, inhibition of the enzyme results in enhanced tau accumulation [78]. One study has reported inhibitory action of methanol extract of C. cassia bark on arginase enzyme using in vitro inhibition assay [79]. This study shows that this species may enhance tau pathology as it blocks the expression of arginase enzyme. Although the species has shown significant AChE inhibition potential along with protection against amyloid insult, but the possible involvement of this species in tau pathology has made it a doubtful candidate for management of Alzheimer. Further exploration of this species is required in order to identify the possible phytoconstituent responsible for arginase inhibition.
Effect on brain insulin resistance
Insulin resistance has been incriminated in the pathogenesis of AD and the term “type 3 diabetes” has been used to portray AD. Massive instabilities in brain insulin and insulin-like growth factor signalling mechanisms denote early and progressive anomalies and could be a reason for the molecular, biochemical, and histopathological lesions in AD. Brain diabetes fabricated experimentally by intracerebral administration of streptozotocin shares several features with AD, including cognitive damage and disruptions in acetylcholine homeostasis [80].
Effect of cinnamon on brain insulin signalling and Alzheimer associated changes was evaluated by feeding C. burmanni powder (20 g/kg) in the diet comprising of high proportions of fat and fructose, to male Wistar rats. The diet fed rats exhibited impaired cognition in Y-maze and increased anxiety in elevated plus maze. The diet had negative effects on mRNA coding for proteins associated with AD and memory loss thus causing increase in tau and APP. Cinnamon led to the reversal of all the negative effects of diet. It also led to increase in the coding of proteins involved in insulin signaling [81].
Role in PD
PD is the second most widespread neurodegenerative disorder. The characteristic hallmarks of PD are fibrillar aggregates known as Lewy bodies [9] and deterioration of dopaminergic cells inside the substantia nigra and the consequent dopamine reduction in the striatum [82]. Lewy bodies are said to be composed of α-synuclein as one of their major component [83]. Cinnamon has shown satisfactory results against PD as given below.
Effect on alpha synuclein
Alpha synuclein comprises considerable portion of total proteins in Lewy bodies. The fabrication of wild type α-synuclein in transgenic mice leads to motor deficits and neuronal inclusions indicative of PD [84].
CEP (0-0.05 mg/ml) inhibited the formation of soluble and insoluble aggregates of α-synuclein in vitro. Drosophila fly model expressing mutant A53T α-syn exhibited a common behavioural phenotype for the flies i.e. age-dependent defective locomotion while normal flies tend to climb up the side of the rearing tube. Flies expressing A53T α-syn remain at the bottom. CEP (0.75 mg/ml) exhibited a significant therapeutic effect on the behavioural indications of the flies and on α-syn aggregation in their brain [85].
Effect on DJ-1 and parkin
The DJ-1 is a gene contributing to familial PD [86]. Enhanced oxidation of DJ-1 has been observed in patients with sporadic PD [87]. It plays a defensive role against oxidative stress-induced cell death. Loss of function and diminished function of DJ-1 elicit the beginning of oxidative stress-related diseases, including PD. Activity of DJ-1 is controlled by oxidative status of cysteine 106 of DJ-1. Compounds that bind to C106 region of DJ-1 displayed neuroprotective activity in PD [88]. Parkin is an ubiquitin E3 ligase that regulates number of cellular procedures via monoubiquitination and polyubiquitination of proteins. Damage to E3 ligase activity of parkin is thought to have a pathogenic role in both inherited and sporadic PD. Mutations in the gene encoding for parkin causes autosomal recessive PD. It is the second most frequent cause of PD [89]. Cinnamon has exhibited protective role in PD by upregulating both DJ-1 and parkin. NaB, a metabolite of cinnamon, at concentration of 5 mM was proficient in increasing the level of DJ-1 in primary mouse and human astrocytes along with human neurons, thus emphasizing its neuroprotective effect [90].
In vitro and in vivo studies were done to evaluate the effect of NaB and C. verum powder on dopaminergic neurons by Khasnavis and Pahan [91]. Following results were obtained: sodium benzoate (1 mM) upregulates parkin and DJ-1 in in vitro study by suppression of IL-1β-induced expression of iNOS in astrocytes thus exhibiting protection of both; C. verum powder and sodium benzoate protect dopaminergic neurons in a mouse model of PD. MPTP (1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine) intoxication in mice led to increase in the level of iNOS and decreased level of parkin/DJ-1 in vivo in the nigra. Cinnamon powder (100 mg/kg) and sodium benzoate (50 mg/kg), administered orally via gavage, exhibited decrease in the expression of iNOS and upregulated parkin and DJ-1 in nigra. Functional impairment was also improved by cinnamon and sodium benzoate as evidenced from rotarod and open field experiments [91].
Effect on dopaminergic neurons
The preferential and progressive loss of dopaminecontaining neurons in the substantia nigra pars compacta and the consequent loss of their projecting fibres in the striatum are the major pathological changes in PD [92,93]. TCA, one of the main components of C. cassia was evaluated in 6-hydroxydopamine (6-OHDA)-induced dopaminergic degeneration in mice. TCA (30 mg/kg, ip) maintained the number of tyrosine hydroxylasepositive dopaminergic neurons, (which had undergone severe loss in 6-OHDA-treated mice) in the striatum and substatntia nigra regions of the 6-OHDA-treated mice [43].
Role in multiple sclerosis (MS)
MS is an immune-mediated demyelinating ailment of the human central nervous system. Permanent neurological disability in MS patients is caused by neurodegeneration [94]. Cinnamon has shown beneficial effect in the management of MS.
Effect on experimentally allergic encephalomyelitis (EAE)
It is the most frequently used investigational model for the human inflammatory demyelinating disease-MS. In EAE model there approximates the basic pathological aspects of MS i.e. inflammation, demyelination, axonal loss and gliosis, due to the collaboration between a range of immunopathological and neuropathological mechanisms [95].
Sodium benzoate ameliorated clinical symptoms and disease progression in acute and chronic phases of EAE, in both in vitro and in vivo studies as shown by Brahmachari and Pahan [96]. Following were the beneficial effects of NaB: NaB exhibited inhibition of encephalitogenicity of myelin basic protein-primed T cells in vitro at 1 mM; it also displayed reticence of both passively and actively induced EAE in mice at dose range of 2.5-10 mg/ml. It haltered the generation of encephalitogenic T cells in vivo in donor mice. NaB at 5 mg/ml dose also exhibited infiltration of mononuclear cells, inflammation, and demyelination in the CNS of passively induced EAE in mice.
C. verum powder was evaluated against EAE in mice by Mondal and Pahan [97]. Oral administration of the powder (50 and 100 mg/kg) led to the reticence of clinical indications of acute and chronic EAE in mice as given below: Cinnamon prevented severity of disease and adoptive transfer of EAE in transgenic mice and adoptive transfer mouse model, respectively; it also inhibited perivascular cuffing, maintained the reliability of blood-brain barrier and blood-spinal cord barrier, blocked inflammation, stabilized the expression of myelin genes, and impeded demyelination in the central nervous system of EAE mice; there was up regulation of T regulatory cells via reduction of NO production with cinnamon treatment.
Huntington disease
The disease is a NDD with autosomal-dominant inheritance. It is caused by a CAG trinucleotide repeat expansion positioned in the first exon of the Huntington disease gene [98]. Patassini et al. [99] have reported that defective urea regulation causes an increase in levels of urea in the brain of Huntington disease patients. It has been identified as a major metabolic defect in the disease. l-arginine (a semi essential amino acid in mammals) is metabolized by arginase enzymes to form ornithine and urea [100]. Thus it can be inferred that the entities inhibiting arginase enzymes can offer novel therapeutic targets for Huntington disease as they will hamper the metabolism of arginine. Cinnamon can be a beneficial agent in this context as discussed below.
Methanol extract of C. cassia bark was explored using in vitro arginase inhibition assay. N-hydroxy-Larginine (NOHA) was used as a positive control in the study. Extract was found to be more potent inhibitor than NOHA. IC50 value of former was found out to be 61.72±2.20 μg/ml whereas for latter it was 156.78± 7.76 μg/ml [79].
The frequency of occurrence of different NDD has increased over time. Changes in the lifestyle of people and increase in the stress-borne conditions have made the people more prone to such disorders. A well-established fact is that all such diseases are marked by multifactorial reactions that play a variety of roles in progression of these diseases and thus makes their management difficult. Although there are synthetic drugs available in the market that provide some relief from these disorders, full cure is yet to be achieved. Plants are promising agents with fewer side effects and having synergistic actions due to a number of phytoconstituents present in them, also different phytoconstituents may act on different targets in the neurodegenerative progression. Cinnamomum is a genus with wide utility in traditional and modern systems of medicine. Different plant parts namely stem bark, root bark and leaves are found to be effective in various pharmacological activities.
This review has given us a clear idea about the effectiveness of Cinnamomum species in the management of neurodegenerative diseases. Various species of the genus have multiple pharmacological activities relevant to neuroprotection as evident in in vitro and in vivo models. These species have been able to diminish selective pathological and histological hallmarks in different NDD e.g. amyloid and tau in AD, alpha synuclein in PD, neuroinflammation in MS. Various extracts, volatile oil and phytoconstituents isolated from Cinnamomum species have been able to inhibit the major neuropathological characters such as inhibition of oxidative stress, inhibiting the production of inflammatory mediators, ischemic injury inhibition, inhibition of different hallmarks of AD, PD and MS.
Although researchers have explored this genus to a large extent, we have not been able to implement this culinary plant in the treatment of any of the NDDs so far. There are many reasons responsible for this. Research should not be limited to extracts only but should be carried out with major constituents of different species of the genus. This will help to narrow down the zone of research to a particular class of compounds and hence will make possible further modifications, in those compounds, required for the activity. Emphasis should be given on mechanismbased in vitro bioassays combined with in vivo models which truly mimic the conditions for the disorder. The entities responsible for the formation of pathological hallmarks of a neurodegenerative disorder should be targeted so that their production can be restricted.
Cinnamon must be subjected to detailed experimental studies and clinical trials in order to develop a drug that can efficiently and safely cease the detrimental consequences of NDD on the human population.
Conflicts of interest
There are no conflicts of interest among the authors.
References
- Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 2010;14:457-87.
- Nieoullon A. Neurodegenerative diseases and neuroprotection: current views and prospects. J Appl Biomed 2011;9:173-83.
- Cacciatore I, Baldassarre L, Fornasari E, Mollica A, Pinnen F. Recent Advances in the Treatment of Neurodegenerative Diseases Based on GSH Delivery Systems. Oxid Med Cell Longev 2012;2012:1-12.
- Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol Rep 2015;67:195-203.
- Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci Biobehav Rev 2011;35:1397-409.
- Francis PT. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr 2005;10:6-9.
- Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes 2014;5:889-93.
- Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson’s disease. Trends Mol Med 2006;12:521-8.
- Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of a-synuclein aggregates. Neuropathology 2007;27:494-506.
- Dodson MW, Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Curr Opin Neurobiol 2007;17:331-7.
- Warby SC, Montpetit A, Hayden AR, Carroll JB, Butland SL, Visscher H,et al. CAG Expansion in the Huntington disease gene is associated with a specific and targetable predisposing haplogroup. Am J Hum Genet 2009;84:351-66.
- Craufurd D, Snowden J. Neuropsychological and neuropsychiatric aspects of Huntington’s disease. In: Bates GP, Harper PS, Jones L, editors. Huntington’s disease. UK: Oxford University Press; 2002. p. 63-94.
- Novak MJ, Tabrizi SJ. Huntington’s disease. BMJ 2010;341:34-40.
- Shook SJ, Pioro EP. Racing against the clock: recognizing, differentiating, diagnosing, and referring the amyotrophic lateral sclerosis patient. Ann Neurol 2009;65:10-6.
- Neary D, Snowden J, Mann D. Frontotemopral dementia. Lancet Neurol 2005;4:771-80.
- Snowden JS, Neary D, Mann DMA. Frontotemporal dementia. Br J Psychiatry 2002;180:140-3.
- Lleo A, Greenberg SM, Growdon JH. Current Pharmacotherapy for Alzheimer’s disease. Annu Rev Med 2006;57:513-33.
- Chen JJ, Swope DM. Pharmacotherapy for Parkinson’s Disease. Pharmacotherapy 2007;27:161S-73S.
- Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur J Neurosci 2000;12:1882-90.
- Lebeau A, Esclaire F, Rostene W, Pelaprat D. Baicalein protects cortical neurons from β-amyloid (25-35) induced toxicity. Neuroreport 2001;12:2199-202.
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001;21:8370-7.
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res 2004;75:742-50.
- Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J Biol Chem 2004;279:26846-57.
- Joo SS, Lee DI. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: enhancement of type a macrophage scavenger receptor expression. Arch Pharm Res 2005;28:1164-9.
- Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer's amyloid β peptide after oral administration of ginsenosides. FASEB J 2006;20:1269-71.
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur J Neurol 2006;13:981-5.
- Aggarwal BB, Harikumar KB, Dey S. Prevention and Treatment of neurodegeneration by spice-derived phytoconstituents. In: Packer L, Sies H, Eggersdorfer M, Cadenas E, editors. Micronutrients and Brain Health. Florida: CRC Press; 2004. p. 281-308.
- Srinivasan K. Traditional Indian Functional Foods. In: Shi J, Ho C, Shahidi F, editors. Functional foods of the East. Florida: CRC Press; 2010. p. 51-84.
- Ho SC, Chang KS, Chang PW. Inhibition of neuroinflammation by cinnamon and its main components. Food Chem 2013;138:2275-82.
- Jain S, Sangma T, Shukla SK, Mediratta PK. Effect of Cinnamomum zeylanicum extract on scopolamine-induced cognitive impairment and oxidative stress in rats. Nutr Neurosci 2015;18:210-6.
- Barceloux DG. Cinnamon (Cinnamomum Species). Dis Mon 2009;55:327-35.
- Ranasinghe P, Jayawardana R, Galappaththy P, Constantine GR, de Vas Gunawardana N, Katulanda P. Efficacy and safety of 'true' cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and meta-analysis. Diabet Med 2012;29:1480-92.
- Thankamani C, Sivaraman K, Kandiannan K, Peter K. Agronomy of tree spices (clove, nutmeg, cinnamon and allspice)-A review. JOSAC 1994;3:105-23.
- Rao PV, Gan SH. Cinnamon: A Multifaceted Medicinal Plant. Evid Based Complement Alternat Med 2014;2014:1-12.
- Wong YC, Ahmad-Mudzaqqirand MY, Wan-Nurdiyana WA. Extraction of Essential Oil from Cinnamon (Cinnamomum zeylanicum). Orient J Chem 2014;30:37-47.
- Hamidpour R, Hamidpour M, Hamidpour S, Shahlari M. Cinnamon from the selection of traditional applications to its novel effects on the inhibition of angiogenesis in cancer cells and prevention of Alzheimer's disease, and a series of functions such as antioxidant, anticholesterol, antidiabetes, antibacterial, antifungal, nematicidal, acaracidal, and repellent activities. J Tradit Complement Med S2015;16:66-70.
- Senanayake UM, Wijesekera ROB. Chemistry of Cinnamon and Cassia. In: Ravindran P, Nirmal Babu K, Shylaja M. Cinnamon and Cassia-The Genus Cinnamomum. Boca Raton, USA: CRC Press; 2004. p. 80.
- Gruenwald J, Freder J, Armbruester N. Cinnamon and Health. Crit Rev Food Sci Nutr 2010;50:822-34.
- Prasad KN, Yang B, Dong X, Jiang G, Zhang H, Xie H, et al. Flavonoid contents and antioxidant activities from Cinnamomum species. IFSET 2009;10:627-32.
- Nabavi SF, Di Lorenzo A, Izadi M, Sobarzo-Sanchez E, Daglia M, Nabavi SM. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015;7:7729-48.
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140:918-34.
- Brahmachari S, Jana A, Pahan K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Reduces Microglial and Astroglial Inflammatory Responses. J Immunol 2009;183:5917-27.
- Hwang H, Jeon H, Ock J, Hong SH, Han YM, Kwon BM, et al. Hydroxycinnamaldehyde targets low-density lipoprotein receptor-related protein-1 to inhibit lipopolysaccharide-induced microglial activation. J Neuroimmunol 2011;230:52-64.
- Pyo JH, Jeong YK, Yeo S, Lee JH, Jeong MY, Kim SH, et al. Neuroprotective Effect of trans-Cinnamaldehyde on the 6-Hydroxydopamine-Induced Dopaminergic Injury. Biol Pharm Bull 2013;36:1928-35.
- Anderson JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med 2004;5:S18-S25.
- Hsu FL, Li WH, Yu CW, Hseih YC, Yang YF, Liu JT, et al. In Vivo Antioxidant Activities of Essential Oils and Their Constituents from Leaves of the Taiwanese Cinnamomum osmophloeum. J Agric Food Chem 2012;60:3092-7.
- Kumar S, Vasudeva N, Sharma S. GC-MS analysis and screening of antidiabetic, antioxidant and hypolipidemic potential of Cinnamomum tamala oil in streptozotocin induced diabetes mellitus in rats. Cardiovasc Diabetol 2012;11:1-11.
- Pandey AK, Mishra AK, Mishra A. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell Mol Biol 2012;58:142-7.
- Yang CH, Li RX, Chuang LY. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules 2012;17:7294-304.
- Abdelwahab SI, Mariod AA, Taha MME, Zaman FQ, Abdelmageed AHA, Khamis S, et al. Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm. (Lauraceae). Arab J Chem 2017;10:131-5.
- Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia 1988;26:685-700.
- Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis 2001;6:69-81.
- Lee EJ, Chen HY, Lee MY, Chen TY, Hsu YS, Hu YL, et al. Cinnamophilin reduces oxidative damage and protects against transient focal cerebral ischemia in mice. Free Radic Biol Med 2005;39:495-510.
- Lee EJ, Chen HY, Hung YC, Chen TY, Lee MY, Yu SC, et al. Therapeutic window for cinnamophilin following oxygen–glucose deprivation and transient focal cerebral ischemia. Exp Neurol 2009;217:74-83.
- Panickar KS, Polansky MM, Anderson RA. Cinnamon polyphenols attenuate cell swelling and mitochondrial dysfunction following oxygen-glucose deprivation in glial cells. Exp Neurol 2009;216:420-7.
- Panickar KS, Polansky MM, Graves DJ, Urban JF Jr, Anderson RA. A procyanidin type A trimer from cinnamon extract attenuates glial cell swelling and the reduction in glutamate uptake follwing ischemia-like injury in vitro. Neuroscience 2012;202:87-98.
- Li TJ, Qiu Y, Mao JQ, Yang PY, Rui YC, Chen WS. Protective Effects of Guizhi-Fuling-Capsules on Rat Brain Ischemia/Reperfusion Injury. J Pharmacol Sci 2007;105:34-40.
- Jung HW, Mahesh R, Bae SH, Kim YH, Kang JS, Park YK. The antioxidant effects of Joongpoongtang 05 on brain injury after transient focalcerebral ischemia in rats. J Nat Med 2011;65:322-9.
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron 1991;7:695-702.
- Arenas E, Persson H. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature 1994;367:368-71.
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci 1995;15:7929-39.
- Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: Therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol 2013;8:739-55.
- Lista S, Dubois B, Hampel H. Paths to Alzheimer’s disease prevention: from modifiable risk factors to biomarker enrichment strategies. J Nutr Health Aging 2015;19:154-63.
- Francis P, Palmer A, Snape M, Wilcock G. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 1999;66:137-47.
- Boga M, Hacibekiroglu I, Kolak U. Antioxidant and anticholinesterase activities of eleven edible plants. Pharm Biol 2011;49:290-5.
- Kumar S, Brijeshlata, Dixit S. Screening of traditional Indian spices for inhibitory activity of acetylcholinesterase and butyrylcholinesterase enzymes. Int J Pharm Biol Sci 2012;3:59-65.
- Dalai MK, Bhadra S, Chaudhary SK, Chanda J, Bandyopadhyay A, Mukherjee P. Anticholinesterase activity of Cinnamomum zeylanicum L. leaf extract. TANG (Humanitas Medicine, HTM) 2014;4:21-6.
- Dalai MK, Bhadra S, Chaudhary SK, Mukherjee P. Anti-cholinesterase potential of Cinnamomum tamala (Buch.-Ham.) T. Nees & Eberm. Leaves. Indian J Tradit Know 2014;13:691-7.
- Malik J, Munjal K, Deshmukh R. Attenuating effect of standardized lyophilized Cinnamomum zeylanicum bark extract against streptozotocin-induced experimental dementia of Alzheimer’s type. J Basic Clin Physiol Pharmacol 2014;26:275-85.
- Hardy J, Selkoe DJ. The Amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to Therapeutics. Science 2002;297:353-6.
- Kim DSHL, Kim JY, Han YS. Alzheimer’s Disease Drug Discovery from Herbs: Neuroprotectivity from -Amyloid (1-42) Insult. J Altern Complement Med 2007;13:333-40.
- Frydman-Marom A, Levin A, Farfara D, Benromano T, Scherzer-Attali R, Peled S, et al. Orally Administrated Cinnamon Extract Reduces β-Amyloid Oligomerization and Corrects Cognitive Impairment in Alzheimer's disease Animal Models. PLoS One 2011;6:1-11.
- Ramshini H, Ebrahim-Habibi A, Aryanejad S, Rad A. Effect of Cinnamomum verum Extract on the Amyloid Formation of Hen Egg-white Lysozyme and Study of its Possible Role in Alzheimer’s Disease. Basic Clin Neurosci 2015;6:29-37.
- Ramshini H, Ayoubi F. Inhibitory Effect of Cinnamomum zeylanicum and Camellia sinensis Extracts on the Hen Egg-White Lysozyme Fibrillation. J Kerman Univ Med Sci 2014;21:290-310.
- Bulic B, Pickhardt M, Schmidt B, Mandelkow EM, Waldmann H, Mandelkow E. Development of Tau Aggregation Inhibitors for Alzheimer’s Disease. Angew Chem Int Ed Engl 2009;48:1740-52.
- Tolnay M, Probost A. The Neuropathological Spectrum of Neurodegenerative Tauopathies. IUBMB Life 2003;55:299-305.
- Peterson DW, George RC, Scaramozzino F, LaPointe NE, Anderson RA, Graves DJ, et al. Cinnamon Extract Inhibits Tau Aggregation Associated with Alzheimer’s disease in vitro. J Alzheimers Dis 2009;17:585-97.
- Hunt JB, Nash KR, Placides D, Moran P, Selenica MB, Abuqalbeen F, et al. Sustained arginase 1 expression modulates pathological Tau deposits in a mouse model of Tauopathy. J Neurosci 2015;35:14842-60.
- Goswami SK, Inamdar MN, Jamwal R, Dethe S. Effect of Cinnamomum cassia methanol extract and Sildenafil on arginase and sexual function of young male Wistar rats. J Sex Med 2014;11:1475-83.
- de la Monte SM, Wands JR. Alzheimer’s disease Is Type 3 Diabetes-Evidence Reviewed. J Diabetes Sci Technol 2008;6:1101-13.
- Anderson RA, Qin B, Canini F, Poulet L, Roussel AM. Cinnamon Counteracts the Negative Effects of a High Fat/High Fructose Diet on Behaviour, Brain Insulin Signalling and Alzheimer-Associated Changes. PLoS One 2013;8(12):e83243.
- Bergman H, Deuschl G. Pathophysiology of Parkinson’s disease: from Clinical Neurology to Basic Neuroscience and Back. Mov Disord 2002;17:S28-S40.
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha synuclein in Lewy bodies. Nature 1997;388:839-40.
- Breydo L, Wu JW, Uversky VN. α-Synuclein misfolding and Parkinson's disease. Biochim Biophys Acta 2012;1822:261-85.
- Shaltiel-Karyo R, Davidi D, Frenkel-Pinter M, Ovadia M, Segal D, Gazit E. Differential inhibition of α-synuclein oligomeric and fibrillar assembly in Parkinson's disease model by cinnamon extract. Biochim Biophys Acta 2012;1820:1628-35.
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 2003;299:256-9.
- Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem 2006;281:10816-24.
- Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, Iguchi-Ariga SMM. Neuroprotective Function of DJ-1 in Parkinson’s disease. Oxid Med Cell Longev 2013;2013:1-8.
- Dawson TM, Dawson VL. The Role of Parkin in Familial and Sporadic Parkinson’s disease. Mov Disord 2010;25:S32-S39.
- Khasnavis S, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J Neuroimmune Pharmacol 2012;7:424-35.
- Khasnavis S, Pahan K. Cinnamon Treatment Upregulates Neuroprotective Proteins Parkin and DJ-1 and Protects Dopaminergic Neurons in a Mouse Model of Parkinson’s disease. J Neuroimmune Pharmacol 2014;9:569-81.
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol 1987;45:19-34.
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci 1999;22:123-44.
- Trapp BD, Nave KA. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu Rev Neurosci 2008;31:247-69.
- Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 2011;164:1079-106.
- Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol 2007;179:275-83.
- Mondal S, Pahan K. Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T Cells: implications for multiple sclerosis therapy. PLoS One 2015;10:1-26.
- Moncke-Buchner E, Reich S, Mucke M, Reuter M, Messer W, Wanker EE, et al. Counting CAG repeats in Huntington’s disease gene by restriction endonuclease EcoP15I cleavage. Nucleic Acids Res 2002;30:1-7.
- Patassini S, Begley P, Reid SJ, Xu J, Church SJ, Curtis M, et al. Identification of elevated urea as a severe, ubiquitous metabolic defect in the brain of patients with Huntington's disease. Biochem Biophys Res Commun 2015;468:161-6.
- Morris SM Jr. Enzymes of arginine metabolism. J Nutr 2004;134:2743S-7S.