- Corresponding Author:
- D. A. Shah
Anand Pharmacy College, Opp. Town Hall, Anand - 388 001, India, 1A. R. College of Pharmacy, P. Box No. 19, Vallabh Vidyanagar - 388 120, India
E-mail: dimalgroup@yahoo.com
| Date of Submission | 17 October 2007 |
| Date of Revision | 26 April 2007 |
| Date of Acceptance | 29 December 2012 |
| Indian J Pharm Sci, 2007, 69 (5): 700-703 |
Abstract
A simple, specific and accurate reverse phase liquid chromatographic method was developed for the simultaneous determination of atorvastatin calcium and nicotinic acid in tablet dosage forms. A phenomenex Luna C-18, 5 mm column having 250 × 4.6 mm i.d. in isocratic mode, with mobile phase containing 0.02 M potassium dihydrogen phosphate: methanol: acetonitrile (20:40:40, pH 4) was used. The flow rate was 1.0 ml/ min and effluents were monitored at 240 nm. The retention times of atorvastatin calcium and nicotinic acid were 3.6 min and 2.4 min, respectively. The linearity for atorvastatin calcium and nicotinic acid were in the range of 2-24 μg/ ml and 60-250 μg/ ml, respectively. The recoveries of atorvastatin calcium and nicotinic acid were found to be in the range of 97.93-101.16% and 98.82-101.30%, respectively. The proposed method was validated and successfully applied to the estimation of atorvastatin calcium and nicotinic acid in combined tablet formulations.
Atorvastatin calcium (ATV) is chemically [R– (R*,R*)]–2-(4-flurophenyl)-β,δ-dihydroxy-5-(1- methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]–1Hpyrrole- 1-heptanoic acid calcium salt trihydrate. Atorvastatin calcium is an inhibitor of 3-hydroxy-3- methylglutaryl coenzyme A (HMG-Co A) reductase. This enzyme catalyses the conversion of HMGCo A to mevalonate, an early and rate limiting step in cholesterol biosynthesis [1-2]. Nicotinic acid (NIC) is chemically pyridine-3-caroboxylic acid. Nicotinic acid lowers plasma triglyceride and cholesterol concentration. It acts as an antilipolytic drug in adipose tissue, increases the activity of lipoprotein lipase and decreases esterification of triglycerides in liver. It is used for Pellagra and in hyperlipoproteinaemia.
A literature survey regarding quantitative analysis of these drugs revealed that attempts were made to develop analytical methods for ATV using extractive spectrophotometry [3], HPLC [4-8], GC-MS [9], LC-MS [10], LC- electrospray tandem mass spectrometry [11-13] and HPTLC [14] methods, while for estimation of ATV and aspirin combination HPLC [15] method had been reported. Estimation of NIC had been reported by methods like HPLC [16-19], capillary electrophoresis [20] and light-induced photo transformation [21] of nicotinic acid with laser and UV.
ATV and NIC combination is available in the market in the form of a tablet. This paper describes reverse phase high performance liquid chromatographic method for the estimation of ATV and NIC combination in mixture in tablet dosage form.
A High performance liquid chromatographic instrument (Shimadzu HPLC class VP series) with LC-10AT VP pump, variable wavelength programmable UV/Vis detector SPD-10AVP and Rheodyne injector (7725i) with 20 µl fixed loop was used. Chromatographic analysis was performed using Spinchrom Software on a Phenomenex Luna C-18 column with 250×4.6 mm i.d. and 5 µm particle size. Chromatographic estimation was performed on an equilibrated C18 column using mobile phase 0.02 M potassiumdihydrogen phosphate:methanol:acetonitrile (20:40:40, pH 4) and detection was performed at 240 nm. The sample was injected using a 20 µL fixed loop, and the total run time was 10 min. Analytically pure ATV and NIC were obtained as gift samples from M/s Blue Cross Labs. Ltd., (Mumbai, India) and M/s Torrent Pharmaceutical Ltd., (Ahmedabad, India). Acetonitrile, methanol, water (E. Merck, Mumbai, India) were of HPLC grade, while ortho-phosphoric acid and potassium dihydrogen phosphate (S. D. Fine Chemicals, Mumbai, India) were of analytical grade. Tablet formulation A (Avas Plus, Micro Labs. Ltd., India) and B (Tonact Plus, Lupin Lab., India) containing labeled amount of 10.34 mg ATV and 375 mg of NIC were procured from a local pharmacy store.
A 1000 µg/ml stock solution of ATV and NIC in methanol was prepared separately. ATV solution was further diluted with methanol to obtain final concentration of 100 µg/ml. Appropriate aliquots of ATV and NIC stock solutions were diluted in mobile phase to obtain final concentrations of 2, 4, 8, 12, 20, 24 µg/ ml of ATV and 60, 80, 100, 150, 200, 250 µg/ ml of NIC. The solutions were injected using a 20 µL fixed loop system and chromatograms were recorded. Calibration curves were constructed by plotting average peak area versus concentrations and regression equations were computed for ATV and NIC.
Twenty tablets were weighed and finely powdered. Powder equivalent to ATV 10.34 mg and 375 mg NIC was accurately weighed and transferred to a 50 ml volumetric ß ask and 20 ml of mobile phase was added to the same. The ß ask was shaken, and the volume was diluted to the mark with the same mixture. The above solution was filtered and appropriate volume of the aliquot was transferred to a 50 ml volumetric flask. The volume was made up to the mark with mobile phase to obtain 4.13 µg/ml of ATV and 150 µg/ml of NIC. The solution was injected at above chromatographic conditions and peak areas were measured. The quantification was carried out by keeping these values to the straight line equation of calibration curve.
The method was validated for accuracy, precision, specificity, detection limit, quantification limit and robustness. The accuracy of the method was determined by calculating recoveries of ATV and NIC by method of standard additions. The intra day and inter day precision study of ATV and NIC was carried out by estimating the corresponding responses 3 times on the same day and on 3 different days for 3 different concentrations of ATV (4, 8, 12 µg/ml) and NIC (100, 150, 200 µg/ml). The repeatability studies were carried out by estimating response of three different concentrations of ATV (2, 4, 8 µg/ml) and NIC (80, 100, 200 µg/ml) for triplicate and results are reported in terms of relative standard deviation (RSD). For specificity study commonly used excipients (starch, microcrystalline cellulose and magnesium stearate) present in selected tablet formulation were spiked into a pre weighed quantity of drugs. The chromatogram was taken by appropriate dilutions and the quantities of drugs were determined. Limit of detection (LOD) was the concentration that yielded signal to noise ratio (S/N) 3:1 and limit of quantification (LOQ) was the concentration that yielded signal to noise ratio (S/N) 10:1. Robustness of the method was studied by changing the composition of organic phase by ±5% and the pH by ±0.2, and also by observing the stability of the drugs for 24 h at 35±2o temperature in the mobile phase.
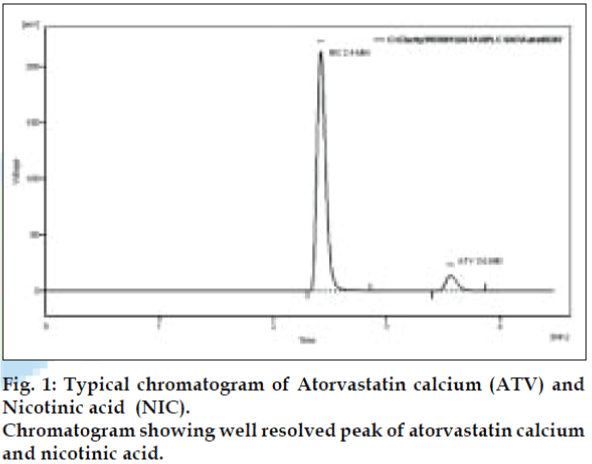
Selection of mobile phase was performed based on resolution, asymmetric factors and theoretical plates obtained for both ATV and NIC. The mobile phase 0.02 M potassium dihydrogen phosphate: methanol:acetonitrile (20:40:40, pH 4) was found to be satisfactory and gave two well-resolved peaks for ATV and NIC. The retention time for ATV and NIC were 3.6 min and 2.4 min, respectively (fig. 1). The resolution was found to be 6.9, which indicates good separation of peaks for both the compounds. The asymmetric factors for ATV and NIC were 1.4 and 1.57, respectively. UV overlain spectra of both the ATV and NIC showed that both the drugs absorbs appreciably at 240 nm so, 240 nm was selected as the detection wavelength in liquid chromatography.
The calibration curve for ATV was obtained by plotting the peak area of ATV versus concentration of ATV over the range of 2-24 µg/ml, and it was found to be linear with r = 0.9998. Similarly, the calibration curve for NIC was obtained over the range of 60-250 µg/ml and was found to be linear with r= 0.9993. The data of regression analysis of the calibration curves are shown in Table 1. The recoveries of ATV and NIC were found to be in the range of 97.93 - 101.16% and 98.82- 101.30%, respectively. The validation parameters are summarized in Table 2. The proposed liquid chromatographic method was applied to the determination of ATV and NIC in their combined dosage forms (Tablet A and B). The results for ATV and NIC were comparable with the corresponding labeled amounts (Table 3).
| Parameters | ATV | NIC |
|---|---|---|
| Linearity range (µg/ml) | 2-24 | 60-250 |
| Slope | 27.737 | 8.574 |
| Standard deviation of slope | 0.0804 | 0.0086 |
| Intercept | -0.803 | -7.99 |
| Standard deviation of intercept | 0.618 | 0.983 |
| Correlation coeffcient (r) | 0.9998 | 0.9993 |
ATV is atorvastatin calcium; NIC is nicotinic acid
Table 1: Regression Analysis of the Calibration Curves for Atv and Nic
| Parameters | ATV | NIC |
|---|---|---|
| Detection limit (µg/ml) | 0.071 | 0.11 |
| Quantitation limit (µg/ml) | 0.215 | 0.332 |
| Accuracy (%) | 97.93-101.16 | 98.82-101.3 |
| Precision (RSDa, %) | ||
| Intraday (n=3) | 0.22-0.60 | 0.03-0.10 |
| Interday (n=3) | 0.39-1.18 | 0.10-0.18 |
| Repeatability (RSDa , n=3) | 0.14-0.78 | 0.02-0.11 |
aRSD indicates relative standard deviation; ATV is atorvastatin calcium; NIC is nicotinic acid
Table 2: Summary of Validation Parameters for the Proposed Method
| Formulations | Labeled amount (mg) | Amount obtained (mg)b | % Recoveryb | |||
|---|---|---|---|---|---|---|
| ATV | NIC | ATV | NIC | ATV | NIC | |
| A | 10.34 | 375 | 10.17±0.110 | 367.91±4.85 | 98.41±1.07 | 98.10±1.29 |
| B | 10.34 | 375 | 10.26±0.095 | 368.52±3.64 | 99.31±0.78 | 98.79±0.57 |
bMean value±standard deviation of three determinations; Tablet A is AVAS PLUS, Micro Labs. Ltd., India and Tablet B is TONACT PLUS, Lupin Lab., India containing labeled amount of 10.34 mg ATV and 375 mg of NIC; ATV is atorvastatin calcium; NIC is nicotinic acid
Table 3: Assay Results of Combined Dosage Using Proposed Method
Thus in proposed study, RP-HPLC method has been developed for determination of ATV and NIC in combined dosage form. The method was validated and found to be simple, sensitive, accurate and precise. The method was successfully applied for determination of drugs in their pharmaceutical formulations hence method can be used for routine analysis of ATV and NIC in combined dosage form.
Acknowledgements
The authors are grateful to M/s Blue Cross Labs. Ltd., Mumbai for providing gift sample of atorvastatin calcium and M/s Torrent Pharmaceuticals Ltd., Ahmedabad for providing gift sample of nicotinic acid.
References
- Budavari S. editor. The Merck Index, 12 th Ed. Whitehouse station, NJ: Merck & Co. Inc.; 1996 p.897.
- Gennaro AE. editor. Remington's The Science and Practice of Pharmacy, 20 th Ed., Vol. II, Easton, PA: Mack Publishing Co.; 2000, p. 1294.
- Erk N. Extractive spectrophotometric determination of atorvastatin in bulk and pharmaceutical formulations. Anal Lett 2003; 36(12): 2699-711.
- Shen HR, Liz D, Zhong MK. HPLC assay and pharmaco-kinetic study of atorvastatin in beagle dog after oral administration of atorvastatin self-micro emulsifying drug delivery system. Pharmazie 2006; 61(1): 18-20.
- Verd JC, Peris C, Alergret M, Diaz C, Hernandez ZG, Sanchez RM. Different effect of simvastatin and atorvastatin on key enzyme involved in VLDL synthesis and catabolism on high fat / cholesterol rabit. Brit J Pharmacol 1999; 127: 1479-85.
- BleskeBE, Willis RA, Anthony M, Casselberry N, Datwani M, Uhley VE. et al . The effect of pravastatin and atorvastatin on coenzyme Q10. Amer Heart J 2001; 142(2): 262.
- Altuntas TG, Erk N. Liquid chromatographic determiation of atrovastatin in bulk drug, tablets and human plasma. J LiqChromatogrRelatTechnol 2004; 27(1): 83-93.
- Erturk S, Aktas ES, Ersoy L, Ficicioglu S. An HPLC method for the determination of atorvastatin and its impurities in bulk drugs and tablets. J Pharm Biomed Anal 2003; 33(5): 1017-23.
- McKenney JM, McCormick LS, Weiss S, Koren M, Kafonek S, Blanck DM. A randomized trial of the effects of atorvastatin and niacin in patients with combined hyperlipidaemic or isolated hypertriglyceridemia, collaborative atorvastatin study group. Amer J Med 1998; 104(2): 137-43.
- Nirogi RV, Kandikere VN, Shukla M, Mudigonda M, Maurya S, Boosi R. et al . Simultaneous quantification of atorvastatin and active metabolites in human plasma by LC-MS using rosuvastatin as internal standard. Biomed Chromatogr 2006; Feb 7.
- Miao XS, Metcalfe CD. Determination of cholesterol lowering stain dugs in aqueous samples using liquid chromatography- electrospray ionization tandem mass spectrometry, J Chromatogr A 2003; 998(1-2): 133-41.
- Jemal M, Ouyang Z, Chen BC, Teitz D. Quantitation of the acid and lactone forms of atorvastatin and its biotransformation products in human serum by high performance liquid chromatography with electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 1999; 13(11):1003-15.
- Bullen WW, Miller RA, Hayes RN. Development and validation of a high performance liquid chromatography- tandem mass spectrometry assay for atorvastatin, ortho-hydroxy atorvastatin and para-hydroxy atorvastatin in human, dog and rat plasma. J AmerSoc Mass Spectrom 1999; 10(1): 55-66.
- Yadav SS. Mhaske DV, Kakad AB, Patil BD, Kadam SS, Dhaneshwar SR. Simple and sensitive HPTLC method for determination of content uniformity of atorvastatin calcium tablet. Indian J Pharm Sci 2005; 67(2): 182-88.
- Manoj K, Shanmugapandiyan P, Anbazhagan S. RP-HPLC method for simultaneous estimation of atorvastatin and aspirin from capsule formulation. Indian Drugs, 2004; 41(5): 284-89.
- Shibata K, Fukuwatari T, Sugimoto E. Reverse phase liquid chromatography of nicotinic acid mononucleotide for measurement of quinolinatephosphoribosyltransferase. J Chromatogr B Biomed sciAppl 2000; 749(2): 281-85.
- Shinde VM, Ramesh R. Simultaneous determination of nicotinic acid and meclozinehydrochorlide in tablet by RP-HPLC. Indian drugs 1998; 35(12): 748-53.
- Casal S, Olieiro MB, Ferreira MA. Development of and HPLC/diode array detector method for simultaneous determination of trigonelline, nicotinic acid and caffeine in coffee. J Liquid ChromatogrRelTechnol 1998; 21(20): 3187-95.
- Gandhimathi M, Ravi TK, Varghese A, Ninan A. RP-HPLC determination of simavastatin and nicotinic acid in tablets. Indian drugs 2003; 40(12): 707-11.
- Iwaki M, Murakami E, Kakehi K. Chromatographic and capillary electrophoretic method for analysis of nicotinic acid and its metabolites. J Chromatogr B Biomed sciAppl 2000; 745(1): 149-57.
- Jain S, Bokadia MM. Light induced phototransformation of nicotinic acid with laser and UV light- A comparative study. Indian J Chem 1999; 38(B2): 232-3.