- *Corresponding Author:
- R. D. Jangle
Department of Chemical Engineering, Advanced Drying Laboratory, Institute of Chemical Technology (Formerly UDCT), N. P. Road, Matunga (E), Mumbai-400 019, India
E-mail: rdjangle@gmail.com
| Date of Submission | 30 October 2012 |
| Date of Revision | 11 March 2013 |
| Date of Acceptance | 05 April 2013 |
| Indian J Pharm Sci,2013;75(3):339-345 | 12-Mar-2013 |
Abstract
A novel, efficient and simple approach for soy phosphatidylcholine analysis according to its fatty acid composition was studied with reverse-phase high-performance liquid chromatography. The reverse-phase high-performance liquid chromatography analysis was performed isocratically using UV detector and simple mobile phase solvents consisting of isopropyl alcohol, methanol, and deionized water in the proportion of 70:8:22 v/v. The uniqueness of the proposed method was the separation of individual fatty acids of soy phosphatidylcholine. The high-performance liquid chromatography method for soy phosphatidylcholine was validated for linearity with correlation coefficient of above 0.99 for all the peaks separated according to their fatty acid composition. The intra-day and the inter-day precision studies provided the relative standard deviation of less than 2%. The limit of detection and limit of quantitation values were also calculated for all the resolved peaks of soy phosphatidylcholine. Also system performance parameters such as number of theoretical plates, capacity factor, tailing factor, separation factor, and peak resolution were studied systematically and found well within the acceptable range. The proposed high-performance liquid chromatography method was successfully applied to soy phosphatidylcholine extracted and purified from deoiled soy lecithin without any interference of impurities or solvent peaks. Individually, the collected peaks of sample soy phosphatidylcholine were subjected for mass spectroscopy. The mass spectra showed all the peaks having different saturated or unsaturated fatty acid chains attached to glyerophosphocholine moiety of soy phosphatidylcholine. The method developed is economic and well suited for estimation of soy phosphatidylcholine with its fatty acid composition.
Keywords
Phosphatidylcholine, fatty acid, mass spectra, phospholipid, resolution
Introduction
Phospholipids are major constituents of cell membrane and are found in all tissues and subcellular compartments as mixtures of various molecular species such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), sphingomyelin (SM), and lysophosphatidylcholine (LPC) depending on the type of polar head groups and the degree of unsaturation of the acyl chains[1-4]. Among these phospholipids, PC represents a major constituent of cell membranes. The demand for lecithin with high PC content from vegetable source is increasing these days, particularly in pharmaceutical, cosmetic, food and other applications due to their emulsifying properties and nonantigenic nature. The application of vegetable lecithins in pharmaceutical and cosmetics domain depends mainly on the PC with its saturated or unsaturated fatty acid content and in many cases; lecithin with more than 50% PC content is used. On the other hand, in some pharmaceutical formulations, especially those used for neurological disorders, liver dysfunction and in the preparation of liposome preparations, lecithin containing 80-90% PC is desired[5].
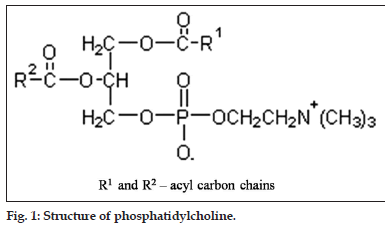
1,2-diacyl-sn-glycero-3-phosphocholine (PC) (fig. 1) consists of polar head group phosphorylcholine attached to the sn-3 position of glycerol and differing saturated and unsaturated fatty acids esterified to the sn-1 and sn-2 position, whereby, the fatty acid in position sn-1 are preferentially saturated as a rule[6]. Different fatty acids attached to glycerol moiety makes PC a very complex molecule and consequently pose difficulty in analyzing PC with its exact fatty acid composition.
Various analytical techniques are reported such as gas chromatography (GC)[7,8], thin layer chromatography (TLC)[9,10], high-performance thin layer chromatography (HPTLC)[11], and highperformance liquid chromatography (HPLC)[12-16] for lipid analysis. Most of these techniques suffer from various drawbacks as being time-consuming, insensitive, destructive, or not related to individual substructures. Now-a-days, techniques such as mass spectrometry (MS) coupled with GC or HPLC are used extensively for better resolution[17-21]. The use of highly sophisticated NMR technique is well suited to quantify phospholipid analogs and needs less sample preparation but still need to be coupled with MS for better molecular analysis[6,22-25]. HPLC offers the separation of lipid classes using the normal phase mode and additionally the separation according to the different fatty acid residues of an individual lipid in the reversed phase mode. There are no reports in literature, which showed separation of PtdCho with its varying fatty acid composition using isocratic reverse-phase HPLC method with UV detector.
This paper presents an efficient, economic RP-HPLC setup to separate PC by their fatty acid composition. In this investigation attempts were made to find out various fatty acid homologs present in phosphatidylcholine. In this respect, the individual peaks were analyzed using mass spectroscopy in the positive and negative ion mode. From several experiments and attempts, the possible fatty acid present in PC can be predicted, however, the position of a particular acid group and that of a double bond in PC is very difficult to predict by this method. Not withstanding these drawbacks, however, the developed method is still a useful technique for discerning various homologues of PC.
Materials and Methods
Phosphatidylcholine (≥99% pure) was procured from Sigma Aldrich, St. Louis, USA. Deoiled soybean lecithin was procured from Sonic Biochem Extraction Ltd., Indore, India. The thin layer chromatographic (TLC) plates and silica gel (100 to 200 mesh size) were obtained from S. D. Fine-Chem Ltd., Mumbai, India. HPLC grade methanol and isopropyl alcohol (IPA) were purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Deionized (DI) water was prepared by Milli-Q system (Millipore, Billerica, MA, USA). All solvents were filtrated through 0.2 µm Pall Ultipor N66 membrane before use. All other reagents used were of analytical grade and procured from S. D. Fine-Chem Ltd., Mumbai, India.
Selection and preparation of mobile phase
Pure PC was dissolved in methanol and injected into the HPLC system and run in different mobile phase systems. Various proportions of mobile phase were tried using isopropyl alcohol, methanol, and water. It was found that isopropyl alcohol, DI water and methanol in the proportion of 70:22:8 v/v has given satisfactory results as compared to the other mobile phases. The isocratic elution was carried out with the flow rate of 0.5 ml/min at 25° and a wavelength of 205 nm was used for detection.
Instrument details and optimization
HPLC system used was (Agilent Technologies, India 1200 Series) equipped with ChemStation software, a model G1322A degasser, a model G1311A quaternary pump, auto sampler, a model G1316A column oven and variable wavelength UV/Vis detector. The separation was performed on Zorbax Eclipse XDB-C18 column (150×4 mm2, 5 μm).
Any solvent remaining in the system from the previous analysis was washed out by passing methanol:water (50:50) for 10 min at 1 ml/min flow rate with purge valve open. After closing the purge valve, 100% methanol was passed for 30 min through column for proper column washing. After this step, for column conditioning and base line stability, isopropyl alcohol, DI water and methanol in the proportion of 70:22:8 v/v mobile phase was passed through HPLC system for 1 h at 1 ml/min flow rate. Injection volume was set to 10 µl.
Standard solution preparation
Standard PC 50 mg was accurately weighed and transferred to a 50 ml volumetric flask and the volume was made with HPLC grade methanol. Solutions of 100, 200, 400, 600, 800, and 1000 μg/ml were made by transferring the aliquot from stock solution and the volume was made with methanol in each case. Further standard solutions were prepared freshly each day by appropriate dilution of stock solution with methanol for intraday as well as interday analysis.
Preparation of sample from deoiled lecithin
Deoiled soy lecithin powder was extracted with 95% ethanol in the ratio of 1:7 w/v in a 500 ml capacity stir tank reactor with overhead stirrer arrangement. The extraction was carried out in air tight reactor with constant stirring at 40° for 2 h with 170 rpm. After stirring, the mixture was filtered and the filtrate was dried in a rotary vacuum evaporator (Buchi Model RII, Switzerland) at 40°. Further, the dried sticky mass of PC rich fraction was purified using silica gel column chromatography and methanol fraction containing purified PC (previously optimized) was collected and methanol from the fraction was evaporated completely to get purified PC in sticky form. For analysis, weighed 10 mg of purified PC in volumetric flask and volume made to 10 ml by dissolving in HPLC grade methanol (1 mg/ml). Then this sample was analyzed by proposed RP-HPLC method for its assay purity.
Mass spectrometry of PC sample
Purified PC fraction collected from silica column was analyzed by proposed RP-HPLC method and carefully collected the elutions of major four peaks of purified PC fractions isolated from HPLC column in different vials and analyzed separately using mass spectrometry and subjected to fragmentation of individual peaks by MS-MS. Mass spectrometer used was of 6420 Series Triple Quadrupole LC/MS, Agilent Technologies (USA), model 410 Prostar Binary LC with 500 MS IT PDA detectors with direct Infusion Mass with ESI.
Validation of the method
Validation of the analytical method was done according to the International Conference on Harmonization guidelines. The method was validated for linearity, precision, accuracy,limit of detection (LOD), and limit of quantitation (LOQ)[26].
Linearity
The linearity of measurement was evaluated by analyzing different concentrations (100 to 1000 μg/ml) of the standard solutions. Calibration curve was constructed for combining all the six peaks as well as individual peaks showed in Table 1 by plotting average peak area against concentration of sample and regression equation was computed. All the samples were analyzed in triplicate.
| Standard PC peak |
RT (Min) |
Correlation coefficient (R2) |
Slope | LOD(µg/ml) | LOQ(µg/ml) |
|---|---|---|---|---|---|
| 1 | 10.92 | 0.995 | 0.159 | 7.26 | 22 |
| 2 | 12.751 | 0.996 | 1.896 | 2.18 | 6.61 |
| 3 | 14.965 | 0.997 | 6.751 | 1.92 | 5.82 |
| 4 | 16.864 | 0.997 | 1.885 | 2.48 | 7.53 |
| 5 | 18.077 | 0.995 | 1.101 | 2.89 | 8.76 |
| 6 | 23.278 | 0.991 | 0.493 | 3.06 | 9.29 |
| Combined | – | 0.998 | 12.382 | 9.36 | 28.36 |
| LOD=Limit of detection, LOQ=Limit of quantitation. | |||||
Table 1: Validation parameters.
Precision
The precision of the method was investigated with respect to repeatability and intermediate precision. The repeatability (intra-day precision) of the method was evaluated by assaying three replicate injections of the PC standard at concentrations of 200, 400 and 600 μg/ml on the same day at different times. The percentage relative standard deviation (% RSD) of the peak area was calculated by combining all the peaks. Intermediate precision (inter-day precision) was demonstrated by evaluating the relative peak area at three different concentration levels as taken in intra-day study that cover the assay method. The precision was expressed as % RSD of the system and was calculated from the combined peak area mean values. The intra-day (n=3) and inter-day (n=3) % RSD are given in Table 2.
| Concentration (µg/ml) | Intra-day | Inter-day | |||
|---|---|---|---|---|---|
| Area | % RSD | Day | Area | % RSD | |
| 200 | 2341.6 | 0.477 | 1 | 2341.6 | 0.714 |
| 2331.2 | 2 | 2375.2 | |||
| 2319.4 | 3 | 2360.8 | |||
| 400 | 4824.7 | 0.283 | 1 | 4824.7 | 0.403 |
| 4845.9 | 2 | 4830.1 | |||
| 4850.3 | 3 | 4860.9 | |||
| 600 | 7579.0 | 0.294 | 1 | 7579 | 0.254 |
| 7545.3 | 2 | 7601.2 | |||
| 7587.4 | 3 | 7617.5 | |||
| N=3, RSD=Relative standard deviation. | |||||
Table 2: Precision study.
Accuracy study
The accuracy of the method was tested by performing recovery studies at three levels of PC reference standard added to the samples. Three different volumes (0.5, 1, and 1.5 ml) of the standard solution (containing 100 μg/ml of PC in methanol) were added to the sample solution of fixed concentration (132.5 μg/ml) and analyzed by the proposed HPLC method. All the samples were determined in triplicate. The % recovery was calculated by using the following formula, % Recovery=[(b-a)/c]×100…(1), where, a – amount of PC found in the sample before addition of standard PC, b – amount of PC found after addition of standard PC, c – amount of standard PC added.
Limit of detection and limit of quantitation
The limit of detection (LOD) and limit of quantification (LOQ) of the developed method were determined by injecting progressively low concentrations of the standard solutions using the developed RP-HPLC method. The LOD is the smallest concentration of the analyte that gives a measurable response (signal-to-noise ratio of 3). The LOQ is the smallest concentration of the analyte, which gives response that can be accurately quantified (signal-to-noise ratio of 10).
System performance parameters
The number of theoretical plates (n) measures the sharpness of the peaks and therefore the efficiency of the column. The number of theoretical plates was calculated according to US pharmacopeia[27] where, the peak width at the base was used for calculation. n=16×(t/Wb)2 …(2), where, Wb=peak width at base, t=retention time of peak.
The capacity factor (k’) gives an indication of how long each component is retained on the column (i.e., how long the component is retarded by the stationary phase than it spends in the mobile phase). The capacity factor was calculated by the Eqn., k’=(tR-tM)/tM ….(3), where, tM=unretained peak’s retention time, tR=retention time of the peak of interest.
The tailing factor (T) measures the asymmetry of the peak and is calculated using the Eqn., T=(W/2f)….(4),where, W=width at 5% of the peak height, f=distance between maximum and the leading edge of the peak.
The separation factor (α) describes the relative position of the two adjacent peaks. The separation factor or relative retention was calculated using the capacity factor (k) as peaks’ separation depends on the components’ interaction with the stationary phase. The separation factor can be calculated by the Eqn.,[27], α=(kB/kA)….(5), where, kA=capacity factor of the 1st peak, kB=capacity factor of the 2nd peak.
Peak resolution (R) is not only a measure of the separation between two peaks, but also the efficiency of the column. It is expressed as the ratio of the distance between the two peak maxima. R=[2(t2-t1)/ (W1+W2)]…(6), where, t1=retention time of the 1st peak, t1=retention time of the 2nd peak, W1=1st peak width at base, W2=2nd peak width at base.
Results and Discussion
The HPLC separation of soy PtdCho was carried out on C18 column by an isocratic elution with isopropyl alcohol, DI water, and methanol in the proportion of 70:22:8 v/v. The flow rate was constant at 0.5 ml/min and the column temperature was at room temperature (25±1°). The UV wavelength was set at 205 nm. There was no interference from impurities present in the PC sample as observed at the detection wavelength. The sharp and symmetrical peaks of PC were obtained when analyzed under these conditions.
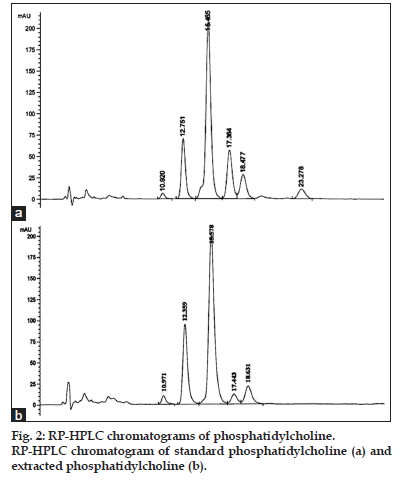
The chromatogram of standard PC peaks is as shown in fig. 2a. Phosphatidylcholine is very complex molecule containing glycerol, fatty acids, phosphate and choline groups. Pure PC contains constant choline and phosphate group with various fatty acids attached to its glycerol backbone. The proposed RP-HPLC method was developed in such a way that it gives various peaks of PC having different fatty acid chains at different retention times. Thereafter, all the PC peaks were considered for validation parameters study and the standard curve was prepared separately for individual peaks as it was related to their separation from each other with better resolution. The retention times for various PC peaks were found to be 10.92, 12.75, 14.97, 16.86, 18.07, and 23.28 min.
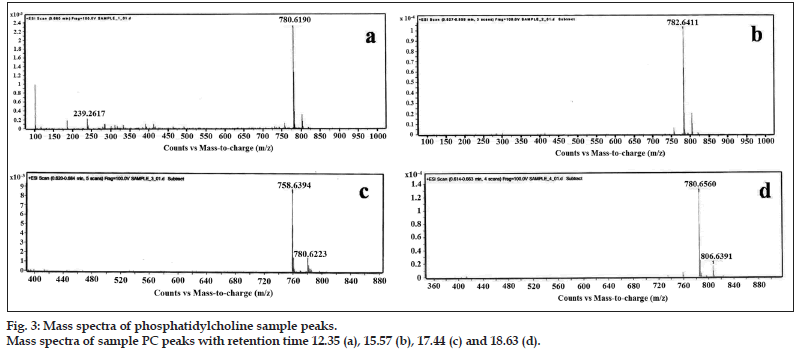
The major four fractions of elution from HPLC graph were individually collected and subjected to mass spectrometry in order to find the type of fatty acid groups present in the extracted and purified PC from deoiled soy lecithin. The major peaks representing the retention time of 12.35, 15.57, 17.44, and 18.63 were collected. The mass spectra of all four peaks and its fragmentation patterns are as shown in fig. 3. The molecular weight of the peak corresponding to the retention time of 12.35 was found to have a mass of 780 (M+23). The fatty acid group giving molecular weight of 780 may consist of 16 carbon atoms or 18 carbon atoms having 3° of unsaturation (double bond). The double bonds present in this core can be associated with one fatty acid or it can be associated with two fatty acids. The peak corresponding to the retention time 15.57 has a mass of 782 (M+23) which may correspond to one fatty acid with 16 number of carbon atoms and one with 18 number of carbon atoms with two double bond present on either of the fatty acid chain or one double bond on each fatty acid. The molecular weight of the peak corresponding to the retention time of 17.44 has a mass of 758 (M+23). The molecular weight 758 is associated with two fatty acids of 16 carbon number without unsaturation. The molecular weight of the peak corresponding to the retention time 18.63 was found to be 784 (M+23), which may contain fatty acid with 16 number of carbon atoms and one with 18 number of carbon atoms with one double bond on either of the fatty acid. The major fragmentation peak at 184 corresponds to phosphocholine. The reason behind quoting of 16 and 18 carbon atoms is due to the fact that the soya lecithin is known to contain majorly oleic, palmitic, palmitoleic, linoleic, α-linolenic and stearic acid, as per the standard literature. PC from natural source is a very complex molecule and mainly contain various fatty acids. The fatty acid side chain present in PC may vary with the source of its origin, and also possibility of extensively peroxidized samples can leave a highly saturated PC residue.
The method was validated for linearity, precision, accuracy, LOD, and LOQ. PC has shown good linearity in the range of 100-1000 µg/ml for individual peaks (Table 1) as this was the working concentration range for the sample analysis. The intra-day and inter-day precisions of PC are presented in Table 2. These results show the acceptable precision of the method, with % RSD values much lower than 2%. The result also shows that an excellent correlation exists between the peak area and the concentration of drugs within the concentration range. The accuracy of the method was evaluated by adding the standard solution of 100, 200, and 300 (µg/ml) to the known concentration of sample solution. The recovery at three different levels of PC concentrations was obtained with an average of 99.26% (Table 3) which was well within the limit of 98 to 102%. The LOD and LOQ for smallest PC peak was found to be 7.26 and 22 μg/ml, respectively (Table 1), which also signifies the high sensitivity of this method based on the signal-to-noise ratio for LOD and LOQ. The signal-to-noise ratios were 6.18 and 20.39, respectively.
| Analyte | Conc of PC before addition(µg/ml) (a) | Std PC added(µg/ml) (c) | Conc of PC after addition(µg/ml) (b) | % Recovery | % Mean | % RSD |
|---|---|---|---|---|---|---|
| 132.5 | 100 | 231.6 | 99.1 | |||
| 132.5 | 100 | 230.1 | 97.6 | |||
| 132.5 | 100 | 231.7 | 99.2 | |||
| 132.5 | 200 | 331.5 | 99.5 | |||
| PC | 132.5 | 200 | 332.1 | 99.8 | 99.26 | 0.714 |
| 132.5 | 200 | 330.8 | 99.15 | |||
| 132.5 | 300 | 430.2 | 99.23 | |||
| 132.5 | 300 | 431.5 | 99.67 | |||
| 132.5 | 300 | 432.8 | 100.1 | |||
| N=3, RSD=Relative standard deviation, PC= Phosphotidylcholine. | ||||||
Table 3: Recovery studies.
Once a method or a new system is proposed, it is important to check the suitability of the method/ system against certain set parameters. The parameters such as number of theoretical plates, capacity factor, tailing factor, separation factor, and peak resolution allow the comparison of the peak shape, its peak and the baseline resolution. Alternatively, these parameters can be calculated experimentally to provide excellent quantitative method of performance analysis (Table 4).
| Factor | Peak | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| No of theoretical Plates (n)(Acceptable limits, n>2000) | 11253 | 13498 | 12651 | 15404 | 12797 | 16145 |
| Capacity factor (k’)(Acceptable limits,10>k’>2) | 1.77 | 2.25 | 2.82 | 3.32 | 3.63 | 5.01 |
| Tailing factor (T)(Acceptable limits T≤2) | 1.164 | 1.167 | 1.162 | 1.138 | 1.086 | 1.243 |
| Separation factor (α)(Acceptable limits α> 1) | 1.27 | 1.25 | 1.18 | 1.09 | 1.38 | |
| Peak resolution (R)(Acceptable limits R>2) | 3.76 | 3.8 | 3.06 | 1.74 | 6.12 | |
Table 4: System performance parameters of developed RPHPLC method of PC.
PC extracted from crude deoiled lecithin by solid– liquid and liquid–liquid extraction followed by gradient column chromatographic separation as mentioned in the literature[28]. This extracted and purified PC was successfully analyzed by the proposed HPLC method with no interference of other impurities present in the lecithin as showed in fig. 2b. The results showed 99% purity of the purified PC. Thus, it can be said that the proposed method of RP-HPLC was very much suitable for quantification and analysis of PC.
A novel, convenient, rapid, accurate, and precise RP-HPLC method was developed for the estimation of PC with various fatty acid chains from soybean source. The assay provides a linear response across a wide range of concentrations. The low intra-day and inter-day with % RSD as low as 2, gives excellent recoveries of the eluted moieties. The proposed method assures prolong life of column and system due to simple organic solvents used in the mobile phase. The proposed method has been developed using UV detector which has made the method more economic than other methods reported in literature. The mass spectrometry results confirmed that various PC peaks from HPLC chromatogram were of different fatty acid chains. PC with its various fatty acid chains can be separated by preparative chromatography in future using the proposed method in economic way. Also, the proposed RP-HPLC method was successfully worked for extracted and purified PC without any interference of other sample impurities.
Acknowledgements
The authors are thankful to University Grant Commission Special Assistanceship Program for providing funding for this research work.
References
- Gross R. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: A fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry 1984;23:158-65.
- Ramanadham S, Bohrer A, Mueller M, Jett P, Gross R, Turk J. Mass spectrometric identification and quantitation of arachidonate-containing phospholipids in pancreatic islets: Prominence of plasmenylethanolamine molecular species. Biochemistry 1993;32:5339-51.
- Dobson G, Deighton N. Analysis of phospholipid molecular species by liquid chromatography-atmospheric pressure chemical ionisation mass spectrometry of diacylglycerol nicotinates. Chem Phys Lipids 2001;111:1-17.
- Hayakawa J, Okabayashi Y. Simultaneous analysis of phospholipid in rabbit bronchoalveolar lavage fluid by liquid chromatography/mass spectrometry. J Pharm Biomed Anal 2004;35:583-92.
- Pardun H. Vegetable lecithins – valuable auxiliary and active substances? Fat Sci Technol 1989;91:45-58.
- Willmann J, Thiele H, Leibfritz D. Combined reversed phase HPLC, mass spectrometry, and NMR Spectroscopy for a fast separation and efficient identification of phosphatidylcholines. J Biomed Biotechnol 2011;385786:1-8.
- Tranchida P, Donato P, Dugo G, Mondello L, Dugo P. Comprehensive chromatographic methods for the analysis of lipids. Trends Anal Chem 2007;26:191-205.
- Meier S, Mjøs S, Joensen H, Grahl-Nielsen O. Validation of a one-step extraction/methylation method for determination of fatty acids and cholesterol in marine tissues. J Chromatogr A 2006;1104:291-8.
- Touchstone J. Thin-layer chromatographic procedures for lipid separation. J Chromatogr B 1995;671:169-95.
- Myher J, Kuksis A. General strategies in chromatographic analysis of lipids. J Chromatogr B 1995;671:3-33.
- Peterson B, Cummings B. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomed Chromatogr 2006;20:227-43.
- Silversand C, Haux C. Improved high-performance liquid chromatographic method for the separation and quantification of lipid classes: Application to fish lipids. J Chromatogr B 1997;703:7-14.
- Dugan L, Demediuk P, Pendley C 2nd, Horrocks L. Separation of phospholipids by high-performance liquid chromatography: All major classes, including ethanolamine and choline plasmalogens, and most minor classes, including lysophosphatidylethanolamine. J Chromatogr B 1986;378:317-27.
- Olsson N, Salem N Jr. Molecular species analysis of phospholipids. J Chromatogr B 1997;692:245-56.
- Becart J, Chevalier C, Biesse J. Quantitative analysis of phospholipids by HPLC with light scattering evaporating detector–application to raw materials for cosmetic use. J High Resolut Chromatogr 1990;13:126-9.
- Kim HY, Wang TL, Ma Y. Liquid chromatography/mass spectrometry of phospholipids using electrospray ionization. Anal Chem 1994;66:3977-93.
- Kuksis A, Myher J, Marai L. Lipid methodology–Chromatography and beyond. Part I. GC/MS and LC/MS of glycerolipids. J Am Oil Chem Soc 1984;61:1582-9.
- Heller D, Murphy C, Cotter R, Fenselau C, Uy O. Constant neutral loss scanning for the characterization of bacterial phospholipids desorbed by fast atom bombardment. Anal Chem 1988;60:2787-91.
- Chilton F, Averill F, Hubbard W, Fontech A, Triggiani M, Liu M. Antigen-induced generation of lyso-phospholipids in human airways. J Exp Med 1996;183:2235-45.
- Christie W. Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J Lipid Res 1985;26:507-12.
- Miguel D, Roueche A, Betbeder D. Separation of dipalmitoyl phosphatidyl choline, cholesterol and their degradation products by high-performance liquid chromatography on a perfluorinated stationary bonded phase. J Chromatogr A 1999;840:31-8.
- Loening N, Chamberlin A, Zepeda A, Gonzalez R, Cheng L. Quantification of phosphocholine and glycerophosphocoline with 31P edited 1H NMR spectroscopy. NMR Biomed 2005;18:413-20.
- Schiller J, Muller M, Fuchs B, Arnold K, Huster D. 31P NMR spectroscopy of phospholipids: From micelles to membranes. Curr Anal Chem 2007;3:283-301.
- Leibfritz D. An introduction to the potential of 1H-, 31P and 13C-NMR-spectroscopy. Anticancer Res 1996;16:1317-24.
- Willker W, Leibfritz D. Assignment of mono- and polyunsaturated fatty acids in lipids of tissues and body fluids. Magn Reson Chem 1998;36:S79-84.
- ICH, Q2B validation of analytical procedures: Methodology, International Conference on Harmonization, November 1996.
- United States Pharmacopeia XXI I (United States Pharmacopeial Convention, Rockvilie, MD, 1990.
- Jangle RD, Magar VP, Thorat BN. Phosphatidylcholine and its purification from raw deoiled soya lecithin. Sep Purif Technol 2013;102:187-95.