- *Corresponding Author:
- S. F. Liu
School of Public Health, Shandong University, Jinan, 250012, P. R. China
E‑mail: liushufang@sdu.edu.cn
| Date of Submission | 10 March 2013 |
| Date of Revision | 27 August 2013 |
| Date of Acceptance | 30 August 2013 |
| Indian J Pharm Sci 2013;75(5):614-618 |
Abstract
A sensitive method based on the fluorescence enhancement was developed for the determination of rutin. It was found that the rutin could form a fluorescent complex with ytterium (III), and the fluorescence intensity of which could be enhanced by sodium dodecyl benzene sulfonate. Under the optimum conditions, the enhanced fluorescence intensity was in proportion to the concentration of rutin in the range of 12-1200 ng/ml and the detection limit (S/N=3) was 1.3 ng/ml. This method possessed the properties of simplicity, fast assay process and high sensitivity, and has been satisfactorily applied for the analysis of actual sample. The possible interaction mechanism of this system was also discussed in this manuscript.
Keywords
Fluorescence, rutin, determination, yttrium
Rutin is one of the most active flavonoid compounds found in asparagus, the leaves and petioles of Rheum species and buckwheat, or the fruits of some other plants, and contributes to the antibacterial and antioxidant properties of the plants. It has many physiological activities, such as maintaining the resistance of the blood vessels, reducing their osmose and brittleness, promoting proliferation of the cells and preventing the blood cells from aggregation [1]. In addition, rutin also has the function of anti-inflammatory [2], antitumor [3] and antibacteria [4]. It is mainly used for the clinical treatment in capillary haemorrhage and also recommended for the adjuvant treatment in hypertensive encephalopathy, acute haemorrhagic nephritis, postpartum haemorrhage, purpura haemorrhagica, retinal haemorrhage and recurrent epistaxis.
The methods currently used for the determination of rutin include chromatography [5-7], capillary electrophoresis (CE) or microchip-CE [8-10], electrochemical methods [11-13], UV spectrophotometry [14,15], and chemiluminescence [16,17]. Among various methods, due to its high reproducibility and ease of automation, LC has been widely used to determine rutin and coexistent flavonoids in plants, but it also has some shortcomings such as complicate operation, high cost and coexistent interferences in the sample solutions. Fluorescence analysis has the advantages of simple operation, high sensitivity and selectivity with a wide range of applications for drug determination, but very little has been reported for the determination of rutin. Based on the above, this work aims to develop a simple and sensitive fluorimetric method for the determination of rutin.
A stock solution of ytterium (III) (1.0×10−2 mol/l) was prepared by dissolving ytterium oxide (Y2O3, 99.9%) in hydrochloric acid and diluting with water. Stock standard solution (1.0×10−3 mol/l) of rutin (Beijing Institute of Biological Products, Medical Department of China) was made by dissolving rutin in 0.1 mol/l NaOH and diluting with water. Stock solutions of surfactants (1×10−2 mol/l) were prepared by directly dissolving them in water. A 0.05 mol/l Tris (hydroxymethyl)aminomethane(Tris)–HCl buffer solution was prepared by dissolving appropriate Tris in water and adjusting the pH with hydrochloric acid. All the chemicals used were of analytical reagents grade and doubly deionised water was used throughout. All fluorescence spectra were recorded on a LS-55 spectrofluorimeter (PE) in a 1 cm quartz cell. All pH measurements were made with a Delta 320-s pH meter (Mettler Toledo, Shanghai).
To a dry 10 ml test tube, solutions were added as following order: definite standard rutin (or sample solution), certain volumes of Y3+(1.0×10−3 mol/l), surfactant solution (1×10−2 mol/l) and 1.0 ml of Tris-HCl buffer (pH 9.5), respectively, and then the mixture was diluted to 10.0 ml with water. The fluorescence intensity was measured in a 1.0 cm quartz cell with excitation and emission wavelengths of 380 nm and 540 nm, respectively. The excitation and emission slits were both 10 nm.
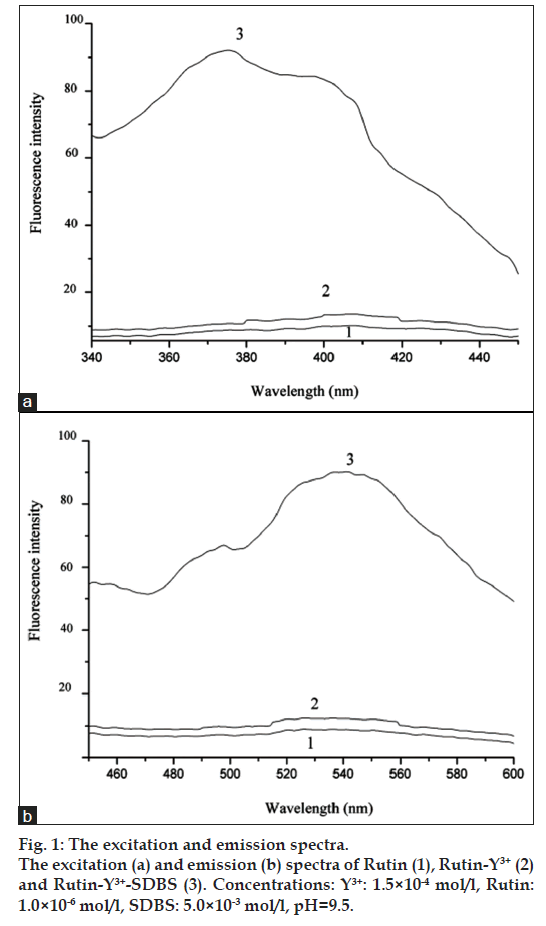
The excitation and emission spectra of rutin (1), rutin-Y3+ (2) and rutin-Y3+-Sodium dodecylbenzenesulfonate (SDBS) (3) systems were shown in fig. 1. Under excitation of 405 nm, the rutin-Y3+ system emitted weak fluorescence in the range of 490-600 nm, which can be greatly enhanced by anionic surfactant SDBS, and the maximum excitation wavelength shifted from 405 nm to 380 nm, indicating that interaction between rutin-Y3+ and SDBS occurred. The excitation wavelength 380 nm and emission wavelength 540 nm were selected for further experiments.
The selection of optimum conditions is a critical part of the work. The effects of pH, buffer solutions (such as NH4Ac-HAc, Na2B4O7-HCl, Tris-HCl and hexamethylenetetramine (HMTA)-HCl), Y3+ concentrations and surfactant types (such as alkylphenol ethoxylate-10(OP-10), sodium dodecyl sulfate (SDS), SDBS, cetyl trimethyl ammonium bromide (CTAB) and cetylpyridinium bromide (CPB) and concentrations were investigated, and to obtain the maximum fluorescence enhancement (ΔIf(%)=(If- If0)/If0;, where If is The fluorescence intensity of rutin-Y3+-SDBS system, If0 is the fluorescence intensity of Y3+-SDBS system), the pH value of 9.5, Tris-HCl buffer solution, 1.5×10−4 mol/l Y3+ and 5.0×10−3 mol/l SDBS were chosen. The addition order and stability of this system were also tested, and the results indicated that the order of rutin, Y3+, SDBS, and Tris-HCl buffer had the biggest fluorescence enhancement of the system. The system reached maximum fluorescence intensity within 10 min after all the reagents were mixed and remained stable for 2 h.
The interference of foreign substances was tested for 4.0×10−7 mol/l rutin, and the results were shown in Table 1. It can be seen that most of the foreign substances tested except Fe2+ have little effect on the determination of rutin under the permission of ±5% relative error.
| Foreign Substances | Coexisting concentration (×10−5 mol/l) | The change of ΔIf(%) |
|---|---|---|
| MgSO4 | 1.4 | −4.4 |
| Na2CO3 | 1.0 | −4.2 |
| Fe2+ | 0.4 | −4.5 |
| Zn2+ | 2 | −4.1 |
| NaCl | 2 | −4.3 |
| Al3+ | 3 | −4.1 |
| Ca2+ | 12 | −4.4 |
| Vitamin B1 | 7 | −4.2 |
| Vitamin C | 15 | −4.5 |
| D‑galactose | 200 | −4.1 |
| D‑xylose | 150 | −4.7 |
| L‑sorbose | 300 | −4.5 |
Concentrations=Y3+: 1.5×10-4 mol/l, rutin=4.0×10-7 mol/l, SDBS=0.5×10-3 mol/l, pH=9.5
Table 1: Interference From Foreign Substances
Under optimum conditions defined here, the calibration graph for rutin was obtained. There was a linear relationship between the fluorescence intensity and the concentration of rutin in the range of 12-1200 ng/ml with a correlation coefficient of 0.998, and the detection limit (S/N=3) was 1.3 ng/ml.
Table 2 showed the comparison of this proposed method with other reported methods for the determination of rutin. It can be seen that the proposed method had wider linear range and higher sensitivity compared to most of reported methods. Although the sensitivity of proposed fluorescence method was higher than that of reported LC-MS/ MS method [6] and one of the chemiluminescence methods [17], compared to the two methods, apparently it has the advantages of low-cost and easy to operate and can meet the general requirements of rutin determination.
| Methods | Detection limit (mol/l) | Linear range (mol/l) | References |
|---|---|---|---|
| ECL | 2.0×10−7 | 5.0×10−7–5.0×10−4 | [11] |
| 1.0×10−8 | 4.0×10−8-1.0×10−4 | [12] | |
| SP | - | 3.3×10−6‑1.6×10−5 | [14] |
| HPLC | 8.4×10−8 | 1.6×10−5‑8.2×10−5 | [5] |
| LC‑MS/MS | 8.8×10−10 | 2.0×10−9‑9.8×10−7 | [6] |
| CE | 3.3×10−7 | 4.1×10−6‑1.3×10−4 | [9] |
| CL | 3.3×10−9 | 1.3×10−8-1.6×10−7 | [16] |
| 3.9×10−11 | 1.6×10−10‑4.9×10−8 | [17] | |
| FL (Reportedpreviously) | 7.0×10−8 | 1.0×10−7‑3.0×10−5 | [18] |
| FL (Proposedin this paper) | 5.9×10−7 | 1.2×10−6‑4.8×10−5 | [19] |
| 2.2×10−9 | 2.0×10−8‑2.0×10−6 | - |
ECL=Electrochemical analytical method, SP=Spectrophotometric method, HPLC=High-performance liquid chromatography, CE=Capillary electrophoresis, CL=Chemiluminescence, FL=Fluorimetry
Table 2: Comparison Of Existing Method Forthe Determination Of Rutin With The Proposed Method
Considering the effects of foreign substances on the fluorescence intensity of the system, the standard addition method was used for the determination of rutin in compound rutin tablets (The Third Pharmaceutical Factory of Jinan, China). The sample was prepared as follows, rutin tablets (10 tablets) were carefully ground, and then 0.2 g powder was dissolved with 0.1 mol/l NaOH solution. The obtained solution was centrifuged for 10 min at 5000 rpm and the supernatant was filtered through filter paper and the filtrate was diluted to 150 ml with distilled water. The obtained solution was determined by the proposed method for 5 times. The results were comparable with the corresponding labelled amount. The validation parameters were summarised in Table 3. It can be seen that the accuracy and precision of this method were satisfactory.
| Parameters (units) | Rutin |
|---|---|
| Lineartiy range (ng/ml) | 12‑1200 |
| Correlation coeffcient | 0.998 |
| LOD (ng/ml) | 1.3 |
| Recovery (%) | 100.05 |
| Precision (Repeatablity, RSD%) | 1.6 |
| Robustness | Robust |
| LOD=Limit of detection, RSD=Relative standard deviation | |
Table 3: Summary Of Validation Parameters
In this work, the mechanism of fluorescence enhancement was also proposed. From fig. 1, it can be seen that the emission peak of rutin-Y3+ system was at the same position as that of rutin system, indicating that the luminescence of the complex belongs to L*-L luminescence mechanism. The formation of rutin-Y3+ complex enlarges the rigid structure plane of rutin and results in the enhancement of the fluorescence intensity of the system. The fluorescence intensity enhancement of rutin-Y3+ system by anionic surfactant SDBS and the blue shift of maximum excitation wavelength indicated that there was interaction between rutin-Y3+ and SDBS. We speculate that rutin-Y3+ complex is positively charged and can be easily dissolved in the micelle of SDBS with negative charge through electrostatic and hydrophobic force, which provides an optimum hydrophobic environment for rutin-Y3+complex, and results in the enhancement of the fluorescence intensity of the rutin-Y3+ system. In addition, the hydrophobic microenvironment can also prevent the collision between rutin-Y3+ complex and water molecules, and decrease the energy loss of the system. Thus, the fluorescence quantum yield is improved and the fluorescence intensity of rutin-Y3+ complex is significantly enhanced.
Acknowledgements
This work was supported by Natural Science Foundations of China (20575035) and Shandong Province (Y2003B02), and by Visiting Scholar Foundation of Key Lab in the University.
References
- The Public Health Department of People’s Republic of China, Pharmacopoeia of the People’s Republic of China, Vol. 11. Beijing: Chemical Industry; 2005, p. 311.
- Gené RM, Cartaña C, Adzet T, Marín E, Parella T, Cañigueral S. Anti-infammatory and analgesic activity of Baccharis trimera: Identification of its active constituents. Planta Med 1996;62:232-5.
- Ramanathan R, Das N, Tan C. Inhibitory effects of 2-hydroxy chalcone and other flavonoids on human cancer cell-proliferation. Int J Oncol 1993;3:115-9.
- van der Watt E, Pretorius JC. Purification and identification of active antibacterial components in Carpobrotusedulis L. J Ethnopharmacol 2001;76:87-91.
- Nour V, Trandafir I, Cosmulescu S. HPLC Determination of Phenolic Acids, Flavonoids and Juglone in Walnut Leaves. J Chromatogr Sci 2013;51:883-90.
- Chang L, Ren Y, Cao L, Sun Y, Sun Q, Sheng N, et al. Simultaneous determination and pharmacokinetic study of six favonoids from Fructus Sophorae extract in rat plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2012;904:59-64.
- Danila AM, Kotani A, Hakamata H, Kusu F. Determination of rutin, catechin, epicatechin, and epicatechin gallate in buckwheat Fagopyrum esculentum Moench by micro-high-performance liquid chromatography with electrochemical detection. J Agric Food Chem 2007;55:1139-43.
- Xie F, Zhang Y, Zheng B, Xu F, Su J, Lu Y, et al. Rapid and sensitive analysis of three polyphenols in tobacco by CE using homemade C(4) D with a mini detection cell. Electrophoresis 2012;33:2433-40.
- Peres RG, Micke GA, Tavares MF, Rodriguez-Amaya DB. Multivariant optimization, validation, and application of capillary electrophoresis for simultaneous determination of polyphenols and phenolic acids in Brazilian wines. Sep Sci 2009;32:3822-8.
- Xu J, Zhang H, Chen G. Carbon nanotube/polystyrene composite electrode for microchip electrophoretic determination of rutin and quercetin in Flos Sophorae Immaturus. Talanta 2007;73:932-7.
- Wei Y, Wang GF, Li MG, Wang C, Fang B. Determination of rutin using a CeO2 nanoparticle-modified electrode. Mikrochim Acta 2007;158:269-74.
- Liu M, Deng J, Chen Q, Huang Y, Wang L, Zhao Y, et al. Sensitive detection of rutin with novel ferrocene benzyne derivative modified electrodes. Biosens Bioelectron 2013;41:275-81.
- Ziyatdinova G, Ziganshina E, Budnikov H. Surfactant media for constant-current coulometry. Application for the determination of antioxidants in pharmaceuticals. Anal Chim Acta 2012;744:23-8.
- Hassan HN, Barsoum BN, Habib IH. Simultaneous spectrophotometric determination of rutin, quercetin and ascorbic acid in drugs using a Kalman Filter approach. J Pharm Biomed Anal 1999;20:315-20.
- Wang S, Du L, Yao X, Niu X, Zhuang H. Kinetic spectrophotometric determination of rutin by its inhibitory effect on the oxidation of amaranth by potassium periodate. Ann Chim 2005;95:87-94.
- Yang D, Li H, Li Z, Hao Z, Li J. Determination of rutin by flow injection chemiluminescence method using the reaction of luminol and potassium hexacyanoferrate (III) with the aid of response surface methodology. Luminescence 2010;25:436-44.
- Song Z, Hou S. Sensitive determination of sub-nanogram amounts of rutin by its inhibition on chemiluminescence with immobilized reagents. Talanta 2002;57:59-67.
- Chen YH, Tian FS. Highly sensitive spectrofuorimetic determination of rutin based on its activated effect on a multi-enzyme redox system. Luminescence 2012;27:59-62.
- Yang JH, Zou HB, Jie NQ, Ge HM, Yang RJ. Study on the fuorescence system Be-rutin-SDS-Triton X-100 and the determination of rutin. Fresenius J Anal Chem 1994;348:377-9.