- *Corresponding Author:
- G. A. Kurian
School of Chemical and Biotechnology, SASTRA University, Thanjavur-613 401, India
E-mail: ginokurian@hotmail.com
| Date of Submission | 31 March 2015 |
| Date of Revision | 17 January 2016 |
| Date of Acceptance | 26 February 2016 |
| Indian J Pharm Sci 2016;78(1):151‑158 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Oxidative stress plays a significant role not only in cardiovascular disease but also in non-communicable diseases, where it plays a significant role the mortality rate. Hydrogen sulfide, the biological gaseous signaling molecule that preserves mitochondria in its mode of action, is an effective cardioprotective drug. However, cardiac mitochondria comprise of two distinct populations, namely interfibrillar and subsarcolemmal mitochondria, which respond distinctly in cardiovascular disease. This study was designed to determine the direct impact of cobalt chloride-induced oxidative stress in isolated mitochondrial subpopulations with an intention to examine the efficacy of hydrogen sulfide in preserving interfibrillar and subsarcolemmal mitochondria functional activities when they were incubated as pretreated, co-treated and post-treated agent. Mitochondrial subpopulations were isolated from the heart of male Wistar rats and subjected to cobalt chloride treatment (500 μM) for 20 min, followed by incubation with 10 μM sodium hydrosulfide in three different ways (Pre, Co, and Post-cobalt chloride treatment). Mitochondrial oxidative stress was measured by the concentration of thiobarbituric acid reactive species, reduced glutathione and the activities of enzymes like superoxide dismutase, catalase and glutathione peroxidase. Mitochondrial membrane potential, swelling behavior and enzyme activities were measured to assess its function. The increased level of lipid peroxidation and the decreased level of reduced glutathione in cobalt chloride-induced group confirm the induction of oxidative stress and were more predominant in the subsarcolemmal mitochondria. Hydrogen sulfide treatment to interfibrillar and subsarcolemmal mitochondria preserved their functional activities, but the effect was prominent only with co-treated group. In conclusion, the present study demonstrated that subsarcolemmal mitochondria are more prone to oxidative stress and the co-treatment of the mitochondria with hydrogen sulfide preserved the enzyme activity in the in vitro conditions.
Keywords
Mitochondria, cobalt chloride, antioxidants, hydrogen sulfide
Reactive oxygen species (ROS) are molecules containing unpaired electrons and includes singlet oxygen, superoxide, peroxides, hydroxyl radicals and hypochlorous acid [1]. These unpaired electrons, are highly reactive and can damage the cell. Oxidative stress results from the imbalance between the production of ROS and the efficiency of the antioxidant system, leading to an altered state which can contribute to endothelial dysfunction and cell death [2]. The reactive nature of oxygen species made it necessary to develop a defense mechanism to protect cellular damage. Oxidative stress has been given more importance because of its role in aging, cell death, cardiovascular disease, diabetes, cancer, ischemia reperfusion injury [3].
In mitochondria, enhanced levels of ROS are generated during oxidative phosporylation which ultimately leads to the mitochondrial dysfunction [4]. Majority of ROS are generated by complexes I and III because of the increased supply of electrons by NADH and FADH, generated by different sources during pathological condition, into the ETC [5]. In active mitochondria, oxidative phosphorylation resulted in enhanced oxygen consumption that leads to the formation of superoxide (O2) radical [6]. Superoxide gets transformed into hydrogen peroxide (H2O2) by the enzymes manganese superoxide dismutase (MnSOD) or copper/zinc superoxide dismutase (Cu/Zn SOD) [7] and then to water by glutathione peroxidase (GPX) or peroxiredoxin III (PRX III) [8]. Yet, when these enzymes cannot convert ROS such as the superoxide radical to H2O fast enough, then oxidative damage occurs and ROS accumulates in the mitochondria.
Heart mitochondria consists of two distinct mitochondrial subpopulations, one beneath the sarcolemma, subsarcolemmal mitochondria (SSM) and another along with myosin fibers interfibrillar mitochondria (IFM) [9]. SSM is released by the initial tissue homogenization followed by centrifugation while IFM requires protease treatment for the disruption of myofibrils [10]. It has been reported that cobalt is toxic in its ionic form and increases the reactive oxygen species in neuroblastoma cells [11]. Similarly it can induce hepatotoxicity in rat’s in vivo [12]. Oxidative stress can also be induced by cobalt chloride in the human placental and goat heart mitochondria in in vitro studies [13]. Hydrogen sulfide can protect the apoptosis induced by oxidative stress [14]. So far no studies have reported the role of hydrogen sulfide on IFM and SSM.
In the present study, these mitochondrial subpopulations were isolated from the rat heart and the direct impact of cobalt chloride-induced oxidative stress was studied in vitro. The oxidative stress induced mitochondrial subpopulations were subjected to sodium hydrosulfide treatment in the following ways, pretreated, co-treated and post-treated, to assess its efficacy. The antioxidant activity, mitochondrial functional and enzymatic activities were evaluated for those mitochondrial subpopulations.
Materials and Methods
All chemicals used were of analytical grade purchased from Himedia, Mumbai, India except sodium hydrosulfide which was from Sigma-Aldrich, USA.
All animal experiments were conducted in accordance with the CPCSEA (Committee for the purpose of conduct and supervision of experiments on animals) guidelines, approved by the institutional animal ethics committee (IAEC No. 214/SASTRA/IAEC), Central Animal Facility, SASTRA University. We used male albino Wistar rats aged 7 to 8 weeks (180-200 g). Animals were kept in polycarbonate cages at a controlled temperature of 25±3° and 60±10% relative humidity with a 12 h each of dark and light cycle. Rats were acclimatized for one week with standard laboratory diet and tap drinking water before the start of the experiment.
Experimental protocols
Hearts from male Wistar rats were collected, after perfusing it with KH buffer. Interfibrillar and sub sarcolemmal mitochondria were isolated. The isolated mitochondria were subjected to the following set of experiments: Group 1 (Normal control): IFM and SSM were kept at 37° for 30 min after adding respiration buffer and stored at -80°. Group 2 (Induction control): Mitochondrial subpopulations were exposed to 500 μM cobalt chloride (CoCl2) for 20 min and centrifuged at 9000 g for 10 min. The pellet was stored in the respiration buffer at -80°. Groups 3 (NaSH pretreated): Isolated mitochondria were treated with 10 μM of sodium hydrosulfide (NaSH) for 10 min, which was then centrifuged at 9000 g for 10 min. Cobalt chloride (500 μM) was added to the pellet and incubated for 20 min at 37o. The samples were centrifuged and the pellet was stored in the respiration buffer at -80°. Group 4 (NaSH co-treated): Both IFM and SSM were simultaneously treated with 500 μM cobalt chloride and 10 μM sodium hydrosulfide for 20 min at 37° and the pellet was stored in the respiration buffer at -80° after centrifugation at 9000 g. Group 5 (NaSH post-treated): Cobalt chloride of 500 μM was added to mitochondrial subpopulations and incubated for 20 min at room temperature. After centrifugation at 9000 g for 10 min, the pellet was exposed to 10 μM of sodium hydrosulfide for 10 min. The sample was centrifuged at 9000 g and the pellet was stored in respiration buffer at -80°.
Isolation of mitochondrial sub population
Rat heart mitochondrial sub populations were isolated by differential centrifugation [15].
Isolation of subsarcolemmal mitochondria
The homogenization of cardiac tissues was carried out on the medium that contains 100 mM KCl, 40 mM Tris HCl: pH 7.5, 10 mM Tris base, 5 mM MgCl2, 1 mM EDTA, and 1 mM ATP in a proportion of 10 ml/g heart. Centrifugation of the homogenate was done at 800 g for 5 min at 4º, the resultant supernatant was again centrifuged at high speed of 9000 g for 10 min at 4º. The pellet from the above step was suspended in a solution containing 100 mM KCl, 10 mM Tris HCl: pH 7.4, 10 mM Tris base, 1 mM MgSO4, 0.1 mM EDTA, 0.02 mM ATP and 1.5% BSA (fatty acid free) and centrifuged at 8000 g for 10 min. Solution containing 100 mM KCl, 10 mM Tris HCl: pH 7.4, 10 mM Tris base, 1 mM MgSO4, 0.1 mM EDTA and 0.02 mM ATP were added to the pellet and recentrifuged at 6000 g for 10 min. The pellet was suspended in the medium that contains 220 mM sucrose, 70 mM mannitol, 10 mM Tris HCl, pH 7.4 and 1 mM EDTA.
Isolation of interfibrillar mitochondria
The homogenization of cardiac tissue was carried out on the medium that contains 100 mM KCl, 40 mM Tris HCl: pH 7.5, 10 mM Tris base, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP in a proportion of 10 ml/g heart (same as above mentioned). The resultant pellet was suspended with 10 fold of the above mentioned solution and homogenized for 5 s and recentrifuged at 800 g for 5 min. Trypsin was added to the pellet at a concentration of 5 mg/g wet tissue and kept undisturbed for 10 min at 4°. The reaction was stopped by adding 20 fold of same buffer and the suspension was centrifuged for 5 min at 5000 g. The resulting pellet was homogenized in a solution containing 100 mM KCl, 10 mM Tris HCl: pH 7.4, 10 mM Tris base, 1 mM MgSO4, 0.1 mM EDTA, 0.02 mM ATP and 1.5% BSA (fatty acid free) and centrifuged at 800 g for 10 min. The resulting supernatant was transferred to a clean tube to be spun at high speed (9000 g for 10 min at 4º). The pellet was cleaned by re suspension in a solution containing 100 mM KCl, 10 mM Tris HCl: pH 7.4, 10 mM Tris base, 1 mM MgSO4, 0.1 mM EDTA and 0.02 mM ATP. The tubes were centrifuged at 6000 g for 10 min. The pellet was collected and stored in buffer containing 220 mM sucrose, 70 mM mannitol, 10 mM Tris HCl, pH 7.4 and 1 mM EDTA [13].
Biochemical parameters
Thiobarbituric acid reactive substances (TBARS) were measured as a marker of lipid peroxidation [16]. The levels of various antioxidant enzymes like manganese superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) [17] were measured using Biotek Synergy H1 multimode reader. Protein concentration was measured with Folin phenol reagent, following the procedure described by Lowry [18]. Assay of malate dehydrogenase, succinate dehydrogenase and NADH dehydrogenase were also carried out. Total glutathione content (GSH) in IFM and SSM was measured as per the standard procedures [19].
Mitochondrial swelling
Ca2+-induced swelling was used to assess the activation of the mitochondrial permeability transition pore [20]. Mitochondria were incubated at 30° in buffer containing 125 mM sucrose, 50 mM KCl, 5 mM HEPES, 2 mM KH2PO4 and 1 mM MgCl2. Swelling of mitochondria was determined in presence of 5 mM glutamate-malate. The rate of change in the absorbance was recorded at 540 nm.
Mitochondrial membrane potential
Mitochondrial membrane potential was estimated using the uptake of the positively charged fluorescent dye, rhodamine 123 [20]. Mitochondrial aliquots were centrifuged at 9000 g for 10 min. Pellets were then resuspended in fresh incubation buffer devoid of EDTA. Rhodamine 123 (1.0 μM) was added and the resuspensions were incubated at 37° in a thermostatic bath for 15 min with gentle shaking. Mitochondrial pellets were separated by centrifugation at 9000 g for 10 min and the concentration of rhodamine 123 was measured in the pellet as well as in supernatant fluorimetrically at an excitation wavelength of 549 nm and emission wavelength of 574 nm. Membrane potentials (negative inside) were calculated by the Nernst equation: Δψ=59*log [(Rh 123)in/(Rh 123)out].
Statistical analysis
The data were expressed as mean±SD. The comparison between groups, at various time points during the experiment was conducted using ANOVA followed by multiple comparison tests, particularly Dunnett’s test using Graph Pad Prism software version 5.0.
Results
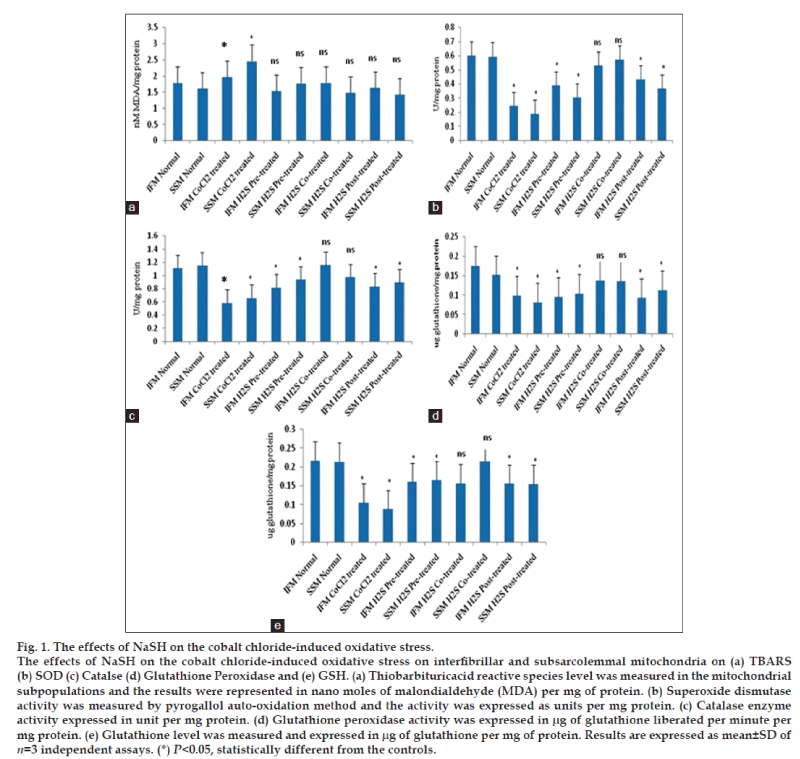
The interfibrillar and sub sarcolemmal mitochondria were isolated from normal rat hearts and the integrity was assessed by the respiratory control ratio and P/O. It is already known that cobalt chloride mediates mitochondrial oxidative stress by inducing reactive oxygen species generation. Lipid peroxidation levels of IFM and SSM after subjected to CoCl2 incubation for 20 min suggest that both mitochondrial sub populations have similar sensitivity (fig. 1) towards oxidative stress in in vitro condition. In concurrence to the elevated TBARS levels (fig. 1a), antioxidant enzymes like SOD (fig. 1b), catalase (fig. 1c), GPx activities (fig. 1d) were declined as compared to the normal control groups.
Effect of hydrogen sulfide pretreatment on isolated IFM and SSM
The antioxidant effect of hydrogen sulfide under oxidative stress was reported in cardiomyocytes [21] and are mediated through the mitochondrial signaling pathway. In this study, we evaluate the potential of H2S in ameliorating the oxidative stress insulted in isolated mitochondrial subpopulation by cobalt chloride incubation. Pretreatment of IFM and SSM with H2S for 20 min reduced the lipid peroxidation in both IFM and SSM as compared to the cobalt chloride treatment group. Compared to SSM, IFM showed 17% decrease in TBARS levels (fig. 1a) and corresponding reduction in thiol levels (fig. 1e) were observed. The antioxidant enzymes like catalase, superoxide dismutase and glutathione peroxidase activities were recovered to near normal level, indicating the effectiveness of hydrogen sulfide in ameliorating the mitochondrial oxidative stress.
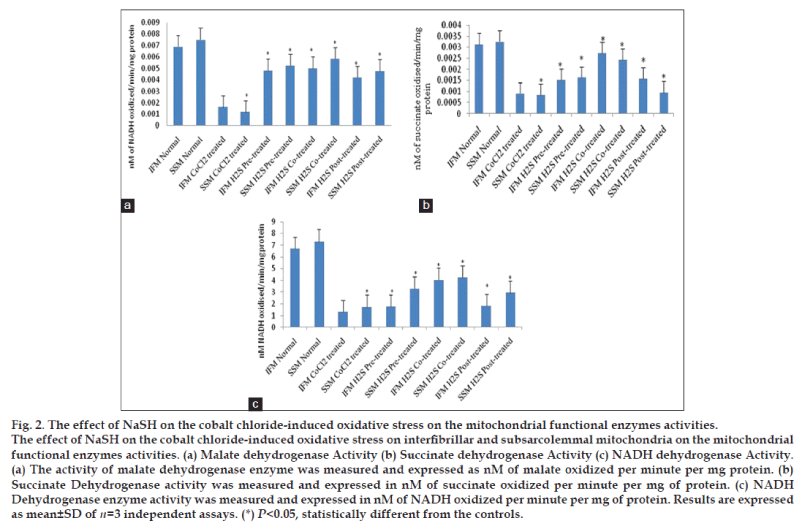
Malate dehydrogenase, considered to be one of the indicators for TCA cycle metabolic pathway regulation was found to have elevated activity (fig. 2a). On the other hand, succinate dehydrogenase (fig. 2b) and NADH dehydrogenase (fig. 2c) that represents complex I and complex II activity did not show a corresponding increase in their activity to near normal level (fig. 2), suggested limited ETC activity.
Effect of hydrogen sulfide co-treatment on isolated IFM and SSM
According to the fig. 1a, lipid peroxidation marker namely TBARS level was low (62%) in H2S co-treated group as compared to the cobalt chloride control group. A supportive observation in the GSH level was found in H2S co-treated groups, where its level was increased by 42% as compared to the cobalt chloride control group. Between the mitochondrial subpopulations, SSM observed 81% decline and IFM showed a 60% decrease in TBARS levels. On the other hand, GSH levels in SSM and IFM were elevated 40 and 15%, respectively as compared to the cobalt chloride control group.
Antioxidant enzymes like catalase, SOD, GPx and GR normally reduce the free radical level in the biological system were found to be almost normal level in H2S co-treated group, suggesting the limited pathological impact of CoCl2 on the mitochondrial subpopulations. Fig. 2a shows the enzyme activities of malate dehydrogenase, one of the main regulatory enzymes of TCA, succinate dehydrogenase (fig. 2b) and NADH dehydrogenase (fig. 2c), that measure the partly complex II (measure subunit A) and complex I (measure retention insensitive part) respectively. All enzymes showed near normal activity, provide sufficient data for the functional integrity of mitochondria. There were no significant changes between the subpopulation in their enzymatic activities.
Effect of hydrogen sulfide post-treatment on isolated IFM and SSM
In post-treated groups, cobalt chloride treatment of mitochondrial subpopulations incubated with NaSH for 10 min resulted in the reduction of lipid peroxidation levels in IFM and SSM as compared to CoCl2 induction group. The corresponding GSH levels were elevated 10%, in IFM and 18% in SSM (fig. 1e). Antioxidant enzymes like catalase, SOD, GPx activities were improved to the near control level (fig. 1), suggesting the effectiveness of H2S post treatment. Similarly, mitochondrial enzymes like MDH, SDH and NADH dehydrogenase activities were also significantly improved as compared to the cobalt chloride control group. However, the observed improvements in antioxidant, mitochondrial TCA and ETC enzymes were no as that of mitochondria that were co-treated with H2S.
Discussion
Mitochondria being the source and a target of reactive oxygen species, the direct link between mitochondrial dysfunction and cardiomyocytes oxidative stress has been well established [22]. In this manuscript, we assessed the direct impact of cobalt chlorideinduced oxidative stress on cardiac mitochondrial sub populations viz., interfibrillar and subsarcolemmal mitochondria and found that subsarcolemmal mitochondria is more sensitive towards cobalt chloride-induced oxidative stress, indicating the distinct response of cardiac subpopulations towards chemically induced oxidative stress. In addition, we found that exogenous H2S incubation with mitochondrial subpopulations, preserve the mitochondrial functional activity and the protection was more prominent with H2S co-treatment, rather than pre or post treatments.
Cobalt chloride-induced oxidative stress, increases the formation of reactive oxygen species (ROS) [23] by acting on the mitochondrial transition pore. Lipid peroxidation, the product of ROS, is considered to be one of the primary markers/ indicators to assess oxidative stress. In fact, the imbalances in redox couples such as reduced to oxidized glutathione (GSH/GSSG ratio) or NADPH/NADP+ ratio [24] are the primary reason for the oxidative stress. Fig. 1 shows an elevated TBARS levels in the mitochondrial sub populations and corresponding decline in the reduced glutathione levels in the cobalt chloride treated group, confirming the existence of oxidative stress. In the normal conditions, mitochondria are enriched with antioxidants and antioxidant enzymes, because of its free radical generation capacity and have the potential to counter the free radical release as well. ROS could be reduced by intracellular antioxidant enzymes, including superoxide dismutase, glutathione peroxidase and catalase as well as some nonenzymatic antioxidant molecules like glutathione and vitamin E. However, during pathological conditions, the increased flux of free radicals may not counterpart with the normal antioxidant defense mechanism. Generally, the mitochondrial oxidative stress results in the impairment of mitochondrial functional activities. In the present study the functional activity of the mitochondria was studied by measuring the enzyme activity of malate dehydrogenase, succinate dehydrogenase and NADH dehydrogenase activities. As predicted, the mitochondrial enzyme activities were declined in CoCl2 treated group.
Cardiac mitochondria is heterogenous in nature; exist as interfibrillar and subsarcolemmal mitochondria [15]. SSM is located beneath the sarcolemma in cardiac and skeletal muscle, while IFM are located between the myofibrils in cardiac and skeletal muscle [10]. Functional and morphological difference between the subpopulations was reported. Similarly, oxidative stress experienced by these subpopulations is also distinct. For instance, preferential loss of IFM function with age was also reported [25]. In this study, the cobalt chloride-induced significant decline in the mitochondrial antioxidant capacity and functional enzyme activity in both IFM and SSM. However, the comparison between the subpopulations showed a prominent difference in the sensitivity of IFM and SSM towards oxidative stress, and our results suggest that SSM is more sensitive towards cobalt chloride-induced oxidative stress in in vitro condition. Our observation agrees with the findings of other published work stating that decreased ETC function and elevated oxidative stress have been reported in SSM after myocardial I/R, with no differences observed in IFM.
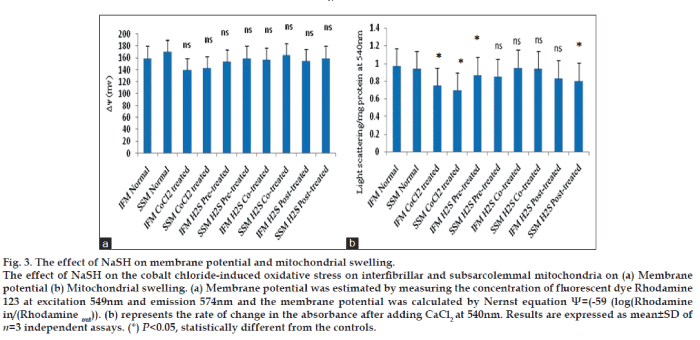
Evidences from the previous study revealed that low concentration of hydrogen sulfide can mediate the stimulatory effect on mitochondria as it can donate electrons to complex II and IV [26]. On the other hand, higher concentration of hydrogen sulfide imparts inhibition on cytochrome C oxidase and thereby contribute to its toxicity [26]. Kimura et al. [27] have shown that subnormal concentration of hydrogen sulfide increase glutathione production and suppresses oxidative stress in mitochondria. In order to study, whether the antioxidant potential reported with H2S is inherent or mediated through other biomolecules, we incubate the isolated IFM and SSM with H2S in three different stages of cobalt chloride treatment, namely, before the treatment (pretreated), along with the treatment (co-treatment) and after the treatment (post-treatment). The oxidative stress measured in terms of TBARS (fig. 1a) and GSH level (fig. 1e) indicates that co-treatment of IFM and SSM with H2S render maximum protection, compared to pre-treated and post-treated mitochondrial sub populations. In agreement with this finding, the antioxidant levels of IFM and SSM were improved to near normal levels. H2S can improve mitochondrial ATP production not only by preserving the mitochondrial membrane, but also reduce metabolic demand by serving as a means for energy supplementation [26]. Moreover, previous studies established that hydrogen sulfide can provide electrons to complex II and IV of mitochondrial electron transport chain. Hence, co-treatment of mitochondria with hydrogen sulfide may preserve the mitochondrial membrane potential, as shown by fig. 3a and thereby reduce the prolonged uncoupling along with associated depolarization [28]. In fact, Co2+ induces mitochondrial swelling (fig. 3b) and in parallel, it also induces the collapse of electrical membrane potential [29].
According to the fig. 3, H2S co-treated IFM and SSM showed minimal resistance to swelling behavior as compared to other experimental groups. In addition, mitochondrial membrane potential was preserved in this group as well.
Financial support and sponsorship
This study was supported by grant from the Department of Science and Technology, New Delhi, India ( No. SR/S0/HS-0255/2012).
Conflicts of interest
There are no conflicts of interest.
References
- Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res 2015;116:531-49.
- Shah AM. Parsing the role of NADPH oxidase enzymes and reactive oxygen species in heart failure. Circulation 2015;131:602-4.
- Kurian GA, Phil M, Paddikkala J. Antioxidant status of South Indian patients undergoing coronary artery bypass graft surgery: A role of intra operative magnesium supplementation. Int J Cardiol 2008;128:139-41.
- Kurian GA, Paddikkala J. N-acetylcysteine and magnesium improve biochemical abnormalities associated with myocardial ischaemic reperfusion in South Indian patients undergoing coronary artery bypass grafting: A comparative analysis. Singapore Med J 2010;51:381-8.
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J BiolChem 2003;278:36027-31.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47-95.
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet 2005;39:359-407.
- Rebrin I, Zicker S, Wedekind KJ, Paetau-Robinson I, Packer L, Sohal RS.Effect of antioxidant-enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free RadicBiol Med 2005;39:549-57.
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J BiolChem 1977;252:8731-9.
- Palmer JW, Tandler B, Hoppel CL. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol 1986;250(5 Pt 2):H741-8.
- Olivieri G, Hess C, Savaskan E, Ly C, Meier F, Baysang G, et al. Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased beta-amyloid secretion. J Pineal Res 2001;31:320-5.
- Garoui el M, Fetoui H, AyadiMakni F, Boudawara T, Zeghal N. Cobalt chloride induces hepatotoxicity in adult rats and their suckling pups. ExpToxicolPathol 2011;63:9-15.
- Salvolini E, Vignini A, Nanetti L, Raffaelli F, Di Primio R, Mazzanti L, et al. Glutamate in vitro effects on human term placental mitochondria.J Matern Fetal Neonatal Med 2012;25:952-6.
- Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. Am J Physiol Heart CircPhysiol 2010;298:H1310-9.
- Kurian GA, Berenshtein E, Saada A, Chevion M. Rat cardiac mitochondrial sub-populations show distinct features of oxidative phosphorylation during ischemia, reperfusion and ischemic preconditioning. Cell PhysiolBiochem 2012;30:83-94.
- Kurian GA, Paddikkala J. Methanol extract of Desmodiumgangeticum DC root mimetic post-conditioning effect in isolated perfused rat heart by stimulating muscarinic receptors. Asian Pac J Trop Med 2012;5:448-54.
- Kurian GA, Paddikkala J. Administration of aqueous extract of Desmodiumgangeticum(L) root protects rat heart against ischemicreperfusion injury induced oxidative stress. Indian J ExpBiol 2009;47:129-35.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J BiolChem 1951;193:265-75.
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 2006;1:3159-65.
- Kurian GA, Berenshtein E, Kakhlon O, Chevion M. Energy status determines the distinct biochemical and physiological behavior of interfibrillar and sub-sarcolemmal mitochondria. BiochemBiophys ResCommun 2012;428:376-82.
- Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, et al. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch 2008;455:607-16.
- Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, et al. Contribution of impaired myocardial insulin signaling tomitochondrial dysfunction and oxidative stress in the heart. Circulation 2009;119:1272-83.
- Saxena S, Shukla D, Saxena S, Khan YA, Singh M, Bansal A, et al. Hypoxia preconditioning by cobalt chloride enhances enduranceperformance and protects skeletal muscles from exercise-induced oxidative damage in rats. ActaPhysiol (Oxf) 2010;200:249-63.
- Yang MS, Chan HW, Yu LC. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology 2006;226:126-30.
- Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free RadicBiol Med 2003;35:1064-72.
- Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, et al. Regulation of mitochondrial bioenergetic function by hydrogensulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 2014;171:2099-122.
- Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 2010;12:1-13.
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F1FOATP synthase is the mitochondrial permeability transition pore. ProcNatlAcadSci U S A 2014;111:10580-5.
- Battaglia V, Compagnone A, Bandino A, Bragadin M, Rossi CA, Zanetti F, et al.Cobalt induces oxidative stress in isolated livermitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int J Biochem Cell Biol 2009;41:586-94.