- Corresponding Author:
- K. K. Srinivasan]
Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, MAHE, Manipal - 576104, India. E-mail: pansrini@yahoo.co.in
| Date of Submission | 18 November 2005 |
| Date of Revision | 7 June 2007 |
| Date of Acceptance | 19 July 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 540-545 |
Abstract
A derivative spectrophotometric procedure has been developed for the simultaneous determination of individual combination of aceclofenac and tramadol with paracetamol in combined tablet preparation. Tablet extracts of the drugs were prepared in distilled water. The zero crossing point technique and the compensation technique were used to estimate the amount of each drug in the combined formulations, and were compared. The results were found to be accurate and free from interferences. The procedure is rapid, simple, nondestructive, and does not require solutions of equations. Calibration graphs are linear (r=0.9999), with a zero intercept up to 24 mg/ml of each drug in combination with paracetamol. Detection limits at the p = 0.05 level of significance were calculated to be 0.5 mg/ml of aceclofenac, tramadol and paracetamol respectively.
Keywords
Derivative spectrophotometry, aceclofenac, tramadol, paracetamol
Derivative spectrophotometry has only recently become a practical analytical method in the general laboratory because of the rapid progress in microcomputers technology. This technique, if properly understood and applied, it will be a valuable tool for problem solving in several areas of analytical chemistry. It can lead to quicker and more accurate quantitation of multicomponent mixtures that previously would have required, e.g., separation by HPLC. In recent years; derivative spectrophotometry has received increasing attention with regard to the assay of drugs in their formulation and in systems of clinical and biological interest. The fundamental principles and convention of derivative spectrophotometry have been described in the works of O’Haver et al. and Fell and Smith [1,2]. The zero crossing technique has found practical application more recently and has become the most often used procedure to resolve binary mixtures by spectrophotometry. Several papers have been published on zero-crossing technique using various orders of derivative spectrophotometry [3-6].
Aceclofenac is an orally administered non-steroidal antiinflammatory drug [7,8], which possesses good analgesic properties and good tolerability profile in a variety of painful conditions. Chemically aceclofenac is 2-[[2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl] oxy]acetic acid. Several methods have been reported for the assay of aceclofenac [9-11].
Tramadol is an orally administered non-steroidal antiinflammatory drug [12,13], which possesses good analgesic properties and good tolerability profile in a variety of painful conditions. The chemical name for tramadol hydrochloride is (±) cis- 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl cyclohexanol hydrochloride. Several methods have been reported for the assay of tramadol [14-16].
Chemically paracetamol is N-(4- hydroxyphenyl)acetamide. Paracetamol exhibits antiinflammatory, analgesic and antipyretic activities, which are due to the inhibition of cyclooxygenase–2 (COX-2). Literature survey revealed that there are UV and HPLC methods reported for the estimation of paracetamol in pharmaceutical formulations [17-20].
The review of the literature revealed that no method is yet reported for the simultaneous estimation of aceclofenac and tramadol in individual combination with paracetamol in combined dosage forms. This paper describes simple, rapid, accurate, reproducible and economical methods for the simultaneous estimation of individual combination of aceclofenac and tramadol with paracetamol in tablet formulations using second derivative zero crossing point technique.
Materials and Methods
All spectrophotometric measurements were made using a Shimadzu UV/Vis spectrophotometer 1601 model with a spectral bandwidth (resolution) of 0.1 nm and wavelength accuracy (with automatic wavelength correction) of 0.5 nm. An ultrasonicator was used for proper dissolution of the samples.
Analytical procedure
Suitable volumes of stock solution, containing up to 24 μg/ml of paracetamol and aceclofenac, were placed in a 10 ml calibrated flask and brought to volume with distilled water. Then the second derivative spectrum of the mixture against water was recorded and values of the derivatives were measured at 279.4 and 289.9 nm for aceclofenac and 259.7 and 295.8 nm for paracetamol, respectively. Similarly suitable volumes of stock solution containing up to 24 μg/ml of paracetamol and tramadol, were placed in a 10 ml calibrated flask and made up to volume with distilled water. Then the second derivative spectrum of the mixture against water was recorded and values of the derivatives were measured at 265.1 nm for paracetamol and 279.1 nm for tramadol, respectively.
Analysis of tablet formulation
Twenty tablets were weighed accurately, average weight was determined and then all the 20 tablets were ground to a fine powder. A quantity equivalent to 100 mg of aceclofenac and 500 mg of paracetamol were transferred to a 100 ml volumetric flask. The contents were ultrasonicated for 10 min with distilled water, made to volume and filtered through Whatmann filter paper. The solution was further diluted with distilled water, to give concentrations of 4 and 20 μg/ml of aceclofenac and paracetamol, respectively. Second derivative absorbances of these solutions were measured at 279.4 and 289.4 nm for aceclofenac (A) and 259.7 and 295.8 nm for paracetamol (B). Similarly, quantity equivalent to 100 mg of tramadol and 500 mg of paracetamol were transferred to a 100 ml volumetric flask, ultrasonicated for 10 min with distilled water, made to volume and filtered through Whatmann filter paper. The solution was further diluted with distilled water to give concentrations of 2 and 10 μg/ml of tramadol and paracetamol, respectively. Second derivative absorbances of these solutions were measured at 279.1 nm for tramadol (B) and 265.1 nm for paracetamol (A).
Results and Discussion
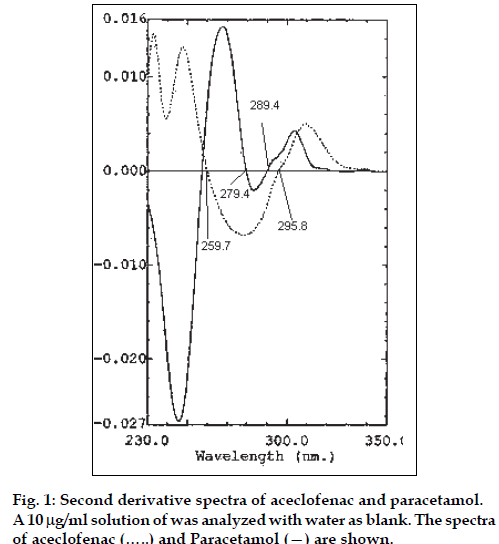
The zero absorption spectra of aceclofenac and paracetamol (10 μg/ml each) show significant differences in the absorption values at similar concentration, hence the traditional Vierordt’s and modified Vierordt’s methods for the assay of binary mixtures gave erroneous results. Several tests were made to select the more suitable order of the derivative, the type of measurement, i.e., graphical or zero-crossing measurements, and the working wavelength exhibiting the best linear response to analyte concentration and higher sensitivity, and while not being affected by any other components. The first derivative spectra of both showed considerable differences in certain areas; which prevents, in the present instance, suitable use of this technique. The corresponding second derivative spectra of aceclofenac and paracetamol are represented in fig. 1. On the contrary zero-crossing second derivative spectrophotometry offers an extremely valuable means of simultaneously determining both the drugs in a mixture.
The second derivative spectra of a mixture of aceclofenac and paracetamol were recorded against water and the values of derivative were measured at 279.4 and 289.4 nm for aceclofenac (zero-crossing wavelength of second derivative of paracetamol) and 259.7 and 295.8 nm (zero-crossing wavelength of second derivative of aceclofenac) for paracetamol and the concentration of aceclofenac and paracetamol was calculated from the calibration graphs. Fig. 2. depicts a typical set of second derivative spectra of a laboratory mixture containing 4 μg/ml of aceclofenac and increasing concentrations of paracetamol ranging from 10-24 μg/ml. Fig. 3. exhibits a typical set of second derivative spectra of a laboratory mixture containing 20 μg/ml of paracetamol and increasing concentrations of aceclofenac ranging from 4-10 μg/ml.
The height of peaks at 279.4 and 289.4 nm, the zero crossing wavelengths of paracetamol, was denoted as h2 (fig. 2), and the height of peaks at 259.7 and 295.8 nm, the zero crossing wavelengths of aceclofenac, was denoted h1 (fig. 3). These heights, h1 and h2 were proportional to aceclofenac and paracetamol concentrations, respectively. Moreover, the values of h1 and h2 were not affected by the presence of other excipients present in the tablet formulation. An interaction study was performed, and the results indicated that when one component is kept constant (2 μg/ml) and the concentration of the other is varied, the H1 (at 279.4 nm) and H2 (at 259.7 nm) values are unaltered up to 30 μg/ml of the second component. Hence, accurate quantitation of the two drugs was achieved even when the ratio of concentration was 1:15.
It is also interesting to note there are two distinct isobestic points each in fig. 2. (at 279.4 and 289.4 nm zero crossing wavelengths of paracetamol) as well as in fig. 3 (at 259.7 and 295.8 nm zerocrossing wavelength of aceclofenac), irrespective of the concentration of paracetamol and aceclofenac, respectively.
The second derivative spectra of tramadol and paracetamol are represented in fig. 4. The values of derivative were measured at 279.1 nm for tramadol (zero-crossing wavelength of second derivative of paracetamol) and 265.1 nm (zero-crossing wavelength of second derivative of tramadol) for paracetamol. Then, the concentrations of tramadol and paracetamol were calculated from the calibration graphs. Fig. 5. shows a typical set of second derivative spectra of a laboratory mixture of 5 μg/ml of tramadol and increasing concentrations of paracetamol ranging from 10-24 μg/ml. Similarly fig. 6. shows a typical set of second derivative spectra of a laboratory mixture of 20 μg/ml of paracetamol and increasing concentrations of tramadol ranging from 4-24 μg/ml.
The height of peak at 279.1 nm, the zero crossing wavelength of paracetamol, was denoted as h2 (fig. 6), and the height at 265.1 nm, the zero crossing wavelength of tramadol, was denoted h1 (fig. 5). These heights, h2 and h1 were proportional to tramadol and paracetamol concentrations, respectively. Moreover, the values of h1 and h2 were not affected by the presence of other excipients present in tablet formulation. An interaction study was performed, and the results indicate that when one component is kept constant (2 μg/ml) and the concentration of the other is varied, the H1 (at 265.1 nm) and H2 (at 279.1 nm) values are unaltered up to 30 μg/ml of the second component. Hence, accurate quantitation of the two drugs was achieved even when the ratio of concentration was 1:15.
It is also interesting to note distinct isobestic points in fig. 5. at 279.1 (zero crossing wavelengths of paracetamol) and in fig. 6. at 265.1 nm (zero crossing wavelength of tramadol) irrespective of the concentration of paracetamol and tramadol, respectively.
The linear regression equations calculated for individual mixtures of aceclofenac and tramadol with paracetamol are assembled in Tables 1 and 2 together with the correlation coefficients, the variances, and the detection limits at a level of significance of p=0.05 for 11 standard samples. Beer’s law is followed for concentrations up to 24 μg/ml of each drug. Two different tests of significance of the intercepts of line of regression (H= a+bc) were performed to establish whether the experimental intercept ‘a’ differed significantly from the theoretical value, zero. These tests are very useful in the case of mixtures to verify if the analytical method is free from procedural errors depending on the concentrations of one of the two components. The first procedure to estimate the difference ‘a–0’ follows from the determination of the quantities ‘t= a/Sa’ (Sa’ is an estimate of the accuracy of the determination of ‘a’) and their comparison with the tabular data for t-distribution. The values calculated for ‘t’ are for aceclofenac and for paracetamol (i.e., they do not exceed the 95% criterion of tp= 2.26 for n= 11 samples); this indicates that the intercepts of line of regression are not significantly different from zero.
| Regression equations | λ (nm) | r | SD, Intercept, Slope, Sb |
LOD µg/ml | LOQ µg/ml | t=a/Sa | |
|---|---|---|---|---|---|---|---|
| A | D2'A=4.25x10-4+5.34x10-4 CA | 259.7 | 0.9997 | 1.13x10-5, 3.43x10-7 | 0.32 | 1.06 | 1.03 |
| A | D2'A=1.00x10-4+1.46x10-4 CA | 295.8 | 0.9994 | 1.28x10-5, 4.12x10-7 | 0.37 | 1.23 | 0.96 |
| B | D2'B=7.99x10-5-6.41x10-4 CB | 279.4 | 0.9989 | 5.95x10-5, 2.83x10-6 | 0.47 | 1.56 | 0.88 |
| B | D2'B=-1.43x10-4-3.22x10-4 CB | 289.9 | 0.9999 | 5.37x10-5, 3.36x10-6 | 0.52 | 1.73 | 0.85 |
A is paracetamol, B represents aceclofenac, CA and CB denote concentrations of drugs (μg/ml). Number of samples n=10, aTheoretical value of t at P= 0.05 level of significance is 2.31
Table 1: Statistical data for the calibration graphs of paracetamol and aceclofenac zero crossing derivative spectrophotometry.
| Regression equations | λ (nm) | r | SD Intercept, Sa Slope, Sb | LOD µg/ml | LOQ µg/ml | t=a/Sa | |
|---|---|---|---|---|---|---|---|
| A | D2'A=-0.12x10-1+0.132x10-1CA | 265.1 | 0.9997 | 1.14x10-4, 2.43x10-6 | 0.29 | 1.16 | 1.02 |
| B | D2'B= 6.32x10-3-7.1x10-3CB | 279.1 | 0.9999 | 4.27x10-3, 2.36x10-5 | 0.42 | 1.63 | 0.82 |
A is paracetamol, B represents tramadol. CA and CB denote concentrations of drugs (μg/ml). Number of samples, n= 10. aTheoretical value of t at P= 0.05 level of significance is 2.31
Table 2: Statistical data for the calibration graphs of tramadol and paracetamol by zero crossing derivative spectrophotometry
To study accuracy, reproducibility, and precision of the proposed methods, five successive determinations on synthetic mixtures of paracetamol and aceclofenac were carried out. The results reported in Tables 3 and 4 show that accuracy and precision are very satisfactory. The complex problem of quantitating components of above mixture with widely differing absorption values could be solved. The conceptual and experimental straightforwardness of the proposed second derivative method vouches for its suitability for the routine analysis of pharmaceutical dosage forms. The method described is simple and confirms that the technique of derivative spectrophotometry, if properly used, can give precise, sensitive, and rapid analysis of mixtures of drugs. In the proposed method, the values of coefficient of variation were satisfactorily low and recovery was close to 100% for both the drugs. Hence, it can be employed for routine analysis in quality control laboratories
| Mixture | Nominal value µg/ml |
Mean value±SD µg/mla |
RSD % |
|---|---|---|---|
| Paracetamol and aceclofenac | 20.0 | 19.89±0.034 | 0.18 |
| 4.5 | 4.46±0.052 | 0.16 | |
| Paracetamol and aceclofenac | 10.0 | 9.94±0.024 | 0.13 |
| 18.0 | 18.02±0.031 | 0.12 | |
| Paracetamol and tramadol | 20.0 | 19.79±0.024 | 0.16 |
| 4.5 | 4.49±0.032 | 0.15 | |
| Paracetamol and tramadol | 10.0 | 9.98±0.025 | 0.12 |
| 18.0 | 18.03±0.031 | 0.13 |
aMean of five determinations
Table 3: Replicate determinations on individual synthetic mixtures of aceclofenac. and tramadol with paracetamol
| Combined tablet dosage form | Label claim, mg | Mean recovery ±SD, %b |
|---|---|---|
| Paracetamol and aceclofenac | 500.0 | 99.96±0.042 |
| 100.0 | 99.86±0.067 | |
| Paracetamol and aceclofenac | 500.0 | 101.04±0.012 |
| 100.0 | 100.12±0.061 | |
| Paracetamol and tramadol | 500.0 | 99.98±0.032 |
| 100.0 | 99.96±0.077 | |
| Paracetamol and acelofenac | 500.0 | 100.04±0.016 |
| 100.0 | 100.15±0.051 |
bMean of five determinations, assay as percentage of label claim.
Table 4: assay of aceclofenac and tramadol with paracetamol in combined tablet dosage form.
Acknowledgements
Authors are thankful to Indoco Remedies, Mumbai for the gift samples of drugs.
References
- Fell AF, Smith G. Principles of Derivative Spectrophotometry. Anal Proc 1982;19:28-32.
- O'Haver TC, Green GL. Derivative Spectrophotometry and its Applications. Anal Chem 1976;48:312-5.
- Morelli B. Determination of a Quaternary Mixture of Vitamins B6, B1, and B12 by Derivative Spectrophotometry. J Pharm Sci 1995;84:34-7. [PUBMED]
- Morelli B. Determination of Anafranil in Pharmaceuticals by Difference and Difference-Derivative Spectrophotometry. Anal Lett 1996;29:1551-5.
- Morelli B. Determination of Noramidopyrine Methane Sulfonate Sodium Salt, Pitophenone Hydrochloride and Fenpiverine Bromide in Pharmaceuticals by Ratio-Spectra Zero-Crossing Derivative Spectrophotometry. Anal Lett 1998;31:2431-5.
- Morelli B. Determination of Mixtures of Cephacetrile and Ceftezole in a Pure form and Injections, by 'Zero- Crossing' Derivative Spectrophotometry. Anal Lett 1988;21:43-6.
- Brogden RN, Wiseman LR. New non-steroridal anti-inflammatory agent. Drugs 1996;52:113-5.
- Grau M, Grauch JL, Montero A, Felipe A, Julia S. Pharmacology of the potent new non-steroridal anti-inflammatory agent aceclofenac. ArzneimForsch 1991;41:1265-76.
- Lee HS, Choi SJ, Jeong CK. Simultaneous determination of aceclofenac and diclofenac in human plasma by narrow bore HPLC using Column-switching. J Pharm Biomed Anal 2000;23:775-81.
- Posac JR, Vazquez MD, Tascon ML, Acuna JA, Dela F, Fuente C, et al . Determination of Aceclofenac Using Adsorptive Stripping Voltammetric Techniques on Conventional and Chemically Modified Carbon Paste Electrodes. Talanta 1995;42:293-304.
- Zawilla NH, Abdul AM, El-Kousy NM, El-Moghazy AS. Determination of Aceclofenac in Bulk and Pharmaceutical Formulations. J Pharm Biomed Anal 2002;27:243-51.
- Gutstein HG, AkilH.. Opioid analgesics. In : Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gillman's The Pharmacological basis of Therapeutics. 10 th ed. McGraw-Hill: London; 2001. p. 569-620.
- Rang HP, Dale MM, Ritter JM, Moore RK, editors. Antiinflammatory Agents: Rang and Dale's Pharmacology. 5 th ed. Church Livingstone: London; 2003. p. 580-5.
- Meyyanathan SN, Pradeep K, Suresh B. Analysis of Tramadol in Pharmaceutical Preparations by High Performance Thin Layer Chromatography. J Separation Sci 2003;26:1359-62.
- Gan SH, Ismail R. Validation of a High Performance Liquid Chromatography Method for Tramadol and O-desmethyltramadol in Human Plasma using Solid-phase Extraction. J Chromatogr B Biomed SciAppl 2001;759:325-35.
- Ho ST, Wan JJ, Liaw WJ, Ho CM, Li JH. Determination of tramadol by capillary gas Chromatography with flame ionization detection. Application to human and rabbit pharmacokinetic studies. J Chromatogr B Biomed SciAppl 1999;736:89-96.
- Ravisankar S, Vasudevan M, Nanjan MJ, Biju K, Suresh B. Reverse Phase HPLC Method for the Estimation of Paracetamol, Chlorzoxazone and Diclofenac Sodium in Formulations. Indian Drugs 1997;34:663-65.
- Erk N, Ozkar Y, Banoglu E, Ozkan SA, Senturk Z. Simultaneous Determination of Paracetamol and Mthocarbamol in Tablets by Ratio Spectra Derivative Spectrophotometry and LC. Pharm Biomed Anal 2001;24:469-75.
- Monser L, Darghouth F. Simultaneous LC Determination of Paracetamol and Related Compounds in Pharmaceutical Formulations using a Carbon-based Column. J Pharm Biomed Anal 2002;27:851-60.
- Kartel M. LC Method for the Analysis of Paracetamol, Caffeine and Codeine Phosphate in Pharmaceutical Preparations. J Pharm Biomed Anal 2001;26:857-64.