- *Corresponding Author:
- lakshmi S
Department of Pharmaceutical Analysis, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur - 603 203, India
E-mail: lakshmiss@hotmail.com
| Date of Received : | 21 July 2005 |
| Date of Revised : | 24 March 2007 |
| Date of Accepted : | 2 October 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 674-676 |
Abstract
The present work describes a simple reverse phase HPLC method for the determination of omeprazole and domperidone from tablet formulations. The determination was carried out on a Hypersil, ODS, C-18 (150×4.6 mm, 5 micron) column using a mobile phase of methanol:0.1 M ammonium acetate (pH 4.9) (60:40). The flow rate and runtime were 1 ml/min and 10 min, respectively. The eluent was monitored at 280 nm. The method was reproducible, with good resolution between omeprazole and domperidone. The detector response was found to be linear in the concentration range of 10-60 µg/ml for omeprazole and 5-30 µg/ml for domperidone.
Omeprazole is chemically (RS)-5-methoxy-2-[[(4- methoxy-3,5-dimethylpyridin-2yl) methyl] sulphinyl]- 1H-benzimidazole. In pharmaceutical preparations, the compound is used as a proton pump inhibitor in the treatment of peptic ulcer [1,2]. Domperidone is chemically 5-chloro-1-[1-[3-2,3-dihydro-2-oxo-1Hbenzimidazol- 1-yl)propyl]-piperidin-4-yl]-2,3-dihydro- 1H-imidazol-2-one and is used as an antiemetic [3,4]. There have been numerous publications describing various methods for the quantification of these compounds individually or in combination with other drugs. Recently omeprazole has been successfully quantified in formulation by high performance liquid chromatography with coulometric detection [5]. HPLC using solid phase extraction was reported for the analysis of omeprazole and its metabolites in human plasma [6]. (RP)-ion pair HPLC method was utilized successfully in the separation of domperidone and cinnarazine in pharmaceutical preparations [7]. Whilst all of the above listed procedures have been successfully validated and applied in routine analysis, none of them addresses simultaneous quantification of both the components in one step. The present paper describes the development of RP-HPLC method using isocratic mobile phase that offers certain advantages in its simplicity and time saving.
Standard samples of omeprazole and domperidone, which were prepared from reference standard procured from a pharmaceutical company (Sipra Laboratories Ltd, Hyderabad). HPLC grade methanol manufactured by E. Merck was procured from commercial sources. Double distilled water was prepared in the laboratory. Tablet formulations, Domstal–O, Domstal–RD (Torrent laboratories, Ahmedabad) and Domril–O (Monokem Laboratories, Ahmedabad) containing both omeprazole and domperidone were obtained from local market.
A Shimadzu HPLC (Kyoto, Japan) system was used coupled with SPD 10A UV detector. Separations were carried out on a Hypersil® BDS C18 column (250×4.6 mm I.D) packed with 5 µ particle size as the stationary phase. The mobile phase consisting of methanol and ammonium acetate buffer (60:40) was pumped at a flow rate 1 ml per min, the detection was monitored at 280 nm and the run time was 10 min.
Omeprazole and domperidone (50 mg each) were weighed accurately in two 100 ml volumetric flasks separately and both standards were dissolved in about 30 ml of solvent solution (60 volumes of water and 40 volumes of methanol). The volume was made up to 100 ml with solvent solution (stock solution). In case of omeprazole varying amounts (1, 2, 3, 4, 5 and 6 ml) of the above solution (500 µg/ml) was taken in six different 50 ml volumetric flasks and the volume was made upto the mark with the solvent solution. An aliquot of 20 μl of the solution from each flask was injected two times. In case of domperidone 10 ml was taken from stock solution (500 µg/ml) and diluted to 100 ml with the solvent solution (50 µg/ml). Varying amounts (1, 2, 3, 4, 5 and 6 ml) of the above solution (50 µg/ml) was taken in six different 10 ml volumetric flasks and the volume was made upto the mark with the solvent solution. An aliquot of 20 μl of the solution from each flask was injected two times. Calibration curves were constructed by plotting mean peak areas against the corresponding drug concentrations. The detector response was found to be linear in the concentration range of 10-60 µg/ml for omeprazole and 5-30 µg/ml for domperidone.
Twenty tablets were powdered finely. A quantity equivalent to two tablets was transferred to a 100 ml volumetric flask and 30 ml of solvent solution was added. The flask was shaken for 15 min and then contents were diluted to 100 ml and filtered through Whatman No.1 filter paper. One ml of this solution was then diluted to 10 ml with solvent solution. Results of the triplicate analysis are given in Table 1.
| Formulation | Label content (mg/tablet) | Mean amount Found (mg/tablet) | Mean % drug | Standard deviation found |
|---|---|---|---|---|
| Omeprazole | ||||
| Brand 1 | 10 | 10.04 | 100.4 | 0.56 |
| Brand 2 | 20 | 20.12 | 100.7 | 0.57 |
| Brand 3 | 20 | 19.97 | 99.8 | 0.62 |
| Domperidone | ||||
| Brand 1 | 10 | 9.96 | 99.6 | 0.63 |
| Brand 2 | 10 | 9.94 | 99.4 | 0.55 |
| Brand 3 | 10 | 10.13 | 101.3 | 0.62 |
Table 1: Analysis of Tablets Containing Omeprazole and Domperidone.
This method was validated for statistical parameters i.e. precision, accuracy, specificity, linearity and range, stability of analytical solutions and ruggedness criteria. Results of the method validation experiments are given in Table 2. The precision of the method was determined by knowing percentage RSD of means of three replicate solutions of all the three independent samples.
| Performance parameters | Results | Acceptance limit | |
|---|---|---|---|
| Precision | Omeprazole | 1.94% | NMT 2.0% RSD |
| Domperidone | 1.96% | ||
| Accuracy | Omeprazole | 3.15% | % Bias NMT 5% |
| Domperidone | 2.13% | ||
| Linearity (Regression Coefficient - r) | Omeprazole | Linear (r=0.996) | Linear NLT 0.995% |
| Domperidone | Linear (r=0.998) | ||
| Stability of analytical solutions (Normal Conditions) | Omeprazole | 0.88% | NMT 2.0% RSD |
| Domperidone | 0.95% | ||
| Stability of analytical solutions (in a dark refrigerator) | Omeprazole | 0.65% | NMT 2.0% RSD |
| Domperidone | 0.76% | ||
| Ruggedness | Omeprazole | 0.56% | NMT 2.0% RSD |
| Domperidone | 0.63% |
Table 2: Results of Method Validation Experiments of Omeprazole and Domperidone.
The accuracy of method is determined by adding known amount of standard to that of sample (above and below the normal level) at 3 different levels to cover both above and below (75 to 125%) the normal levels expected in the sample. The normal expected level for the assay of omeprazole and domperidone is about 20 µg/ml. So the study range was 15, 20 and 25 µg/ml for both.
The linearity of analytical method was studied by analyzing response of standard with predetermined concentration range, linearity curve was plotted for response areas against the concentration of the solution. Regression coefficient was calculated using above plot. For omeprazole, prepared solutions were within concentration range of 10 to 50 µg/ml at 5 constant consecutive concentration levels i.e. 10, 20, 30, 40 and 50 µg/ml. For domperidone, prepared solutions were within concentration range of 5 to 30 µg/ml at constant consecutive concentration levels i.e. 5, 10, 15, 20, 25 and 30 µg/ml. The regression coefficient of area of above consecutive concentrations was calculated.
The stability of analytical solutions of the method studied by a series of samples and standards were prepared and analysed immediately. They were stored at normal lab conditions and in a dark refrigerator, then reanalyzed 120 h later against freshly prepared standard solutions. The ruggedness of analytical method for omeprazole and domperidone in assay determination was studied by analyzing the samples by two sets. (i.e. different analyst, different reagents and solutions and different days).
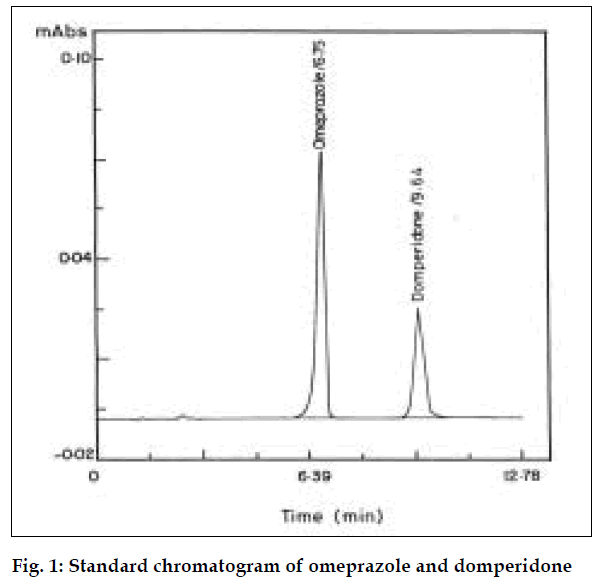
A typical chromatogram obtained in the present investigation is shown in fig. 1. The results obtained were summarized in Table 1. Prior to the analysis, the method was subjected to system suitability tests.
The resolution factor was found to be 6.55, which indicated that there is good resolution between omeprazole and domperidone. This method is highly sensitive to estimate omeprazole and domperidone in tablet formulations.
The statistical parameters in method validation studies for precision, accuracy, specificity, stability of analytical solutions and ruggedness were justified the validity of the proposed method. The results of the assay and method validation studies given in Tables 1 and 2 have shown that the method is simple, accurate and precise and non-interference from tablet excipients.
Acknowledgements
The authors are thankful to CEAL Labs, Chennai for providing facilities.
References
- Indian Pharmacopoeia, Vol. 1, The Controller of Publications, Delhi, 1996, 532.
- The United States Pharmacopoeia, Vol. XXIV, Supplement 7, The U.S. Pharmacopoeia Convention, Inc. Rockville, MD, 2000.
- British Pharmacopoeia, Vol.1, The British Pharmacopoeia Commission, London, 2001.
- Reynolds, J.E.F., Eds., In; Martindale: The Extra Pharmacopoeia, 33 rd Edn., The Pharmaceutical Press, London, 2002.
- Sluggett, G.W., Stong, J.D., Adams, J.H and Zhao, Z., J. Pharm. Biomed. Anal., 2001, 25.
- Motevalian, M., Saeedi, G., Keyhanfar, F., Teyebi, L and Mahmoudian, M., Pharm. Pharmacol. Commun, 1983, 278, 311.
- Argekar, A.P and Shah, S.J., J. Pharm. Biomed. Anal., 1999,19, 813.
- Reviewer Guidance: Validation of Chromatographic Methods, Center for Drug Evaluation and Research (CDER), PDA, Incorporation Publication Service, 1994.