- *Corresponding Author:
- Nandini Pai
D. G. Ruparel College, Mahim, Mumbai-400 016, India
E-mail: desai70@yahoo.com
| Date of Submission | 24 November 2005 |
| Date of Revision | 15 May 2006 |
| Date of Acceptance | 9 February 2007 |
| Indian J Pharm Sci 2007, 69 (1): 118-120 |
Abstract
A simple, precise, fast and selective HPLC method has been developed for the simultaneous estimation of lamivudine, zidovudine and nevirapine from tablets by external standard method. The analytes were resolved by using mobile phase of 50:50 mixture of methanol:buffer (0.1 M ammonium acetate in 0.5% glacial acetic acid) on an Inertsil ODS 3V (250 × 4.6 mm, 5 µ) column as a stationary phase and UV 270 nm as detection wavelength.
Lamivudine, ([2R-cis]-4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone) [1-6], nevirapine, (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyridol-[3,2-b:2’3'-e][1,4]-diazepin-6-one) [2,3] and zidovudine, (3’-azido-3’-deoxythymidine) [4,5,7,8] are well established anti HIV agents [1,2]. Lamivudine is official in IP [1] and USP [6]. Nevirapine is official in IP [1,3]. Zidovudine is official in IP [4,5], BP [9] and EP [7]. For the treatment of AIDS, most antiviral agents are used as part of combination therapy. Since there are several generic combination products available in the worldwide market for polytherapy, an assay method for the estimation of these drugs simultaneously is of utility. No HPLC method has so far been reported for the simultaneous determination of these drugs in pharmaceutical preparations. This report presents an HPLC assay for the simultaneous estimation of lamivudine, zidovudine and nevirapine from tablets.
All the solvents used were of HPLC grade and all reagents used were of AR grade unless otherwise specified. Standard stock solutions were prepared by dissolving 30 mg of lamivudine, 40 mg of nevirapine and 60 mg of zidovudine in 50 ml of mobile phase. Further dilutions were made in mobile phase to obtain a final concentration of the analytes as lamivudine (150 μg/ml), nevirapine (200 μg/ml) and zidovudine (300 μg/ml). A Shimadzu Class 2010A HPLC equipped with auto sampler having a variable volume (1-200 μl) injector was used for the assay. The column used was Inertsil C18-ODS 3V, (250×4.6 mm, 5 μ). The mobile phase was a 50:50 mixture of methanol : buffer (0.1 M ammonium acetate in 0.5% glacial acetic acid). The mobile phase was filtered through 0.45 μ membrane filter and degassed by sonication prior to use. The volume of injection was 20 μl. The elution was carried out isocratically at the flow rate of 1.0 ml/min, at ambient temperature. Detection was performed with an UV detector set at 270 nm.
System suitability was carried out as per USP 25 on freshly prepared standard solution and the parameters were obtained using the above chromatographic conditions. Specificity of the method was established by injecting all the excipients used for the tablet manufacturing. There was no interference of the placebo with the principal peaks and hence the method was established to be specific for the determination of these three drugs.
Six different concentrations of a mixture of lamivudine, nevirapine and zidovudine were prepared for linearity studies and chromatographed (n=6). The responses were measured as peak areas. The calibration curve obtained by plotting peak areas against concentration showed linearity in the concentration range of 75 to 225 μg/ml of lamivudine, 100 to 300 μg/ml for nevirapine and 150 to 450 μg/ml for zidovudine.
For the estimation of lamivudine, nevirapine and zidovudine from tablets, twenty tablets (Duovir-N, Cipla Ltd.) were powdered. Sample powder equivalent to 150 mg of lamivudine was transferred into a 250 ml volumetric flask. About, 200 ml of mobile phase was added to this and was sonicated for about 15 min. This solution was allowed to equilibrate to room temperature and diluted to the mark with mobile phase. The solution was mixed thoroughly and filtered through a Whatman filter paper no. 41. Following this, 5 ml of this filtered solution was diluted to 20 ml with mobile phase and a suitable aliquot injected onto the HPLC.
The precision of the method was established by carrying out (n=6) analysis of the analyte using the proposed method. The low value of standard deviation showed that the method is precise. The results obtained are shown in Table 1. In replicate analysis (n=6), the average content of lamivudine, zidovudine and nevirapine per tablet as determined by the proposed method are 150 mg, 299 mg and 200 mg respectively. The accuracy of experiment was established using recovery technique i.e., by external standard addition method. A known amount of standard was added at three different levels to the placebo (excipients) of sample. Each determination was performed in triplicate. The results of recovery analysis are presented in Table 1. The mean recovery is well within the acceptance limit hence the method is accurate. No change in assay values were observed indicating stability of drug in the solvent used during analysis when evaluated over a time period of 24 h.
| Sr. No. | Zidovudine (300 mg/tablet) | Lamivudine (150 mg/tablet) | ||
|---|---|---|---|---|
| Assay | % Recovery | Assay | % Recovery | |
| 1 | 297.0 | 100.7 | 148.9 | 100.5 |
| 2 | 302.4 | 100.6 | 151.6 | 100.5 |
| 3 | 301.5 | 100.1 | 151.3 | 99.9 |
| 4 | 298.2 | - | 149.4 | - |
| 5 | 298.5 | - | 149.6 | - |
| 6 | 297.2 | - | 149.2 | - |
| Mean | 299.1 | 100.5 | 150.0 | 100.2 |
| %RSD | 0.77 | - | 0.78 | - |
Table 1: Precision And Recovery Analysis
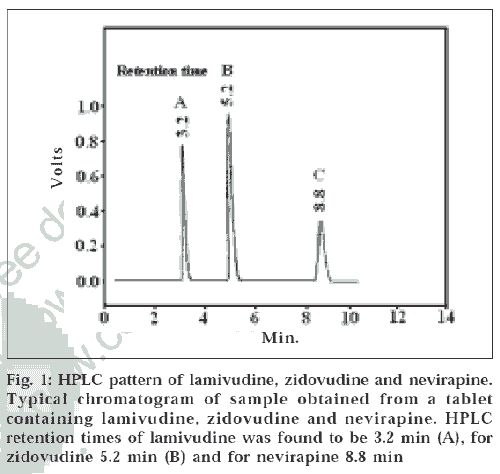
The proposed method gives good resolution between lamivudine, nevirapine and zidovudine (fig. 1). The method used is very simple and rapid and does not involve the use of complex - instruments, sample preparation or mobile phase preparation. High percentage of recovery as shown in Table 1 shows that the method is accurate. The stability data on the drugs carried out by this method shows that the stock solutions are stable for a longer time than that taken for analysis. The linearity, precision, accuracy, ruggedness of the method proves that the method is easily reproducible in any quality control setup.
References
- Indian Pharmacopoeia - Addendum, The Controller of Publications, New Delhi, 2002, 913.
- Indian Pharmacopoeia - Addendum, The Controller of Publications, New Delhi, 2002, 919.
- Indian Pharmacopoeia - Addendum, The Controller of Publications, New Delhi, 2002, 920.
- Indian Pharmacopoeia - Addendum, The Controller of Publications, New Delhi, 2002, 936.
- Indian Pharmacopoeia - Addendum, The Controller of Publications, New Delhi, 2002, 938.
- The United state pharmacopoeia, convention Inc., Twin brook Parkway, Rockville, 2003, 26, 1055.
- European Pharmacopoeia, 4th Edn.Council of Europe, Strasbourg, 2002, 2163.
- The Merck Index, 13th Edn., Merck & Co., Inc., Whitehouse Station. NJ, 2001, 958.
- British Pharmacopoeia, Vol. II, Her Majesty's Stationary Office, London, 2003, 1963.