- *Corresponding Author:
- Vandana B. Patel
Quality Assurance Laboratory, Pharmacy Department, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda, Vadodara, India
E-mail: vbpatel04@yahoo.com
| Date of Submission | 28 May 2005 |
| Date of Revision | 21 March 2006 |
| Date of Acceptance | 10 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 764-767 |
Abstract
Rapid, precise, accurate, and specific ratiospectra derivative spectrophotometry and a simple UV spectrophotometry using simultaneous equation methods are developed for the simultaneous determination of bisoprolol fumarate and hydrochlorothiazide from combined pharmaceutical dosage forms. For ratiospectra derivative spectrophotometry, the amplitudes were measured at 202.6 nm for bisoprolol fumarate and the difference in amplitudes at 212.6 and 230 nm were measured for the determination of hydrochlorothiazide. In the simultaneous equation method, the signals were measured at 223 nm and 271.6 nm. Concentration of each drug was obtained by using the absorptivity values calculated for the drugs at two wavelengths, 223 and 271.6 nm. Commercial tablet formulations were successfully analyzed using the developed methods.
The binary mixture of bisoprolol fumarate (BF) [1],1-[4-[[2-(1-methylethoxy)ethoxy]methyl] phenoxy]-3-[(1-methylethyl) amino]-2-propanolethylene-1,2-dicarboxylic acid; and hydrochlorothiazide (HCTZ) [2],6-chloro-3,4-dihydro-2H- 1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide,were widely used as antihypertensive drugs. The literature reports many analytical methods for the quantitative determination of HCTZ alone or in combination with other anti hypertensive drugs including spectroscopic and chromatographic methods [3–9]. However, there is no evidence in literature for simultaneous determination of HCTZ and BF. Hence, in the present investigation ratiospectra derivative spectrophotometry and simultaneous equation method was developed for the simultaneous determination of BF and HCTZ from their combined dosage form.
Materials and Methods
Spectrophotometric analysis was carried out on a Shimadzu 1601 double beam spectrophotometer with a fixed slit width (2 nm). The system software of the instrument was used for obtaining the ratiospectra and tracing the 1st derivative of the ratiospectra. Pure drug samples of BF and HCTZ were kindly gifted by M/s Sun Pharma Advanced Research Center, Vadodara. Doubledistilled water (laboratory made) and methanol analytical reagent grade (Allied Chemical Corporation, Vadodara) were used as solvent in this work. Commercial pharmaceutical preparation (Lodoz 5® Merck, Mumbai, Bisocar HT 2.5®, Rusan HC, Mumbai) was procured from commercial source.
Preparation of standard stock solutions of BF and HCTZ
About 50 mg each of HCTZ and BF were accurately weighed and dissolved in 50 ml of methanol. Five millilitres of the above solution was diluted to 50 ml with double distilled water to produce 100 μg/ml each of HCTZ and BF in double distilled water.
Ratiospectra derivative spectrophotometry
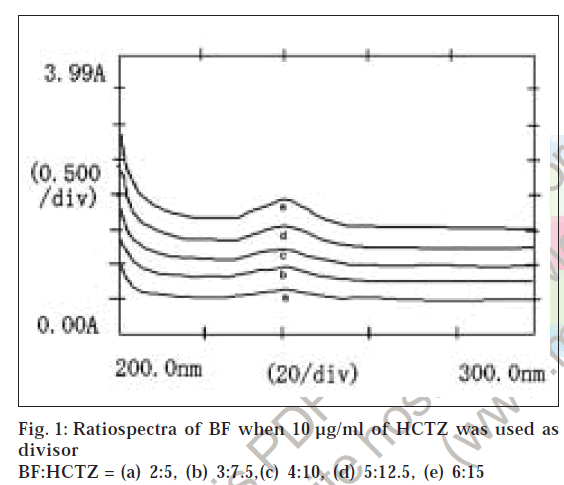
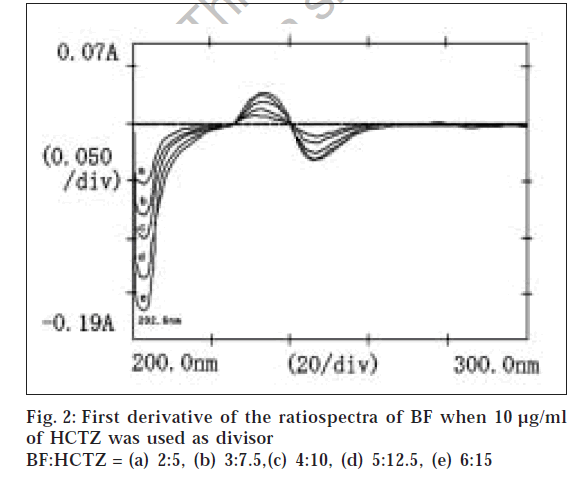
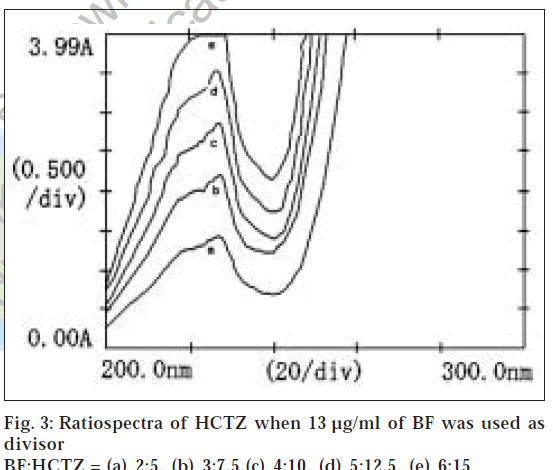
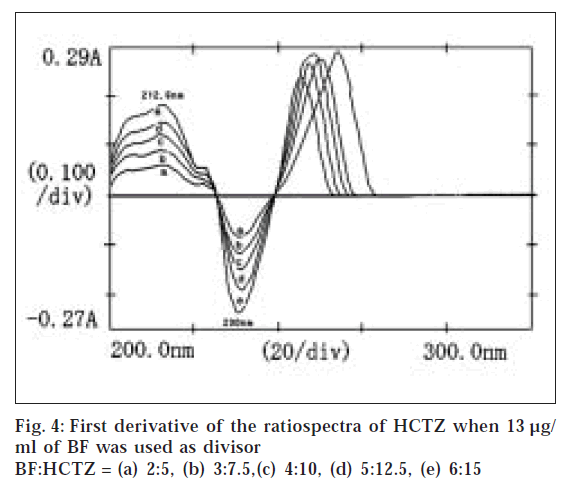
Suitable aliquots of the standard stock solution were diluted with double distilled water to produce standard solutions of 10 μg/ml of HCTZ and 13 μg/ml of BF for obtaining divisor spectra. Different binary mixture solutions containing BF and HCTZ in 2:5 ratio (very near to clinical dose ratio 2.5:6.25 of BF and HCTZ) were prepared by diluting different aliquots of the stock solutions with double distilled water. The absorption spectra of 10 μg/ml of HCTZ and 13 μg/ml of BF were recorded in the range of 200 to 300 nm and stored in the memory of the instrument as divisor spectra. The absorption spectra of the binary mixture solutions of BF and HCTZ were recorded in the range of 200 to 300 nm and were stored in the memory of the instrument. The stored spectra of the binary mixture solutions were divided by a previously stored divisor spectrum of 10 μg/ml of HCTZ to get the ratiospectra (fig. 1). The first derivative of the ratiospectra was traced with Δλ = 3 interval (fig. 2) and the amplitude at 202.6 nm were plotted against the respective concentrations of BF. Similarly the absorption spectra of binary mixtures of BF and HCTZ were divided by a previously stored standard divisor spectra of 13 μg/ml of BF and the first derivative of the ratiospectra (figs. 3 and 4) were traced with Δλ = 3 interval. The differences in amplitudes at 212.6 nm and 230 nm were then plotted against the respective concentrations of HCTZ.
Simultaneous equation method
Stock solutions of BF and HCTZ were diluted with double distilled water to obtain 2-6 μg/ml of BF and 5-15 μg/ml of HCTZ separately. From the overlain spectra two wavelengths, 223 and 271.6 nm, were selected for the formation of simultaneous equation. The absorptivity values, E (1%, 1 cm), of both the drugs at both the wavelengths were determined. Five binary mixture solutions of HCTZ and BF were prepared in 2:5 ratio (very near to clinical dose ratio 2.5:6.25 of BF and HCTZ) and the dilution of 2.5:6.25 μg/ml was prepared. The quantitative estimation of the drugs were carried out by solving the simultaneous equations, Cx = (A2ay1-A1ay2)/(ax2ay1-ax1ay2), Cy = (A1ax2-A2ax1)/(ax2ay1-ax1ay2)....(2),,where A1 and A-2 are absorbances of the mixture at 223 and 271.6 nm, respectively, ax1 and ax2 are absorptivities ax1 at 223 and 271.6 nm respectively, ay1 and ay are absorptivities of y at 223 and 271.6 nm, respectively, and Cx is the concentration of BF and Cy is the concentration of HCTZ. The sample solution for analysis of drugs from tablet was prepared as described in ratiospectra derivative spectrophotometric method and the concentrations of the drugs were calculated using simultaneous equations.
Analysis of tablets
A total of 20 tablets were accurately weighed, average weight determined and finely powdered. An amount equivalent to one tablet (2.5 mg of BF and 6.25 mg of HCTZ) was taken and dissolved in 10 ml of methanol. About 10 ml of water was added and stirred for further 5 min. The mixture was centrifuged at 1000 rpm for 5 min. The supernatant was transferred to a 100 ml volumetric flask through a Whatman No. 40 filter paper. The residue was washed thrice with double distilled water and the combined filtrate was made up to the mark with double distilled water. The sample solution thus prepared was diluted with distilled water to get the solution containing about 2.5 μg/ml of BF and 6.25 μg/ml of HCTZ. The above solution was analyzed for the content of BF and HCTZ using the method described above.
Results and Discussion
spectrophotometry and simultaneous equation methods for simultaneous determination of BF and HCTZ from their binary mixture were successfully developed. The ratiospectra derivative in the range of 2 to 6 μg/ml with co-relation co-efficient of 0.9994 and 5-15 μg/ml for HCTZ with correlation coefficient of 0.9971 (Table 1). The instrumental parameters like divisor spectra and smoothing factor (Δλ) of tracing the 1st derivative of ratiospectra were optimized for the reliable determination of subject components. Some divisor concentrations were tested for selecting the standard solution as divisor at an appropriate concentration, and it was observed that the standard solution of 10 μg/ml of HCTZ was suitable for determination of BF and 13 μg/ml of BF was suitable for determination of HCTZ. The smoothing factor Δλ = 3 was found to be optimum for tracing the first derivatives of the ratiospectra as linearity and sensitivity is concerned. The experiment was repeated five times in a day for intra-day and on five different days for inter-day precision. The method was found to be precise as % RSD for intra-day and inter-day precision were 0.986%, 1.314% for BF and 0.857%, 1.069% for HCTZ, respectively. The reproducibility of the method was determined by using methanol from three different manufacturers (Allied Chemical Corporation, Vadodara, S. D. Fine Chemicals, Mumbai and Qualigens, Mumbai) for the preparation of stock solution of standard drugs. The average value of % RSD, 0.948 for BF and 0.101 for HCTZ reveals the reproducibility of the method. The accuracy of the method was determined by performing recovery studies by standard addition method in which preanalyzed samples were taken and standard drug was added at five different levels. The % recovery±SD lies in the range of 99.6 ± 1.35% to 101.4 ± 1.8% for BF and 98.00 ± 0.82% to 102.0 ± 0.00% For HCTZ (Table 2). The analysis of commercially available tablet formulations revealed satisfactory results as evident from the results shown in Table 3.
| Mixture | Concentration (µg/ml) | Average amplitude | % RSD | ||||
|---|---|---|---|---|---|---|---|
| BF | HCTZ | BF | **HCTZ | *BF | **HCTZ | ||
| a | 2 | 5 | 0.056 | 0.134 | 2.014 | 2.321 | |
| b | 3 | 7.5 | 0.082 | 0.204 | 1.575 | 1.131 | |
| c | 4 | 10 | 0.105 | 0.272 | 0.849 | 0.480 | |
| d | 5 | 12.5 | 0.129 | 0.351 | 1.152 | 0.477 | |
| e | 6 | 15 | 0.155 | 0.402 | 0.981 | 0.938 | |
*Amplitude at 202.6 nm when 10 µg/ml of HCTZ was used as divisor.
**Difference in amplitude at 212.6 and 230 nm when 13 µg/ml of BF was used as divisor
Table 1: calibration curve data for Ratiospectra derivative spectroscopy
| Level of standard Addition (%) | % Recovery±SD | |||
|---|---|---|---|---|
| Method 1 | Method 2 | |||
| BF | HCTZ | BF | HCTZ | |
| 80 | 99.59 ± 1.35 | 102.0 ± 0.00 | 98.81 ± 0.92 | 101.2 ± 1.69 |
| 90 | 101.4 ± 1.80 | 99.56 ± 0.46 | 98.46 ± 1.39 | 98.76 ± 0.45 |
| 100 | 100.3 ± 0.95 | 97.99 ± 0.82 | 98.07 ± 1.20 | 102.6 ± 0.27 |
| 110 | 99.78 ± 1.70 | 99.58 ± 0.00 | 97.26 ± 0.57 | 98.99 ± 0.05 |
| 120 | 100.8 ± 1.50 | 98.43 ± 0.34 | 96.67 ± 6.34 | 101.3 ± 0.98 |
Table 2: Recovery Study Data
For simultaneous equation method, the overlain spectra of both the drugs showed the λmax of 271.6 nm for HCTZ and 223 nm for BF. Hence these wavelengths were selected for estimation of HCTZ and BF. Absorbances were determined at both the wavelengths. HCTZ obeyed linearity in the concentration range of 2-6 μg/ml, BF in the concentration range of 5-15 μg/ml and the correlation coefficient (r2) was <1 in both the case. The absorptivity was then calculated and along with absorbance, these values were submitted in the equations 1 and 2 to obtain concentration of drugs. The experiment was repeated five times in a day for intra-day and on five different days for inter-day precision. The method was found to be precise as % RSD for intra-day and inter-day precision were 3.156%, 3.222% for BF and 0.609%, 0.562% for HCTZ, respectively. The reproducibility of the method was determined by using methanol from three different manufacturers for the preparation of stock solution of standard drugs. The average value of % RSD, 1.251 for BF and 1.023 for HCTZ reveal the reproducibility of the method. The accuracy of the method was determined by performing recovery studies by standard addition method in which preanalyzed samples were taken and standard drug was added at five different levels. The % recovery ±SD lies in the range of 96.7 ± 6.34% to 98.8 ± 0.92% for BF and 98.8 ± 0.45% to 102.6 ± 0.27% for HCTZ (Table 2). The analysis of commercially available tablet formulations revealed satisfactory results as evident from the results shown in Table 3.
| Tablet | Label claim (µg/ml) | Ratiospectra derivative spectrophotometry | Simultaneous equation method | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conc. found (µg/ml) | % recovery | Conc. found (µg/ml) | % recovery | |||||||
| BF | HCTZ | BF | HCTZ | BF | HCTZ | BF | HCTZ | BF | HCTZ | |
| A | 2.5 | 6.25 | 2.49 | 6.29 | 99.50 | 100.71 | 2.17 | 6.28 | 96.57 | 100.51 |
| B | 2.5 | 6.25 | 2.56 | 6.15 | 102.26 | 98.36 | 2.21 | 6.26 | 98.10 | 100.08 |
A = Bisocar HT, B = Lodoz
Table 3: Analysis of Commercial Tablet Formulations
By observing the validation parameters, accuracy, intra-reproducibility (% RSD), specificity, linearity (correlation day and inter-day precision expressed as %RSD,coefficient, r2<1) and range. Both the methods were found to be specific, accurate, precise, and reproducible. Hence both the methods can be employed for routine analysis of tablets for assay.
Acknowledgements
The authors are grateful to M/s. Sun Pharma Advanced Research Centre (SPARC) for providing hydrochlorothiazide and bisoprolol fumarate pure drug as gift sample. One of the authors R. Sahu is grateful to University Grant Commission (UGC) for providing financial support in the form of Junior Research Fellowship (JRF) during this work. The authors owe a great debt of gratitude to Prof. A. N. Misra, Head, Pharmacy Department, The Maharaja Sayajirao University of Baroda for providing the necessary facilities.
References
- Budavari, S., Eds., in; The Merck index, 13th Edn., Merck and Co., Inc., Whitehouse Station, NJ, 1997, 1294.
- Budavari, S., Eds., in; The Merck index, 13th Edn., Merck and Co., Inc., Whitehouse Station, NJ, 1997, 4802.
- Barary, M.H., Indian J. Pharm. Sci., 1984, 46, 224.
- Erram, S.V. and Tipnis, H.P., Indian Drugs, 1993, 30, 462.
- Cieri, U.R., J. AOAC Int., 1994, 77, 1104.
- Jonczyk A. and Nowakowska Z., Actapol Pharm., 2001, 58, 339.
- Erturk, S., Cetin, S.M. and Atmaca S., J. Pharm. Biomed. Anal., 2003, 33, 477.
- Shah, S.A., Rathod, I.S., Suhagia, B.N., Savale, S. S. and Patel, J.B., J. AOAC Int., 2001, 84, 1715.
- Takubo, T., Chromatogr. B. Analyt. Technol. Biomed. Life Sci., 2004, 806, 199.