- *Corresponding Author:
- Benjaporn Buranrat
Faculty of Medicine, Mahasarakham University, Mueang District, Maha Sarakham 44000, Thailand
E-mail: buranrat@gmail.com
| Date of Received | 25 November 2021 |
| Date of Revision | 01 October 2022 |
| Date of Acceptance | 25 March 2023 |
| Indian J Pharm Sci 2023;85(2):435-444 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
New treatments are needed for cholangiocarcinoma, an aggressive cancer with a poor prognosis. Combination of statins and bisphosphonates may be useful in treating cancer through influencing the mevalonate pathway. We studied the synergistic effects of simvastatin and alendronate against cholangiocarcinoma cells, looking first at cytotoxic, apoptotic and anti-migratory effects, then possible mechanisms of action. Individually, both simvastatin and alendronate increased cholangiocarcinoma cell death and the combination was more effective. Additionally, the combination treatment inhibited colony formation, reduced protein-related cell proliferation, NF-κB and cyclin D1, and increased p53 protein-related cell death. Apoptosis was induced by the combination treatment through activation of early apoptotic cells and stimulation of the caspase 3 enzyme. The combination treatment also decreased cancer cell migration by suppressing matrix metalloproteinases, metalloproteinase-2 and metalloproteinase-9 levels. Finally, the combination treatment reduced Rac1 protein expression in the mevalonate pathway. Our study shows that a combination of simvastatin plus alendronate has a direct anticancer and anti-migratory effect on cholangiocarcinoma cells in vitro. Mevalonate inhibitors could be used as therapeutic options for cholangiocarcinoma and perhaps, as chemotherapy-sensitizing agents.
Keywords
Cholangiocarcinoma, simvastatin, alendronate, mevalonate pathway
Cholangiocarcinoma (CCA) is an aggressive cancer of the bile duct that initiates from the epithelial bile ducts and is a major public health problem in Southeast Asian countries including Thailand. In Thailand, CCA risk factors include Opisthorchis viverrini and Clonorchis sinensis infections, which promote chronic inflammation and oxidative stress, and lead to an unregulated intracellular signaling pathway[1,2]. Unfortunately, CCA is not usually detected until stage III or IV, when surgical treatment is no longer possible. Chemotherapeutic agents (anticancer drugs) must be used instead and these have low efficacy and high toxicity[3]. Therefore, alternative strategies for CCA treatment are urgently needed for the management of unresectable CCA.
The Mevalonate (MVA) pathway is the key pathway in cholesterol synthesis, and produces various biochemical agents in intracellular processes[4]. The products of the MVA pathway include Rac, Rho, Ras, and Rap, which control protein function in many cellular steps, such as growth, necrosis, apoptotsis, angiogenesis, invasion, and migration. Drugs that repress the MVA pathway could therefore be useful in the treatment of cancer. Statins and Bisphosphonates (BPs), for example, inhibit β-Hydroxy β-Methylglutaryl-CoA (HMG-CoA) reductase and Farnesyl Pyrophosphate Synthase (FPPS), reduce MVA product formation, and lead to an in vitro suppression of cancer cell proliferation and migration[5,6].
Statins inhibit the rate-limiting enzyme of the MVA pathway, HMG-CoA reductase, which controls cholesterol synthesis[5], and they are broadly used as lipid-lowering drugs in hyperlipidemia patients, significantly decreasing their risk of Coronary Heart Disease (CHD)[7]. Statins may cause decreased levels of MVA and its downstream products, affecting various cell functions including the cellular signaling process, protein synthesis, and cell cycle progression[8]. Several studies have indicated that statins could be beneficial in clinical cancer treatment by regulating tumor initiation and suppressing cancer cell growth invasion and metastasis[9-12], and that statins could reduce the concentration of anticancer drugs needed to treat many cancer cell types.
BPs are approved for treating and preventing osteoporosis and tumor migration to the bones. Many studies have shown that BPs may prevent cancer-associated bone disease. BPs act by repressing the FPPS and MVA enzymes, which can result in changes in Dimethylallyl Pyrophosphate (DMAPP), Isopentenyl Pyrophosphate (IPP) and Farnesyl Pyrophosphate (FPP) products[13,14]. Additionally, BPs affects the downstream production of FPPS and Geranylgeranyl Pyrophosphate Synthase (GGPPS)[15]. BPs have also shown anticancer activities in in vitro studies including inhibition of cell growth, proliferation, apoptosis, invasion, and migration[16]. The underlying mechanisms are via repression of the MVA pathway although other actions cannot be ruled out.
Given the importance of the MVA pathway in cancer cell processes, the effects of statins and BPs on the MVA pathway, and the growing number of studies demonstrating statin and BP activity against many cancer cell types, it seems plausible that these drugs could have additive or synergistic effects in CCA cancer cells. In the present study, therefore, we investigated the effect of simvastatin and alendronate alone and in combination on CCA cell growth and migration using KKU-100 cells.
Materials and Methods
Chemicals and reagents:
All of the cell culture reagents, with the following exceptions, were obtained from Gibco BRL Life Technologies (Grand Island, NY, USA). Simvastatin, alendronate, Radioimmunoprecipitation assay lysis buffer, and sulforhodamine B (SRB) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies against NF-kB, p53, cyclin D1, caspase 3, Rac1, RhoA, beta-actin, and anti-rabbit IgG HRP-link antibody were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell lines and culture conditions:
The KKU-100 human CCA cell line was kindly provided by the Department of Pathology at Khon Kaen University Faculty of Medicine. Cells were cultured in high glucose Dulbecco's Modified Eagle's Medium (DMEM) with antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin) and 10 % Fetal Bovine Serum (FBS).
Cell viability assay:
Cell viability was measured using the SRB) assay as described previously[17]. In brief, cells were exposed to simvastatin (0-200 μM) and alendronate (0-1000 μM) for 24-48 h, then fixed and stained with 0.4 % SRB, before addition of 200 μl 10 mM Tris base. The Optical Density (OD) was read at 540 nm using a spectrophotometer. Treatment with a combination of simvastatin and alendronate was also tested using the SRB assay. In these combination treatment experiments, cells were exposed to various concentrations of simvastatin (0-50 cM) with 50 μM alendronate or various concentrations of alendronate (0-250 μM) with 25 μM simvastatin for 24 h, then cytotoxicity was measured by SRB assay as described above.
Colony formation assay
Cancer cell replication was measured using the colony formation assay described previously[17]. In brief, cells were plated overnight and then simvastatin (25-50 μM) and alendronate (25 μM) were added for 24 h. The next day, the cells were transferred to new DMEM medium and allowed to grow for a further 15 d. After this, the cells were fixed with cold absolute methanol, stained with 0.5 % crystal violet, washed, and colony formation was counted.
Wound healing assay:
Cell migration was assessed using the wound healing assay described previously[17]. In brief, cells were seeded overnight and a wound was then made by scratching each monolayer with a 0.2 ml pipette tip. The cell monolayers were treated with 1 μM simvastatin with or without 25 μM alendronate for 48 h, and closure of the distant wounds was captured from 0-48 h by inverted microscopy (10x magnification).
Gelatin zymography analysis:
Metalloproteinases (MMP)-2 and MMP-9 levels were assessed by gelatin zymography analysis as described previously[17]. In brief, cells were seeded overnight, then exposed to 1 μM simvastatin with or without 25 μM alendronate for 24 h, and the medium was collected. Later, protein samples were mixed with 2X non-reducing buffer and the samples (20 μg) were then pipetted onto a 12 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel comprising 0.01 % (w/v) gelatin. The gel was incubated with developing buffer at 37° for 8 h and stained with 0.5 % Coomassie Brilliant Blue R-250 for 1 h. After that, the gel was washed several times with destaining buffer. The molecular weights of MMP-2 and MMP-9 were observed to be 72 kDa and 92 kDa.
Matrigel migration assay:
We used the matrigel invasion assay to test cell migration. The cells were plated in the upper Transwell chambers with simvastatin alone (0-25 μM), alendronate alone (0-250 μM), and a combination of simvastatin and alendronate (10 μM simvastatin plus 50 μM alendronate) in serum-free medium. Complete DMEM medium was added to the bottom chamber, and it was then incubated with the tested compounds for 24 h. Cells passing through the membrane were fixed in cold absolute methanol for 30 min at -20°, stained with 0.5 % crystal violet, and then captured with an inverted microscope (100x, CKX53, Olympus Corporation).
Apoptosis by flow cytometry:
Cell apoptosis was assessed by flow cytometry as described previously[18]. In brief, cells were seeded overnight and then exposed to 50 μM simvastatin plus 250 μM alendronate for 24 h. After this, the cells were resuspended in buffer, stained with 5 μl Propidium Iodide (PI) and 5 μl Annexin V-FITC, and allowed to incubate in the dark at room temperature for 15 min before addition of 400 μl of assay buffer. Cancer cell apoptosis was assessed by flow cytometry.
Western blotting:
Protein expression was assessed by western blot as described previously[17]. Cells were seeded overnight and then exposed to 50 μM simvastatin with or without 250 μM alendronate for 24 h prior to collection. Cytosol proteins (20 μg) were loaded onto a 10 % SDS-PAGE gel, then transferred onto Polyvinylidene Fluoride (PVDF), and blocked with 2.5 % albumin. The membrane was treated with primary antibodies for NF-κB, p53, cyclin D1, caspase 3, Rac1, RhoA, and beta-actin (1:2,500) overnight at 4°, and then exposed to secondary antibody (1:5000) for 2 h at room temperature. The intensity of the target band was determined using Western substrate (Bio-Rad Laboratories, USA).
Statistical analysis:
All experimental data were analyzed by one-way Analysis of Variance (ANOVA), followed by Tukey's post-hoc test to compare untreated and treated groups. Half maximal inhibitory concentration (IC50) values were calculated using the Prism 5 program (GraphPad Software, San Diego, CA). All data are expressed as mean±Standard Error of Mean (SEM) and we set the statistically significant difference as less than 0.05.
Results and Discussion
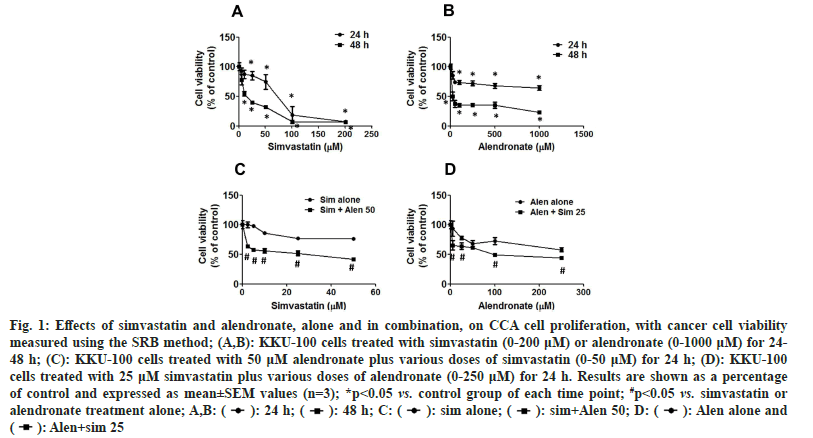
The effects of the MVA pathway inhibitors, simvastatin and alendronate, on CCA cell viability were assessed using the SRB assay. Cells were exposed to simvastatin alone and alendronate alone for 24-48 h. Simvastatin and alendronate treatment alone decreased the cell viability of KKU-100 cells in a time- and dose-dependent manner (fig. 1A and fig. 1B), with IC50 values of 64.18±12.35 μM at 24 h and 15.16±1.51 μM at 48 h for simvastatin, and with IC50 values of 999.77±138.26 μM at 24 h and 48.24±9.36 μM at 48 h for alendronate (Table 1).
Fig. 1: Effects of simvastatin and alendronate, alone and in combination, on CCA cell proliferation, with cancer cell viability measured using the SRB method; (A,B): KKU-100 cells treated with simvastatin (0-200 μM) or alendronate (0-1000 μM) for 24- 48 h; (C): KKU-100 cells treated with 50 μM alendronate plus various doses of simvastatin (0-50 μM) for 24 h; (D): KKU-100 cells treated with 25 μM simvastatin plus various doses of alendronate (0-250 μM) for 24 h. Results are shown as a percentage of control and expressed as mean±SEM values (n=3); *p<0.05 vs. control group of each time point; #p<0.05 vs. simvastatin or alendronate treatment alone; A,B:  Alen alone and
Alen alone and  .
.
| Treatment groups | Incubation time (h) | IC50 μM) |
|---|---|---|
| Simvastatin | 24 | 64.2+12.4 |
| 48 | 15.2+1.5 | |
| Alendronate | 24 | 999.8+138.3 |
| 48 | 48.2+9.4 | |
| Simvastatin (0-50 μM ) | 24 | 121.1+0.8 |
| Simvastatin+50 μM Alendronate | 24 | 14.8+1.1 |
| Alendronate (0-250 μM) | 24 | 229.4+16.1 |
| Alendronate+25 μM Simvastatin | 24 | 85.7+5.8 |
Note: Values represent mean±SEM (n=3). Different character indicates significant difference
Table 1: IC50 values of Simvastatin and alendronate, alone and in combination, against CCA cells.
We also examined the effects of simvastatin and alendronate in combination using the SRB assay, with KKU-100 cells exposed to the combination for 24 h. When various concentrations of simvastatin (0-50 μM) were tested in combination with 50 μM alendronate, the IC50 value of simvastatin was reduced from 121.10±0.83 μM to 14.80±1.06 μM. Also, when various concentrations of alendronate (0-250 μM) were tested in combination with 25 μM simvastatin, the IC50 value of alendronate was reduced from 229.37±16.09 μM to 85.67±5.81 μM (fig. 1D and Table 1). Clearly therefore, the two MVA pathway inhibitors have synergistic cytotoxic effects against KKU-100 cells.
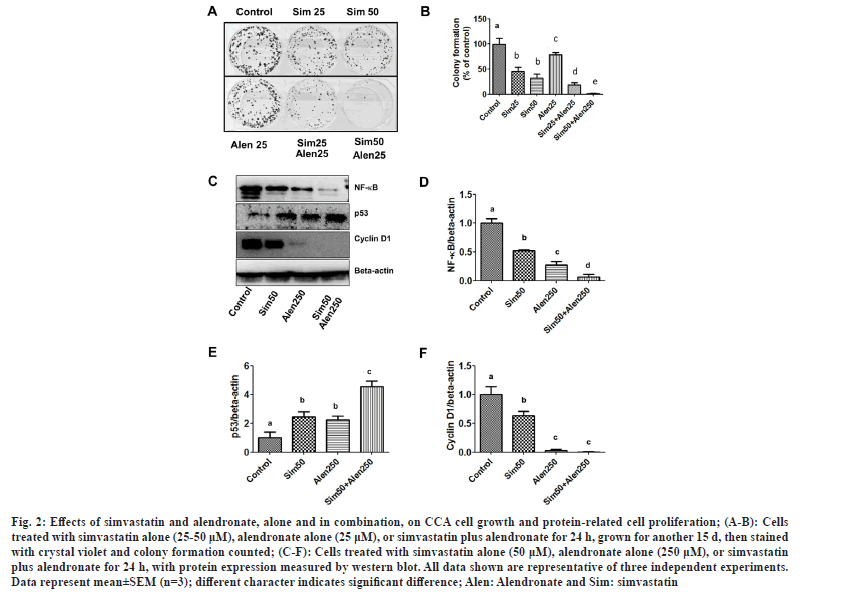
To examine the effects of simvastatin, alendronate, and a combination of simvastatin and alendronate on CCA cell replication and protein expression, the colony formation assay and western blotting were used. We found that 25-50 μM simvastatin and 25 μM alendronate reduced the colony formation of cancer cells with approximate percentages of inhibition of 45.83 %, 32.11 %, and 78.68 %, respectively. Alendronate potentiated the inhibition of colony formation with both simvastatin concentrations (25 and 50 μM), and the approximate percentages of inhibition were 18.87 % and 1.47 %, respectively (fig. 2A and fig. 2B). These results show that a combination of simvastatin and alendronate has an antiproliferative effect on KKU-100 cells.
Fig. 2: Effects of simvastatin and alendronate, alone and in combination, on CCA cell growth and protein-related cell proliferation; (A-B): Cells treated with simvastatin alone (25-50 μM), alendronate alone (25 μM), or simvastatin plus alendronate for 24 h, grown for another 15 d, then stained with crystal violet and colony formation counted; (C-F): Cells treated with simvastatin alone (50 μM), alendronate alone (250 μM), or simvastatin plus alendronate for 24 h, with protein expression measured by western blot. All data shown are representative of three independent experiments. Data represent mean±SEM (n=3); different character indicates significant difference; Alen: Alendronate and Sim: simvastatin.
Whether or not the combination of simvastatin and alendronate inhibited KKU-100 proliferation by altering expression of the proteins NK-κB, p53, and cyclin D1 was investigated using western blot analysis. Our results indicate that simvastatin significantly decreased expression of NF-κB and induced wild type p53 (fig. 2C-fig. 2F). These results show that simvastatin in combination with alendronate inhibited cell proliferation and cell regrowth by reducing protein-related cancer cell growth via NF-κB and cyclin D1, and by increasing protein-associated cell death via p53.
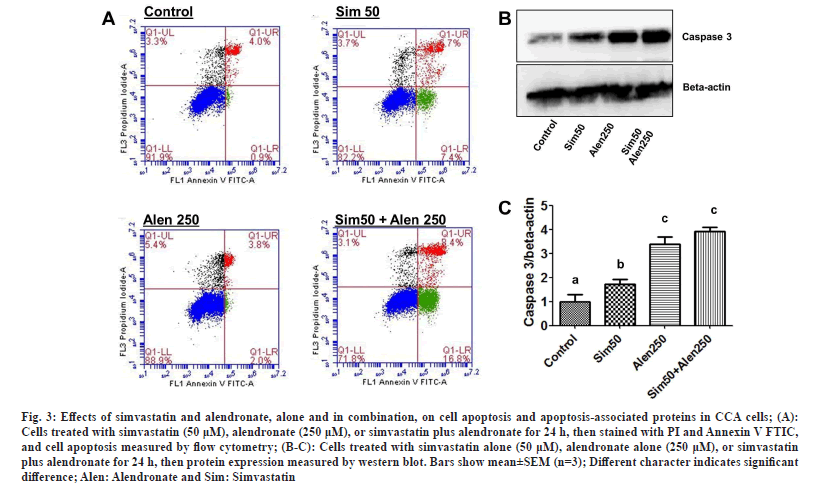
The effects of simvastatin alone, alendronate alone, and simvastatin and alendronate in combination on KKU-100 cell apoptosis and protein-related apoptosis were investigated by flow cytometry and western blotting. The results show that 50 μM simvastatin induced early phase apoptosis in KKU-100 cells and 250 μM alendronate slightly increased apoptosis. In combination, simvastatin and alendronate induced apoptosis more than either drug treatment alone (fig. 3A). Based on these results, simvastatin and alendronate have significant effects on CCA cancer cell apoptosis and cell death.
Fig. 3: Effects of simvastatin and alendronate, alone and in combination, on cell apoptosis and apoptosis-associated proteins in CCA cells; (A): Cells treated with simvastatin (50 μM), alendronate (250 μM), or simvastatin plus alendronate for 24 h, then stained with PI and Annexin V FTIC, and cell apoptosis measured by flow cytometry; (B-C): Cells treated with simvastatin alone (50 μM), alendronate alone (250 μM), or simvastatin plus alendronate for 24 h, then protein expression measured by western blot. Bars show mean±SEM (n=3); Different character indicates significant difference; Alen: Alendronate and Sim: Simvastatin.
Western blotting was used to gain insight into the molecular mechanism by which the two test drugs induce KKU-100 apoptosis. By comparing flow cytometry and western blotting results, we found that simvastatin treatment alone significantly induced caspase 3 protein expression, but the induction was weaker than with alendronate treatment alone. Simvastatin and alendronate in combination induced higher levels of caspase 3 protein expression than the untreated control group, simvastatin alone, or alendronate alone (fig. 3B and fig. 3C). In conclusion, our results show that simvastatin in combination with alendronate stimulates CCA cell apoptosis more than either drug treatment alone.
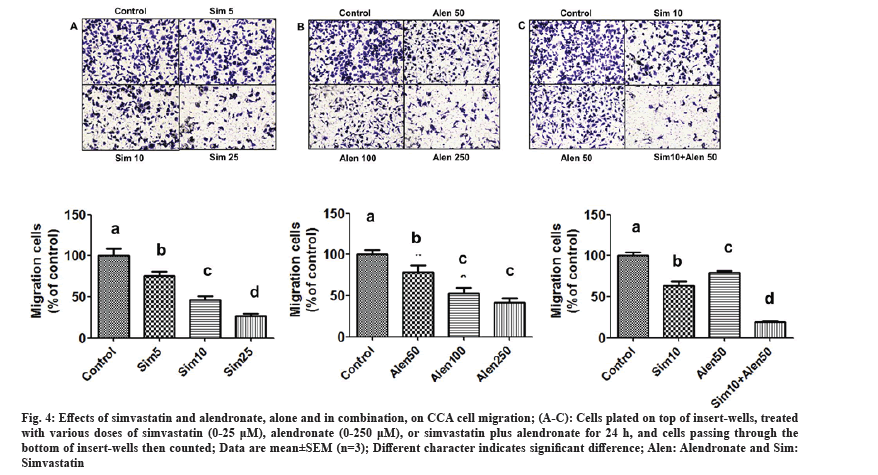
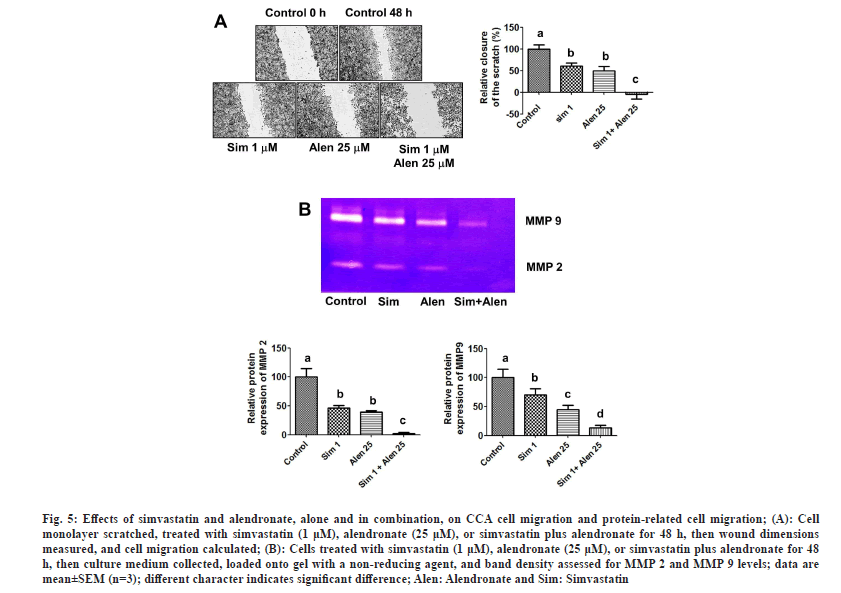
The effects of simvastatin, alendronate, and their combination on KKU-100 cell migration were assessed using matrigel migration, wound healing, and gelatin zymography assays. In the first assay, cancer cells were exposed to simvastatin alone (5-25 μM), alendronate alone (50-250 μM), and alendronate supplemented with 10 μM simvastatin, and simvastatin supplemented with 50 μM alendronate for 24 h in matrigel-coated chambers. Our results showed that simvastatin and alendronate significantly decreased KKU-100 cell migration in a dose-dependent manner, and that combination treatment inhibited migration more than the untreated control and simvastatin and alendronate treatment alone (fig. 4A-fig. 4C). In the wound healing assay, treatment with simvastatin alone and alendronate alone inhibited cancer cell migration (fig. 5A), but combination treatment inhibited CCA migration significantly more.
Fig. 4: Effects of simvastatin and alendronate, alone and in combination, on CCA cell migration; (A-C): Cells plated on top of insert-wells, treated with various doses of simvastatin (0-25 μM), alendronate (0-250 μM), or simvastatin plus alendronate for 24 h, and cells passing through the bottom of insert-wells then counted; Data are mean±SEM (n=3); Different character indicates significant difference; Alen: Alendronate and Sim: Simvastatin.
Fig. 5: Effects of simvastatin and alendronate, alone and in combination, on CCA cell migration and protein-related cell migration; (A): Cell monolayer scratched, treated with simvastatin (1 μM), alendronate (25 μM), or simvastatin plus alendronate for 48 h, then wound dimensions measured, and cell migration calculated; (B): Cells treated with simvastatin (1 μM), alendronate (25 μM), or simvastatin plus alendronate for 48 h, then culture medium collected, loaded onto gel with a non-reducing agent, and band density assessed for MMP 2 and MMP 9 levels; data are mean±SEM (n=3); different character indicates significant difference; Alen: Alendronate and Sim: Simvastatin.
Next, to investigate the mechanism by which simvastatin and alendronate reduce cell migration, we examined MMP-2 and MMP-9 protein expression using the gelatin zymography assay. Simvastatin treatment and alendronate treatment each exerted an inhibitory effect on the levels of MMP-2 and MMP-9 when compared with the untreated control. In combination, simvastatin and alendronate reduced MMP-2 and MMP-9 protein expression levels further still (fig. 5B). Our results suggest that MMP-2 and MMP-9 levels can be down-regulated in CCA cells by treatment with simvastatin and alendronate.
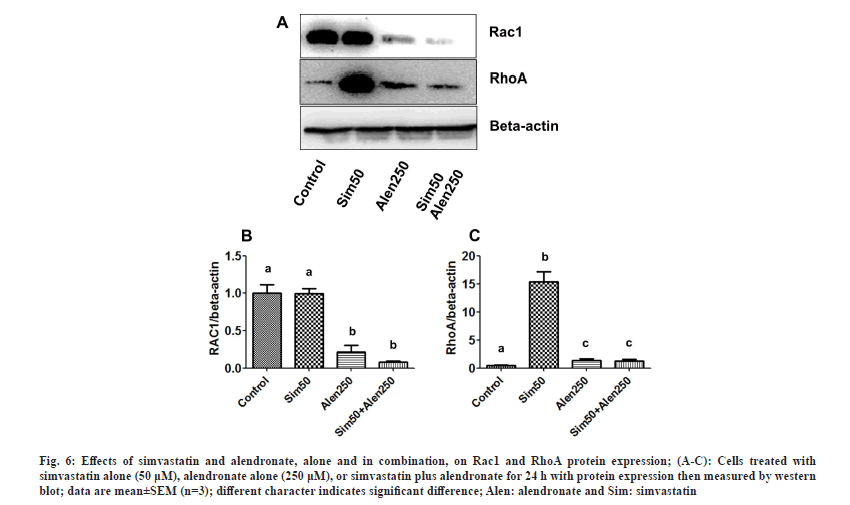
To explore the mechanism by which simvastatin and alendronate induce cancer cell death, increase cell apoptosis, and inhibit cell migration, we examined Rac1 and RhoA protein expression by western blotting. The effects of the different treatments on Rac1 and RhoA protein expression in the KKU-100 cells are shown in fig. 6A-fig. 6C. Simvastatin did not alter Rac1 expression, but alendronate suppressed Rac1 expression compared to the untreated control and simvastatin treatment. Treatment with simvastatin and alendronate in combination caused an augmented reduction in Rac1 expression.
Fig. 6: Effects of simvastatin and alendronate, alone and in combination, on Rac1 and RhoA protein expression; (A-C): Cells treated with simvastatin alone (50 μM), alendronate alone (250 μM), or simvastatin plus alendronate for 24 h with protein expression then measured by western blot; data are mean±SEM (n=3); different character indicates significant difference; Alen: alendronate and Sim: simvastatin.
Whilst simvastatin did not alter Rac1 expression, it did significantly stimulate RhoA protein expression compared to the control, alendronate alone, and combination treatment. Alendronate and the combination treatment increased RhoA levels compared to the control, but not significantly. In conclusion, simvastatin and alendronate modulated Rac1 and RhoA protein expression in the MVA pathway and, although additional studies will be required, this combination could potentially be used to induce cytotoxicity and apoptosis in CCA cells and to inhibit the migration of CCA cells as well.
Simvastatin and alendronate are known to influence cell proliferation and apoptosis in several cancer cell types, perhaps by inhibiting the production of MVA products, such as Rho and Rac. Prior to the current study, however, the effects of simvastatin and alendronate, alone and in combination, on human CCA cells were still unknown. We have shown here that simvastatin and alendronate, in a dose- and time-dependent manner, inhibit CCA cell proliferation, induce cells apoptosis, and suppress the metastatic potential of KKU-100 cells. Moreover, when simvastatin and alendronate were combined, their activity was superior to either drug alone. The combination reduced cancer cell regrowth by reducing expression of NF-κB and cyclin D1, and by increasing expression of the tumor suppressor protein p53. The combination also induced apoptosis by increasing caspase 3 levels, inhibited cancer cell migration by reducing MMP-2 and MMP-9 expression, and modulated the MVA pathway by interfering with Rac1 and RhoA expression. Taken together, these results suggest simvastatin and alendronate could potentially be developed as a therapeutic or preventive treatment for CCA cancer or as an adjunct(s) for potentiating the activity of current anticancer drugs.
The MVA pathway is involved in cholesterol synthesis and protein prenylation, and has been associated with many steps in carcinogenesis, the aggressiveness of tumors, and cancer progression. A few drug classes, such as statins and BPs, suppress the MVA pathway and also act as anticancer agents. We therefore examined the effects of simvastatin and alendronate, alone and in combination, on CCA cells. Both drugs target the HMG-CoA reductase and FPPS enzymes in the MVA pathway and act as cancer suppressors. Our results indicate that both drugs potentiate CCA cancer cell death by reducing NF-κB and cyclin D1 and inducing p53 and caspase 3. Both p53 and NF-κB are important in many steps in the cellular process[19] and, importantly, both can control cancer cell proliferation and migration. NF-κB is associated with the regulation of cell transformation, proliferation, and tumor development; furthermore, NF-κB activates the transcription of cyclin D1 in the G1 phase of the cell cycle[20], stimulating cancer cell growth. Also, stimulation of the p53 tumor suppressor can lead to DNA damage in cancer cells, resulting in apoptosis and cell cycle arrest[21]. Our results show that simvastatin and alendronate inhibit CCA cell proliferation by inducing the tumor suppressor protein p53 and reducing expression of NF-κB and cyclin D1.
The effects of MVA pathway inhibitors are mostly mediated by apoptosis induction. For example, simvastatin and zoledronic acid suppress drug resistance and additively induce apoptosis[22] in multiple myeloma cells. The aim of many studies is to reduce the concentration of BPs needed to kill or manage cancer cells. In human breast cancer, it was found that zoledronic acid combined with the standard anticancer drug, doxorubicin, could positively induce cancer cell death[23-25]. MVA pathway inhibitors kill cancer cells by reducing protein geranylation more than farnesylation[26], but little is known about the downstream mechanisms. These inhibitors decrease downstream protein and lead to a reduction in Rac, Rho, Ras, and Rab protein expression levels. These effects can suppress cell growth, proliferation, and migration.
The anticancer effects of statins have been demonstrated in both in vitro and in vivo studies, and against various cancer cell types including gastric, breast, pancreatic, prostate and colon cancer[27]. Our previous report showed that two statins, simvastatin and atorvastatin, strongly suppress CCA cell growth and migration at low concentrations[18]. Work by Wong et al.[6] confirmed that the mechanism of action of statins against cancer cells involves activation of cell death and apoptosis via an obstruction of the post-translational modification of various small G proteins (Rac, Rho, and Ras)[6]. Importantly, the Ras protein controls the crucial signaling pathway of growth factor, while Rho and Rac mainly control cell migration, cell adhesion, and the cytoskeleton[28]. Repression of the small G proteins may contribute to MVA inhibitor-induced inhibition of cancer cell growth and migration. MVA inhibitors significantly decrease GPP and FPP production, and inhibit cell proliferation both in vitro and in vivo. In the present study, MVA inhibitor-induced repression of cell growth and migration may have been due to altered Rac1 and RhoA protein expression levels, especially Rac1 levels. Our results indicate that MVA inhibitors can modify small G protein, which has a crucial role in the suppression of CCA cancer growth and migration. Another mechanism of action, proposed by Karlic et al.[29] is that simvastatin and ibandronate down regulate energy metabolism by suppressing nicotinamide adenine dinucleotide phosphate, then activate reactive oxygen species, causing cancer cell starvation and death[29]. Statins and BPs may therefore cause cancer cell death via multiple mechanisms, but the primary mechanism is likely to be suppression of the MVA pathway.
In an epidemiologic analysis, El-Refai et al.[30] reported that combination of statins with BPs improved overall survival in patients with cancer (hazard ratio 0.85; 95 % confidence interval, 0.80 to 0.91)[30]. Lee et al.[31] also found that patients with advanced non adenocarcinomatous non-small cell lung cancer who received simvastatin (40 mg/d) plus afatinib (40 mg/d) had a longer overall survival than afatinib treatment alone (approximately 10.0 mo vs. 7.0 mo), but this difference was not statistically significant (p=0.930)[31]. Simvastatin is usually used at a dose of 40 mg/d to treat hyperlipidemia patients and has low toxicity. These findings suggest that MVA inhibitors, both statins and BPs, could be developed as therapeutic options for CCA cancer and perhaps as sensitizing chemotherapeutic agents.
The MVA inhibitors, simvastatin and alendronate, when used in combination, have a cytotoxic effect against human CCA cells. This activity is partly attributable to a reduction in cancer cell growth, induction of apoptosis, and inhibition of cell migration. The combination also inhibited CCA cancer cell proliferation and regrowth by blocking NF-κB and cyclin D1 and by inducing p53 and caspase 3 protein expression. CCA cell migration was inhibited too, caused by simvastatin and alendronate reducing MMP-2 and MMP-9 levels. The above findings indicate that simvastatin and alendronate have promising activities against CCA cancer cells via modulation of Rac1 expression, and may represent a useful new approach for CCA cancer therapy.
Funding:
This study was finally supported by the Thailand Science Research and Innovation (TSRI) 2021.
Conflict of interest:
The authors declared no conflict of interest.
References
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, et al. The tumorigenic liver fluke Opisthorchis viverrini–multiple pathways to cancer. Trends Parasitol 2012;28(10):395-407.
[Crossref] [Google Scholar] [PubMed]
- Sripa B, Pairojkul C. Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol 2008;24(3):349.
[Crossref] [Google Scholar] [PubMed]
- Sookprasert A, Chindaprasert J, Wirasorn K. Systemic therapy for locally advanced and metastatic cholangiocarcinoma. Asian Pac J Cancer Prev 2012;13:3-6.
- Yeganeh B, Wiechec E, Ande SR, Sharma P, Moghadam AR, Post M, et al. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol Ther 2014;143(1):87-110.
[Crossref] [Google Scholar] [PubMed]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990;343(6257):425-30.
[Crossref] [Google Scholar] [PubMed]
- Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002;16(4):508-19.
[Crossref] [Google Scholar] [PubMed]
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al.Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78.
[Crossref] [Google Scholar] [PubMed]
- Altwairgi AK. Statins are potential anticancerous agents. Oncol Rep 2015;33(3):1019-39.
[Crossref] [Google Scholar] [PubMed]
- Bouterfa HL, Sattelmeyer V, Czub S, Vordermark D, Roosen K, Tonn JC. Inhibition of Ras farnesylation by lovastatin leads to downregulation of proliferation and migration in primary cultured human glioblastoma cells. Anticancer Res 2000;20(4):2761-71.
[Google Scholar] [PubMed]
- Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003;9(1):10-9.
[Google Scholar] [PubMed]
- Koyuturk M, Ersoz M, Altiok N. Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent pathway requiring signalling through JNK. Cancer Lett 2007;250(2):220-8.
[Crossref] [Google Scholar] [PubMed]
- Tapia-Pérez JH, Kirches E, Mawrin C, Firsching R, Schneider T. Cytotoxic effect of different statins and thiazolidinediones on malignant glioma cells. Cancer Chemother Pharmacol 2011;67:1193-201.
[Crossref] [Google Scholar] [PubMed]
- Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci 2006;103(20):7829-34.
[Crossref] [Google Scholar] [PubMed]
- Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, et al. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem 2006;1(2):267-73.
[Crossref] [Google Scholar] [PubMed]
- Guo RT, Cao R, Liang PH, Ko TP, Chang TH, Hudock MP, et al. Bisphosphonates target multiple sites in both cis-and trans-prenyltransferases. Proc Natl Acad Sci 2007;104(24):10022-7.
[Crossref] [Google Scholar] [PubMed]
- Stresing V, Daubiné F, Benzaid I, Mönkkönen H, Clézardin P. Bisphosphonates in cancer therapy. Cancer Lett 2007;257(1):16-35.
[Crossref] [Google Scholar] [PubMed]
- Buranrat B, Mairuae N, Konsue A. Cratoxy formosum leaf extract inhibits proliferation and migration of human breast cancer MCF-7 cells. Biomed Pharmacother 2017;90:77-84.
[Crossref] [Google Scholar] [PubMed]
- Buranrat B, Senggunprai L, Prawan A, Kukongviriyapan V. Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci 2016;153:41-9.
[Crossref] [Google Scholar] [PubMed]
- Rocha S, Martin AM, Meek DW, Perkins ND. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-κB subunit with histone deacetylase 1. Mol Cell Biol 2003;23(13):4713-27.
[Crossref] [Google Scholar] [PubMed]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol 1999;19(4):2690-8.
[Crossref] [Google Scholar] [PubMed]
- Hupp TR, Lane DP, Ball KL. Strategies for manipulating the p53 pathway in the treatment of human cancer. Biochem J 2000;352(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Schmidmaier R, Simsek M, Baumann P, Emmerich B, Meinhardt G. Synergistic antimyeloma effects of zoledronate and simvastatin. Anticancer Drugs 2006;17(6):621-9.
[Crossref] [Google Scholar] [PubMed]
- Ottewell PD, Lefley DV, Cross SS, Evans CA, Coleman RE, Holen I. Sustained inhibition of tumor growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. Int J Cancer 2010;126(2):522-32.
[Crossref] [Google Scholar] [PubMed]
- Ottewell PD, Mönkkönen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst 2008;100(16):1167-78.
[Crossref] [Google Scholar] [PubMed]
- Ottewell PD, Woodward JK, Lefley DV, Evans CA, Coleman RE, Holen I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Mol Cancer Ther 2009;8(10):2821-32.
[Crossref] [Google Scholar] [PubMed]
- van de Donk NW, Lokhorst HM, Nijhuis EH, Kamphuis MM, Bloem AC. Geranylgeranylated proteins are involved in the regulation of myeloma cell growth. Clin Cancer Res 2005;11(2):429-39.
[Crossref] [Google Scholar] [PubMed]
- Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist 2006;11(3):306-15.
[Crossref] [Google Scholar] [PubMed]
- Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal 2010;8:23-41.
[Crossref] [Google Scholar] [PubMed]
- Karlic H, Haider F, Thaler R, Spitzer S, Klaushofer K, Varga F. Statin and bisphosphonate induce starvation in fast-growing cancer cell lines. Int J Mol Sci 2017;18(9):1982.
[Crossref] [Google Scholar] [PubMed]
- El-Refai SM, Brown JD, Arnold SM, Black EP, Leggas M, Talbert JC. Epidemiologic analysis along the mevalonate pathway reveals improved cancer survival in patients who receive statins alone and in combination with bisphosphonates. JCO Clin Cancer Inform 2017;1:1-2.
[Crossref] [Google Scholar] [PubMed]
- Lee Y, Lee KH, Lee GK, Lee SH, Lim KY, Joo J, et al. Randomized phase II study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res Treat 2017;49(4):1001-11.
[Crossref] [Google Scholar] [PubMed]