- *Corresponding Author:
- T. Phaechamud
Department of Pharmaceutical Technology, Faculty of Pharmacy, Silpakorn University, Nakorn Pathom 73000, Thailand
E-mail: thawatchaienator@gmail.com
| Date of Submission | 02 January 2014 |
| Date of Revision | 21 October 2014 |
| Date of Acceptance | 18 January 2015 |
| Indian J Pharm Sci,2015;77(1):62-74 |
Abstract
The objective of this investigation was to prepare the shellac wax matrix tablets by fusion and molding technique incorporated with Lutrol in different ratios to modify the hydrophobicity of matrix tablet. The matrix tablets with single drug were loaded either with propranolol hydrochloride or hydrochlorothiazide as hydrophilic and hydrophobic model drugs, and a dual drug formula was also prepared. The single and dual drug release patterns were studied in a dissolution apparatus using distilled water as medium. Propranolol hydrochloride released from matrix was easier than hydrochlorothiazide. Drug release from shellac wax matrix could be enhanced by incorporation of Lutrol. However retardation of drug release from some matrix tablets was evident for the systems that could form dispersion in the dissolution medium. The gel network from high content of Lutrol was hexagonal which was a dense and more compact structure than the other structures found when low amounts of Lutrol were present in the formula. Therefore, the formulae with high content of Lutrol could prolong drug release more efficiently than those containing low content of Lutrol. Hence shellac wax matrix could modulate the drug release with the addition of Lutrol. Sustainable dual drug release was also obtained from these developed matrix tablets. Thus shellac wax-Lutrol component could be used as a potential matrix tablet prepared with fusion and molding technique with excellent controlled drug release.
Keywords
Shellac wax-Lutrol, matrix tablet, drug release, fusion, molding technique
Introduction
Controlled release dosage form is a system to provide drug release in an amount sufficient to maintain the therapeutic drug level over extended periods of time, in which the release profile is controlled by special techniques [1]. The matrix tablet is one of the varieties of controlled release dosage form. It is designed to solve many drawback of the conventional dosage form [2]. The drug release from matrix tablet is mainly controlled by two mechanisms including dissolution control and diffusion control [3]. However, many factors could influence the drug release profiles which several drug release mathematic models are designed to conceptualize the true release mechanism [4-6].
The matrix tablet made from waxy material is a great potential for the time controlled release of drug [7]. The wax matrix tablet could be prepared by sintering method based on heating the waxy material and blending the other excipients into the molten wax [2].Some methods could be used to prepare the wax matrix including hot melt extrusion [8] or injection molding [9,10]. However, these methods compose of many processes and high cost of production. The melting and molding technique is an interesting and easier method to prepare the wax matrix tablet [11]. This method is based on melting waxy carrier and mixing with drug or other excipients before molding and solidifying.
Shellac wax (S) obtains from insect secretion of Laccifer lacca. This wax has been found in India, Thailand and other South East Asia. It is obtained about 5% as a by-product from shellac manufacturing or collected from a first melting of crude as initial substance before processing to be shellac [12]. This wax is used in agricultural manufacture for fruit or vegetable coating [13,14]. In pharmaceutical field, shellac is applied as compression coating for conventional tablet dosage form [15]. However the application of S as matrix base for controlled release has not been reported.
Poloxamers or Lutrols (L) are synthesized triblock copolymers. This group of copolymers consists of ethylene oxide (EO) and propylene oxide (PO) blocks arranged in a tri block structure. These copolymers have amphiphilic properties [16]. The hydrophilic polymer such as this polymer can tune up the drug release profile for waxy matrix due to the hydrophilic property of L hence it could create the pore and channel on the wax matrix which allowed higher content of dissolution medium penetration [17]. The incorporation of this polymer may enhance the drug release of S tablet therefore this L is used to tune up the drug release from S matrix in this experiment.
Propranolol hydrochloride (PRO) is nonspecific β-adrenergic blocker drug popularly used to treat many of cardiovascular diseases such as cardiac arrhythmia, angina pectoris, and myocardial infarction and hypertension. It is soluble in water [18]. It has to be taken orally for two or three times daily to treat the diseases as described above. Therefore, it will be convenient for patient if it is prepared into the controlled drug release dosage forms, which the administration is as once daily. Hydrochlorothiazide (HCT) is a thiazide group diuretic drug used to treat hypertension, edema or diabetes insipidus. This drug is sparingly soluble in water [18]. Both drugs are used together to treat hypertension as a combine formulation and has a market product named Inderide®. Therefore, PRO and HCT were used as hydrophilic and hydrophobic model drug in this investigation, respectively.
In this study, drug release pattern of sole and combined drug-loaded in matrix tablets prepared from fusion and molding technique of shellac wax with various ratio of Lutrol were studied. Physical properties of matrix tablets and physicochemical characterizations of the prepared mixtures were also investigated.
Materials and Methods
Hydrochlorothiazide (HCT, batch No I 1413891 was supplied by Government of Pharmaceutical Organization, Thailand). Propranolol HCl (PRO, lot no M080311, PC Drug Co., Ltd., Bangkok, Thailand), Lutrol F127 (L) (lot no WPDF563B, BASF, Ludwigshafen, Germany) and shellac wax (S) (Ake Shellac Co., Ltd., Lumpang, Thailand) were used as received. Ethylene glycol (lot no.1341646,POCH SA, Sowinskiego, Poland) and formamide (lot no. 0808223, Ajax Finechem Pty Ltd, Auckland, New Zealand) were used as solvent for contact angle determination.
Preparation of matrix tablets
Matrix tablets were prepared in different ratios of L and S at 0:10, 2:8, 3:7, 5:5, 7:3, 8:2 and 10:0. L and S were accurately weighed after deducted displacement value (DV) of each drug. DV of each drug was calculated by using equation as described previously [19,20]. The bases were melted by the order of melting point. The melting temperature was about 100° in order to obtain the soft and pourable molten mixture. PRO and HCT were used as hydrophilic and hydrophobic model drugs, respectively. The 25 mg/tablet of PRO or HCT was then incorporated into the molten mixtures and kept stirring until the drug and molten bases were completely mixed. The drug-loaded molten base was poured into 15 mm diameter stainless steel mold and kept at room temperature until the matrix tablet was solidified. The obtained single layer tablets were withdrawn from the mold and were kept in the desiccator. For combine drug matrix tablets, the 25 mg each of both drugs were combined and then incorporated into the tablet containing L and S at 3:7, 5:5, 7:3 and 10:0 ratios.
Weight variation, hardness, thickness and diameter
Weight variations of tablets were determined by analytical balance. Average weight and standard deviation were calculated (n=20). Ten tablets were observed for their hardness, thickness and diameter using hardness tester (TBH 325 TD, Basel, Switzerland), which simultaneously determined the thickness and diameter. Average and standard deviation of hardness, thickness and diameter were presented (n=10).
Study of water uptake and erosion
In order to evaluate the water uptake and erosion of each tablet, the tablets were individually weighed before dissolution testing as original dry weight. After dissolution test, each tablet was blotted to remove excess water and immediately weighed on analytical balance as wet weight and then all of them were dried at 60° for 24 h and kept in desiccator for at least 3 days and individually weighed as remaining dry weight. Water uptake and erosion were
evaluated gravimetrically according to the following Eqns., % water uptake=(wet weight–remaining dry weight)/remaining dry weight)×100....(Eqn. 1) and % erosion=((original dry weight–remaining dry weight)/original dry weight)×100....(Eqn. 2)
Determination of contact angle and surface free energy (SFE)
Contact angle could describe the wettability of any compound in the formulation. Moreover, it was used to calculate the SFE of those compounds. SFE could be used to describe many properties of compounds such as polarity or the miscibility of mixed component [21]. In this experiment, SFE was calculated using Wu’s Eqn., expressed below.

where cos Ѳ is the contact angle of a solvent; γ1 is the surface free energy of compound 1, respectively; γi d and γi p is dispersion and polar component of compound 1 or 2, respectively.
The contact angle of 0:10, 3:7, 5:5, 7:3 and 10:0 of L:S matrix tablets were determined by goniometer (FTA 1000, First Ten Angstroms, USA) using three solvents including distilled water, ethylene glycol and formamide (n=3). Each of solvent was dropped slowly onto the smooth surface of matrix tablets with collecting time at 10 s and calculated for SFE using Wu’s equation in the equipment program. SFE was calculated by the contact angle from two solvents. In this experiment, the contact angle of two solvents was paired and calculated for the SFE. SFE from each paired solvent were then averaged and reported.
Drug release study
Dissolution of PRO or HCT was studied using dissolution apparatus I (basket apparatus, RC-6, Minhua Pharmaceutical machinery Co., LTD., China) under 100 rpm of rotational speed in 900 ml distilled water at 37° which was used as dissolution medium. The 5 ml of samples were sampled at specific time interval by 5, 15, 30, 45 min, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7 and 8 h, respectively. The volume of sample solution removed was replaced with an equal volume of fresh dissolution fluid. The samples were analyzed by UV spectroscopy in order to measure the amount of drug release. The samples were examined at 289 and 271 nm for PRO and HCT, respectively.The cumulative drug release of PRO or HCT were calculated and plotted against time.
The dissolution of combined PRO and HCT matrix tablets were studied with the method as previously described. However, the amount of drug release was determined using first derivative UVspectroscopy technique (FUV). Drug release amount was determined at 297 and 336 nm for PRO and HCT, respectively. The cumulative drug release of PRO and HCT were calculated and plotted against time. The simultaneous determination of two drugs content was measured with FUV and the obtained spectra (D1) at 297 and 336 nm for PRO and HCT, respectively, was employed for this study. Range of linearity of PRO and HCT was 1.5-7.5 (r2=0.9999) and 3.6-18.0 μg/ml (r2=0.9996), respectively. % Recovery of PRO and HCT was 106.59 and 97.11, respectively. Precision was determined as intraday and interday precision. The RSD of intraday precision was 2.46 and 1.88% for PRO and HCT, respectively. For interday precision, the RSD was 2.23 and 1.57% for PRO and HCT, respectively. LOD of standard curve was found to be 0.10 and 0.49 μg/ml for PRO and HCT, respectively. LOQ was 0.31 and 1.48 μg/ml for PRO and HCT, respectively.
Mechanisms of drug release were evaluated by fitting of cumulative drug release data with mathematical release models. The models used in this experiment were zero order, first order, Higuchi’s model, power law expression and Hixson-Crowell cube root equation. The experimental cumulative drug release data within the range of 10-80% were used to evaluate the kinetic of drug release by least square fitting method. The data were fitted with the mathematical Eqns by nonlinear computer programme, Scientist for Windows, version 2.1 [22]. The coefficient of determination (r2) was used to indicate the degree of curve fitting. Goodness-of-fit was also evaluated using the Model Selection Criterion (msc) [22]. The parameters of each model in the software were T, F, K, Tl and N. The T expressed as time in minute of drug release, F was fractional drug release, K was the constant of each model, Tl was lag time of drug release and N was the n exponent value of power law model.
Determination of particle size and size distribution
Formula containing both L and S were possible to be a self-emulsification tablet according to the surface-active property of L and the wax or lipid component of S. The self-emulsification tablet is the tablet, which could form emulsion using the body fluid and a little vigorous stirring from the gastrointestinal motility. Normally, it contains only two main components, the surface active agent and lipid or wax component [20]. The 3:7, 5:5 and 7:3 L:S ratios were determined the particle size and particle size distribution to observe the size of particle in the dissolution medium which might be the emulsion system. After drug release test for 8 h, the dissolution medium of 3:7, 5:5 and 7:3 were measured for the particle size and size distribution using laser scattering particle analyzer (LA-950, Horiba; Japan) (n=3). The oil in water (o/w) emulsion mode was selected. The samples were investigated under circulation speed No. 3 and agitation speed No. 1. The particle size and size distribution were collected.
Results
Physical properties of matrix tablet containing L:S at different ratios
The physical properties of matrix tablet prepared from various ratios of L:S loaded with PRO, HCT and combined drug are shown in Tables 1 and 2, respectively. Tablet weight increased as the L content was increased. The weight variation of tablets containing the same ratio of L:S but different types of drug loading was not significantly different. The hardness tended to increase as the content of L was increased. However, the hardness of tablet containing 10:0 L:S was deducted. Because of the higher drug loading, the hardness of combined drug loaded formula was rather higher than that of sole drug loaded formulation. Owing to the limit of mold size, the thickness and diameter of obtained tablets were similar.
| Ratio | Weight±SD | Thickness±SD | Hardness±SD | Diameter±SD |
|---|---|---|---|---|
| of L:S | (mg) | (mm) | (Newton; N) | (mm) |
| (n=20) | (n=10) | (n=10) | (n=10) | |
| HCT | ||||
| 0:10 | 1002.2±15.5 | 6.46±0.05 | 149.00±19.65 | 14.74±0.06 |
| 2:8 | 1085.2±14.5 | 6.58±0.06 | 178.10±24.86 | 14.79±0.04 |
| 3:7 | 1138.7±16.8 | 6.62±0.05 | 176.70±15.52 | 14.75±0.02 |
| 5:5 | 1156.9±9.2 | 6.55±0.03 | 176.30±17.03 | 14.72±0.06 |
| 7:3 | 1204.9±5.9 | 6.59±0.04 | 203.50±22.41 | 14.74±0.04 |
| 8:2 | 1218.7±7.6 | 6.54±0.02 | 216.70±22.88 | 14.97±0.22 |
| 10:0 | 1298.4±2.9 | 6.59±0.04 | 196.90±14.79 | 14.93±0.06 |
| PRO | ||||
| 0:10 | 1002.1±10.6 | 6.49±0.03 | 159.60±19.46 | 14.67±0.08 |
| 2:8 | 1075.8±22.5 | 6.48±0.10 | 142.20±15.60 | 14.85±0.14 |
| 3:7 | 1077.2±17.9 | 6.47±0.06 | 141.50±11.32 | 14.77±0.10 |
| 5:5 | 1143.5±5.8 | 6.49±0.02 | 174.80±14.62 | 14.77±0.12 |
| 7:3 | 1192.4±8.3 | 6.57±0.03 | 193.40±21.08 | 14.85±0.04 |
| 8:2 | 1198.3±6.5 | 6.48±0.05 | 198.00±11.19 | 14.80±0.05 |
| 10:0 | 1287.9±9.4 | 6.60±0.03 | 189.60±12.62 | 14.90±0.05 |
Physical properties of hydrochlorothiazide (HCT) and propranolol HCl (PRO) matrix tablets containing various ratios of Lutrol (L):shellac wax (S), SD: Standard deviation
Table 1: Physical Properties Of Hct And Pro Matrix Tablets
| Ratio | Weight±SD | Thickness±SD | Hardness±SD | Diameter±SD |
|---|---|---|---|---|
| of L: S (mg) (n=20) (mm) (n=10) (Newton; N) (n=10) (mm) (n=10) | ||||
| 3:7 | 1098.2±27.4 | 6.64±0.04 | 200.50±47.52 | 14.91±0.04 |
| 5:5 | 1162.2±10.8 | 6.46±0.05 | 186.10±14.49 | 14.75±0.05 |
| 7:3 | 1197.3±8.8 | 6.53±0.04 | 309.70±35.49 | 14.77±0.09 |
| 10:0 | 1317.2±5.7 | 6.64±0.04 | 218.10±7.71 | 14.91±0.04 |
Physical properties of combined drug loaded matrix tablets containing various ratios of Lutrol (L): shellac wax (S). SD: Standard deviation
Table 2: Physical Properties Of Combined Drug Loaded Matrix Tablets
Drug release from single drug matrix formulation
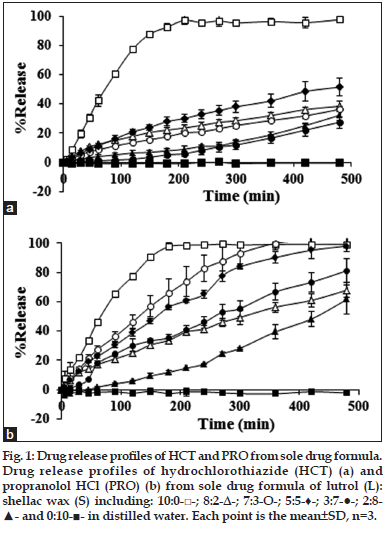
Dissolution profiles of HCT and PRO from tablets comprising different ratios of L:S are shown in fig. 1. The drug release was higher by the increment of L except for the tablets comprising 7:3 and 8:2 L:S which HCT release was lower. The tendency of PRO release also depended on the L content except for the ratio of 8:2, which its release was slower. At the same matrix bases ratio between these two drugs, the PRO release was faster than HCT according to the drug solubility properties. Both drugs released were fastest when they were incorporated in L, which almost drug released within 180 min. Both drugs could not release when they were incorporated in S. Incorporation of L could promote the drug release but the drug release did not only depend on the L content because the higher ratio of L in some case could promote the decrement of drug release.
Fig. 1: Drug release profiles of HCT and PRO from sole drug formula. Drug release profiles of hydrochlorothiazide (HCT) (a) and propranolol HCl (PRO) (b) from sole drug formula of lutrol (L): shellac wax (S) including: 10:0-□-; 8:2-Δ-; 7:3-О-; 5:5-♦-; 3:7-●-; 2:8- ▲- and 0:10-■- in distilled water. Each point is the mean±SD, n=3.
Drug release from combined drug formulation
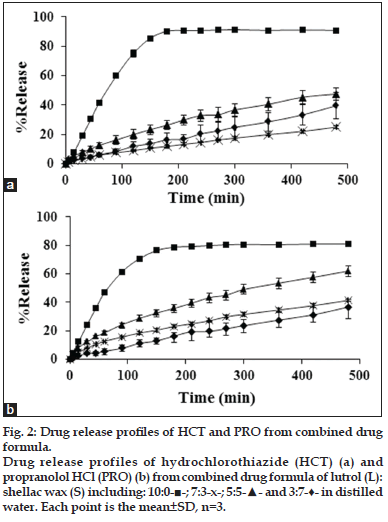
The dual drug release was investigated in order to observe that the combination of both drugs influence on the drug release or not. Both drugs were incorporated into 3:7, 5:5, 7:3 and 10:0 L:S. The 0:10, 2:8 and 8:2 L:S were discarded from the experiment because the drug release was very low. In addition, the drug release from the other two ratios were more closely by the 3:7 and 7:3 L:S which appeared in sole HCT formulation. The drug release from tablet prepared from 7:3 L:S was different from those containing sole drugs therefore it was interesting for more investigation. In the combined drug formulation, HCT release showed the same trend found in sole drug formulation, which a slightly higher drug release was evident (fig. 2). Surprisingly, PRO release did not follow the trend of the sole drug release. There was the release relevant with the HCT release which drug release was slower and found its deduction in 7:3 L:S. However, PRO could release faster than HCT when the L content increased except for 10:0, which both drugs could release with an apparent rapid release rate.
Fig. 2: Drug release profiles of HCT and PRO from combined drug formula. Drug release profiles of hydrochlorothiazide (HCT) (a) and propranolol HCl (PRO) (b) from combined drug formula of lutrol (L): shellac wax (S) including: 10:0-■-; 7:3-x-; 5:5-▲- and 3:7-♦- in distilled water. Each point is the mean±SD, n=3.
Analysis of drug release data; drug release pattern from single drug formulation
The degree of goodness-of-fit for release profiles of HCT and PRO to different mathematic equations is shown in Table 3. HCT did not release from the 0:10 L: S. However, HCT could release when L was incorporated into S. Increasing amount of L in formulation influenced the drug release pattern. The drug release from 2:8, 3:7 and 5:5 L:S were best fitted with zero order. Higuchi’s model release was obtained for the drug released from 7:3 and 8:2 L:S. In case of tablets made from L (10:0 L: S), drug release was found to be the best described by cube root law.
| L:S | Zero order | First order | Higuchi’s | Cube root | Power law | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | msc | r2 | msc | r2 | msc | r2 | msc | r2 | msc | n | |
| HCT | |||||||||||
| 10:0 | 0.9619 | 2.70 | 0.9940 | 4.54 | 0.9921 | 4.28 | 0.9989 | 6.54 | 0.9933 | 4.14 | 0.54 |
| 7:3 | 0.9982 | 5.89 | 0.9987 | 6.23 | 0.9887 | 4.04 | 0.9987 | 6.20 | 0.9988 | 6.03 | 0.84 |
| 5:5 | 0.9753 | 3.39 | 0.9931 | 4.67 | 0.9940 | 5.82 | 0.9886 | 4.16 | 0.9976 | 5.59 | 0.58 |
| 3:7 | 0.9940 | 4.72 | 0.9826 | 3.65 | 0.9406 | 2.42 | 0.9863 | 3.89 | 0.9963 | 5.00 | 1.67 |
| PRO | |||||||||||
| 10:0 | 0.9135 | 1.95 | 0.9918 | 4.31 | 0.9583 | 2.68 | 0.9942 | 4.48 | 0.9844 | 3.41 | 0.47 |
| 7:3 | 0.9858 | 3.94 | 0.9958 | 5.17 | 0.9947 | 4.94 | 0.9933 | 4.69 | 0.9990 | 6.48 | 0.60 |
| 5:5 | 0.9696 | 3.21 | 0.9960 | 5.24 | 0.9985 | 6.20 | 0.9904 | 4.36 | 0.9993 | 6.93 | 0.54 |
| 3:7 | 0.9917 | 4.39 | 0.9898 | 4.19 | 0.9693 | 3.09 | 0.9908 | 4.29 | 0.9917 | 4.19 | 0.95 |
Comparison of degree of goodness‑of‑fit from curve fitting of dissolution profiles of hydrochlorothiazide (HCT) and propranolol HCl (PRO) from matrix tablets containing different base ratios of Lutrol (L): shellac wax (S) in distilled water. r2 is the coefficient of determination and msc is model selection criterion
Table 3: Comparison Of Goodness‑Of‑Fit Of Dissolution Profiles From Matrix Tablets
For 0:10 L:S, PRO could not release from this base hence the release profile was not tested. PRO could release when L was incorporated into S as well as HCT-loaded formula. PRO released from 2:8 was best described by the zero order release kinetic. The 3:7 L:S was fitted well with Higuchi’s model. First order was fitted well for drug release from 5:5 L:S and the cube root law was used to describe drug release from 7:3 L:S. The Higuchi’s model was fitted well for PRO released from 8:2 L:S and the cube root law was best fitted for that of 10:0 L:S.
Dual drug release pattern
The degrees of goodness-of-fit of release profiles of combined drug to different mathematic equations are shown in Table 4. Both PRO and HCT showed the same release pattern from 3:7, 5:5, 7:3 and 10:0 L: S. The release pattern from 3:7 L:S showed the best fitted with the zero order but the release profile from 5:5 L:S fitted well with Higuchi’s model. For 7:3 L:S, the drug release pattern was the best described by first order model. The drug release from 10:0 L: S was fitted well with cube root law for both PRO and HCT as also found in sole drug formulation.
| L:S | Zero order | First order | Higuchi’s | Cube root | Power law | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | msc | r2 | msc | r2 | msc | r2 | msc | r2 | msc | n | ||
| HCT | ||||||||||||
| 10:0 | 0.9857 | 3.58 | 0.9870 | 3.68 | 0.9829 | 3.4 | 0.9981 | 5.61 | 0.9979 | 5.18 | 0.64 | |
| 8:2 | 0.9382 | 2.48 | 0.9860 | 3.97 | 0.9912 | 4.42 | 0.9767 | 3.47 | 0.9962 | 5.12 | 0.43 | |
| 7:3 | 0.9362 | 2.47 | 0.9848 | 3.90 | 0.9873 | 4.08 | 0.9796 | 3.61 | 0.9966 | 5.26 | 0.39 | |
| 5:5 | 0.9943 | 4.81 | 0.9573 | 2.79 | 0.9260 | 2.24 | 0.9736 | 3.30 | 0.9945 | 4.66 | 1.07 | |
| 3:7 | 0.9972 | 5.51 | 0.9702 | 3.11 | 0.9500 | 2.60 | 0.9944 | 4.82 | 0.9984 | 5.90 | 0.88 | |
| 2:8 | 0.9938 | 4.28 | 0.9149 | 2.02 | 0.8795 | 1.62 | 0.9842 | 3.49 | 0.9994 | 6.30 | 2.00 | |
| 0:10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| PRO | ||||||||||||
| 10:0 | 0.9851 | 3.64 | 0.9799 | 3.34 | 0.9564 | 2.56 | 0.9941 | 4.56 | 0.9950 | 4.44 | 0.72 | |
| 8:2 | 0.9494 | 2.65 | 0.9791 | 3.54 | 0.9867 | 3.96 | 0.9793 | 3.54 | 0.9870 | 3.80 | 0.53 | |
| 7:3 | 0.9893 | 4.14 | 0.9884 | 4.07 | 0.9882 | 4.00 | 0.9973 | 5.52 | 0.9990 | 6.33 | 0.70 | |
| 5:5 | 0.9525 | 2.74 | 0.9958 | 5.17 | 0.9950 | 4.99 | 0.9932 | 4.70 | 0.9956 | 4.96 | 0.47 | |
| 3:7 | 0.9903 | 4.30 | 0.9901 | 4.17 | 0.9925 | 4.45 | 0.9842 | 3.49 | 0.9991 | 6.44 | 0.67 | |
| 2:8 | 0.9788 | 3.41 | 0.9382 | 2.34 | 0.8426 | 1.41 | 0.9534 | 2.62 | 0.9938 | 4.54 | 0.98 | |
| 0:10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
Comparison of degree of goodness‑of‑fit from curve fitting of dissolution profiles of hydrochlorothiazide (HCT) and propranolol HCl (PRO) from combined drug formula containing different base ratios of Lutrol (L):shellac wax (S) in distilled water. ND=not determined; r2=coefficient of determination; msc=model selection criterion
Table 4: comparison of goodness‑of‑fit of dissolution profiles from combined drug Formulations
Water uptake and erosion studies
The water uptake and erosion of each sole drug tablets are shown in Table 5. The water uptake and erosion of PRO loaded formulation increased as the content of L increased until the L ratio was a half in formulation. The higher content of L in formulation caused the reducing of the water uptake and erosion as seen in 7:3 L:S, which the water uptake cannot calculate because the tablet was completely eroded. However, the tablet was not completely eroded when the L was increased to 8:2 L:S. The tablets showed a small swelling which % water uptake and erosion were 110.50±8.63% and 70.51±3.70%, respectively. These results for HCT-loaded tablets were similar to that of PRO. Increasing amount of L could promote the higher water uptake and erosion until the half ratio was reached then the erosion was decreased for 7:3 L:S but again increased at 8:2 L:S and finally become completely erosion for 10:0 L:S.However, the water uptake was increased as the L ratio increased until 7:3 L:S was reached. In addition, the % water uptake between 7:3 and 8:2 L:S was not quite different. Moreover, the overall % water uptake and erosion of HCT tablets was lower than PRO tablets and 7:3 L:S tablet still remained in dissolution medium. For 0:10 L:S of both PRO and HCT formulation, the tablet weight did not change at 8 h of dissolution study therefore there were no water sorption and erosion. In case of the 10:0 L:S, both PRO and HCT tablets completely dissolved. The water uptake and erosion pattern of combine drug tablets (Table 6) were similar to that as found in HCT formulation. Increasing of L could promote the higher water uptake and erosion, however, the erosion deducted when 7:3 L:S was used as matrix base.
| L:S | HCT | PRO | ||
|---|---|---|---|---|
| Water uptake | Erosion | Water uptake | Erosion | |
| 0:10 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| 2:8 | 19.54±4.33 | 21.13±2.68 | 27.33±2.32 | 29.52±5.21 |
| 3:7 | 26.31±3.24 | 30.26±4.92 | 77.42±3.10 | 56.07±5.22 |
| 5:5 | 95.66±10.21 | 45.07±5.40 | 230.16±26.29 | 89.9±1.47 |
| 7:3 | 105.41±2.56 | 22.01±3.10 | ND | 100±0.00 |
| 8:2 | 105.84±5.77 | 56.21±6.10 | 110.5±8.63 | 70.51±3.70 |
| 10:0 | ND | 100±0.00 | ND | 100±0.00 |
%Water uptake and erosion of hydrochlorothiazide (HCT) and propranolol HCl (PRO) matrix tablets from sole drug formula containing various bases containing different ratios of Lutrol (L): shellac wax (S) (mean+SD; n=3). ND=not determined
Table 5: %Water Uptake And Erosion Of Hct And Pro Matrix Tablets
| L: S | % water uptake | % erosion |
|---|---|---|
| 3:7 | 13.97±6.38 | 29.73±10.39 |
| 5:5 | 99.29±17.30 | 51.63±5.30 |
| 7:3 | 111.72±5.15 | 29.81±4.26 |
| 10:0 | ND | 100±0.00 |
%Water uptake and erosion of combined drug formula containing various bases containing different ratios of lutrol (L): shellac wax (S) (mean+SD; n=3) ND=Not determined
Table 6: %Water Uptake And Erosion Of Combined Drug Formulations
Contact angle and SFE
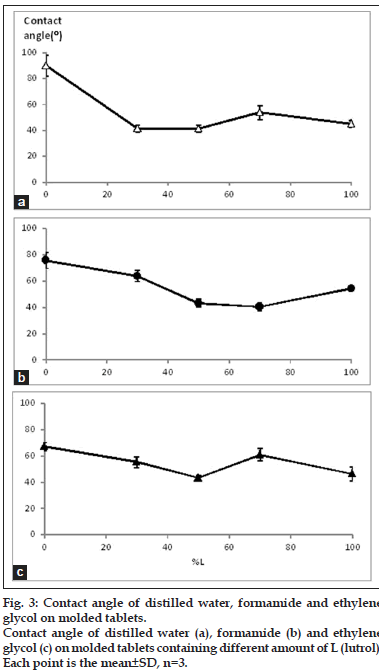
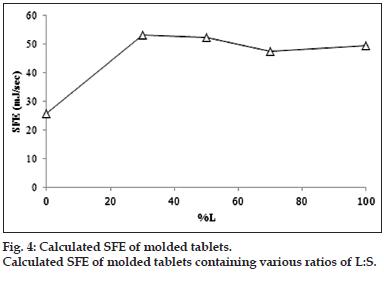
The contact angle on surface of molded tablet was used to determine SFE for determination of the wettability. The higher SFE indicates the higher polarity hence the system with high SFE could be easily spread with the fluid such as dissolution medium. In the other meaning, the low of contact angle could also indicate the easily spreading or miscible well of the medium on the prepared matrix. Contact angle of tablets loaded with the combined model drugs at various ratios of L:S are shown in fig. 3. The high contact angle was found when the ratio was 0:10 L:S. The other formula showed a small value with no difference of contact angle in each formula. Therefore, it could conclude that the distilled water hardly penetrated or spread on the surface of 0:10 L:S tablet because of its high hydrophobicity, but the distilled water could be easier spread on the surface of other formula. The SFE (fig. 4) showed the trend relevant with those of their contact angle which the high contact angle caused the low SFE indicating the lower polarity of the surface. Increment of L tended to increase the SFE. The SFE increased rapidly in 3:7 L:S then it seem constant but slightly decreased in the 7:3 L:S.
Particle size and size distribution of dispersed systems
From visual observation, the dissolution medium obtained from 3:7, 5:5 and 7:3 loaded with combined both PRO and HCT was not the clear solution during dissolution test while the obtained clear solution was evident for those of 10:0 and 0:10. Therefore the emulsion might be occurred from 3:7, 5:5 and 7:3 L:S owing to the surface active property of L and some part of S. Therefore, the determination of o/w particle size was done to prove this hypothesis. The experiment results indicated the evidence of dispersed particles appeared in the dissolution medium at different size and size distribution for 3:7, 5:5 and 7:3 L:S (Table 7). The emulsion from 3:7 L:S showed polydispersion which approximately three size of emulsion was found. In the other hand, the particle size and size distribution of droplets from 5:5 and 7:3 was not different. Both of them showed only two size of emulsion. The particle size from 7:3 L:S was smaller than the particle size from both 3:7 and 5:5 L:S.
| L:S | Mean | Mode |
|---|---|---|
| 3:7 | 39.35±8.27 | 12.19±0.19 |
| 5:5 | 58.13±3.42 | 16.99±1.32 |
| 7:3 | 57.88±1.98 | 16.28±0.02 |
Particle size of dispersed systems from 3:7, 5:5 and 7:3 Lutrol (L): shellac wax (S) in dissolution medium (mean+SD; n=3)
Table 7: Particle Size Of Dispersed Systems
Discussion
Tablets containing single or combined drug exhibited the same physical properties. The increased amount of L enhanced both tablet weight and hardness due to the higher density of L than that of S. Typically, the natural waxes contained many types of fatty compound, which influenced the molecular compact [23] therefore the density of wax comprising of these compounds were lower than that of L which composed only a unique structure. Hence the tablet made from high ratio of L on S was heavier. This also influenced on the hardness. The high fractal ratio from the wax component lowered the matrix hardness [24]. The fractal ratio was obtained from the number of each compound existed in each wax such as fatty acid and fatty alcohol. The arrangement of each compound in wax had a variety pattern, therefore the overall structure of those waxes did not compact well and to be brittle when it was fabricated into tablet. Molecular structure of polyethylene in L on the physical properties varied depending on chain branching and polymer molecular weight [25]. L arranged themselves with better alignment than those of S thus it could more compact and had more density than those of S. Therefore tablet loaded with high amount of L could promote heavier weight and more hardness. However, the decrement of hardness was found on 10:0 L:S tablet. This phenomenon could describe by the visual observation during tablet hardness test. The tablet containing L and S especially for 7:3 and 8:2 L:S could absorb more pressure force from the hardness tester. The tablet shrunk and then cracked unlike those made from S, which cracked easily when it was pressed. This might be the nature of S, which was hard but fragile due to the chemical arrangement as described previously unlike the L where the chemical structure is linear hence it could absorb more force resulting in more flexibility. When S was incorporated together with L, the tablet was both hard from S and flexible from L. Hence it could produce the tablet with higher toughness than the tablet made from 10:0 L:S.Both PRO and HCT in sole drug loaded formulation showed the similar trend of drug release. Increasing content of L promoted the higher drug release. This phenomena occurred only when the ratio of L was lesser than S. In formula with ratio of L higher than S, the drug release rate decreased (7:3 and 8:2 L:S for HCT and 8:2 for PRO). Typically, the drug release should increase as the content of hydrophilic polymer in hydrophobic matrix increased due to its hydrophilic property of the first one which could tune up the matrix erosion [17,26]. The incorporation of L could promote the drug release from lipid matrix such as from glyceryl palmitostearate [17]. Interestingly, this experiment showed the conflict result with the previous reports [17,26]. The sustained drug release profiles from those ratios could be explained with the visual observation of the matrix tablets. The matrix containing the higher ratio of L on S formula during dissolution testing revealed the swelling of tablet unlike those of the lower content of L on S formulation, which the tablet appeared to be eroded. This result could confirm by the water sorption and erosion study. L could form gel depending on its concentration and temperature [16], therefore the swelling of tablet which contained high content of L was owing to the gel formation. According to the swelling of matrix tablet from the higher ratio of L formulation, the drug release was sustained. However, the tablet containing lower content of L did not swell because the main component was S, therefore the polymer concentration was not enough to perform to be the gel structure thus the tablet eroded easily. However, the swelling of L in some matrices was rather strange because the drug release from 10:0 L:S, which was prepared with pure L showed rather fast drug release and the tablet was completely dissolved in the dissolution medium. It might be possible that the other compound could interact with either L or S and hence resulted in the formation of a nonerodable and swollen matrix tablet. Therefore, the physicochemical characterization was examined. The data obtained from differential scanning calorimetry (DSC), powder x-ray diffraction (PXRD), FT-infrared spectroscopy (FTIR) and hot stage microscopy (HSM) showed no interaction occurred between the drug and matrix bases except for the low amount of HCT in L, which could be the solid dispersion (data not shown). Therefore, the chemical interaction in dry state could not describe this behavior. Physical properties were aimed to clarify this result. L is the thermo reversible gel which can become a gel depending on its concentration and the temperature [16]. However, the major drawback of the gel from this polymer is its rapid erosion therefore it is not suitable to be used to prepare the sustained release formulation [27,28]. However, this drawback could be solved by adding hexamethylene diisocyanate into this polymer chain to overcome the rapid erosion of L and that it could prolong the drug release over 40 days [29]. However, the more easy method to provide the sustained release from L was also reported. The sustained release from L could be attained by strengthening the gel structure using the addition of other compounds into the gel structure [19,30]. They strengthened the gel structure by adding carrageenan to prolong the release of vaginal insert formula. The gel structure of L occurred by the rearrangement of PPO and PEO unimer of polymer chain. In the dissolution medium, the PPO firstly dehydrated and formed the inner layer micelle then PEO formed outer layer micelle due to its hydrophilic property. The spherical micelle was then attributed packing each other if it contained sufficient polymer to become a gel [28]. The rapid erosion of L was from the rapid decrease of polymer concentration in the excess amount of dissolution medium. The gel structure was unpacked and became a micelle then dissolved out into the medium. Therefore, the strengthen gel structure was done by supporting the network by adding the polymer such as carrageenan, methylcellulose or dextran. Those polymers supported the micelle network by interacting with hydrophilic PEO block through entanglement, facilitating and scaffolding [28]. From the reason described above, it was possible that S, PRO or HCT might influence on the micelle network of L and therefore the sustained release was occurred. The drug content and carrageenan could affect the sustained release of L based system of vaginal tablet [28]. The experiment found that the content of drug could also significant affect the drug release from poloxamer based system. The drug release rate decreased as content of acyclovir increased. According to the results, it could be concluded that all components physically influenced the micelle network of L and hence the gel was stabilized and promoted the sustained drug release. However, the prolongation of drug release for the PRO loaded formula containing the higher amount of L on S (8:2 L:S) could be described by the enhancement of gel strength by chloride ion as previously reported [16]. The chloride ion was from the salt of PRO, which was liberated after PRO dissolved. Moreover, from the high water solubility of PRO, the many pores inside matrix tablet were presented leading to high content of dissolution medium penetrated into the matrix tablet. Therefore, PRO loaded formula needed to use more content of L to overcome the effect of the liberated ion. In case of the lower content of L formulation, the polymer concentration was not enough to form gel structure or the gel network could not form because the high content of S which was the dissolution barrier hence the matrix tablets with lower content of L gradually eroded after contact to dissolution medium. Therefore, the incorporation of L could promote the higher drug release which was previously reported for an incorporated hydrophilic substance into hydrophobic matrix [17].
The drug release from combined drug loaded formulation was similar to that of the HCT single drug loaded formulation. The 7:3 could sustain both PRO and HCT. The addition of HCT and PRO together could overcome the decrement of gel strength by hydrochloride salt of PRO. The drug release from PRO was faster than that from HCT according to the hydrophilic property of PRO. The drug release from erodible polymer was separated into two cases, surface or bulk eroding polymer [31]. The drug release from L and lower ratio of L formula was surface erosion, which the polymer dissolution was much faster than the water intrusion into the polymer bulk hence the drug released upon the erosion front of the tablet and/or diffusion from the diffusion front of the tablet.
From the reason described above, the hydrophilic drug like PRO could release form both diffusion and erosion but the hydrophobic drug such as HCT was mainly released by erosion only. Therefore, PRO could release much faster than HCT. The release of PRO was significantly faster than HCT as the ratio of L was higher in the formulation. The high ratio of L promoted the high water penetration into the tablet, which promoted the longer diffusion front. Therefore, the solubility of drug could play the more significant impact on the drug release profile.
The water sorption and erosion were determined in order to profoundly understand the drug release behavior. Many researches have used these parameters to describe the drug release [9,10]. The water sorption increased as the L content increased in HCT-loaded tablets except for 10:0 L:S which the tablet was completely eroded. For PRO-loaded tablet, the increasing water sorption was followed by the increment of L with maximum at 5:5 L:S. The 7:3 and 10:0 L:S tablets completely eroded. The tablet prepared from 8:2 L:S could remain in dissolution medium but the water sorption was lesser than that of the 5:5 L:S tablet. The incorporation of L could produce more water uptake into the matrix tablet from its hydrophilicity. However, tablet prepared with some base ratio could not measure for the %water uptake because it completely eroded. The tablet erosion also increased as the L content increased except for HCT-loaded in 7:3 L:S tablet since the erosion decreased from the strength of gel network as described previously. The same result was found in 8:2 L:S PRO-loaded tablet which the erosion was lesser than that of 5:5 L:S tablet as confirmed from the gel formation by visual observation. The tablet comprising high L content (7:3 or 8:2 L:S) could swell in the dissolution medium unlike those of the other formula which the tablet did not swell but erode. The water uptake and erosion in combined formulation were found as the same trend as found in the sole drug loaded tablet. The increased L amount could produce much more water penetration into the tablet, which produced high %water uptake. However in the case of high enough of L concentration (7:3 L:S) the tablet could swell and result in the decrease of the tablet erosion.
The measurement of CA and SFE could apply to estimate the miscibility of many compounds as some experiments were attempted to investigate the micelle of hydrophobic poly(vinylidene fluoride) and hydrophilic poly(vinylpyrrolidone) [21]. The CA and SFE were used to estimate the miscibility of prepared tablets and the dissolution medium in this research work. The results described the more miscibility of tablet and distilled water when L was incorporated.
There was the emulsion like for the dissolution medium of some test tablets. The o/w emulsion was found with two size distributions for 5:5 and 7:3 L:S and three size distributions for 3:7 L:S. The size of system from 3:7 L:S was smallest when compared with those of two remaining bases. The emulsion was presented from the high content of surfactant together with fat compound dispersed in aqueous system with an agitation from the dissolution apparatus. Some types of dosage forms could form into an emulsion after it dispersed in aqueous system that they are called “self-emulsified tablets” [32]. Since oil droplets were dispersed in the water system, the o/w emulsion occurred. As discussed above, S composed of 4 fat compounds, which were fatty acid esters, free fatty alcohols, free fatty acids and hydrocarbons. The fatty alcohol and fatty acid ester containing hydroxyl group and ester group might partially dissolve in water and easily liberate from the wax component, then they might form the o/w emulsion owing to the surface-active property of L. However the medium might not contain only o/w emulsion owing to the limit content of fatty alcohol and fatty ester in S but it was the mixture between emulsions and micelles. The micelles could produce from L itself, if the system contained enough concentration or temperature as mentioned previously. Moreover, these micelles could assemble themselves to be a structure called liquid crystalline [33]. The liquid crystalline obtained from L could form the variety structures depended on the concentration and temperature such as cubic shape or hexagonal shape which from the single micelle and rod-shape micelle, respectively. The high concentration (66-75% by weight) of amphiphilic molecule in the system could produce hexagonal micelle structure, which was more dense and compact structure. In the other hand, cubic structure could be occurred at the lower concentration (18-64% by weight) [33,34]. According to these structures, the size varied depended on the ratio of L on S. the cubic shape and single unit micelle should be presented in 3:7 L:S, in which the size was smaller than those of the 5:5 and 7:3 L:S, in which the larger size was the hexagonal structure. The 5:5 and 7:3 L:S provided two size distributions since the almost structure was the hexagonal and o/w emulsion. In contrast, the 3:7 L:S, in which provided three size distributions might come from the size of single micelle, cubic structure and the o/w emulsion. The variety of shape of liquid crystalline affected the drug release as described previously. The gel network from high content of L was hexagonal which dense and more compact structure than the other structure found when low amount of L presented in the formula. Therefore, the formula with high content of L could prolong the drug release better than the low content of L.
The mathematic models of drug release were based on the real phenomena such as diffusion, dissolution, swelling, erosion, precipitation and/or degradation. The objective was to conclude the real phenomena into the mathematic model to estimate and describe drug release behavior from the selected formulation [35]. The power law expresses the drug release from the dosage forms, which indicates the release kinetic by n value, which depends on shape of dosage form. For cylindrical shape such as tablet, the n value nearly 0.45 indicated the Fickian release kinetic which the drug was released via diffusion control, the n value about 0.89 indicate the case-II transport which the drug is released depending on the swelling and erosion of polymer. The n value between those of 0.45 and 0.89 is indicated the drug release from both diffusion control of drug and swelling and erosion control of the polymer. The Hixon-Crowell cube root law or shortly as cube root law describes the drug release from the erosion of the matrix tablet is consistent with its geometry [5,6,35].
The tablet made from S could not produce the drug release due to its high hydrophobicity. The incorporation of L promoted drug release from S tablet. The release was fitted well with zero order for HCT tablet made from 2:8, 3:7 and 5:5 L:S but the PRO tablet released with zero order only for the systems comprising 2:8 L:S. The increasing of L could promote more porous on the tablet surface hence the hydrophilic drug could more dissolve and diffuse out from the tablet but the concentration gradient might not steady thus the drug release depended on the concentration of PRO as described by first order equation for tablet containing 5:5 L:S. However, the 3:7 L:S was fitted well with Higuchi’s because the porous on the surface of tablet was lesser than that of 5:5 L:S tablet therefore the solubility of PRO slightly affected on drug release. PRO was gradually dissolved and diffused out of tablet with best described by Higuchi’s model. For formula 7:3 and 8:2 L:S, the concentration of L was enough to form the gel structure in tablet. The gel strength depended on the amount of S, which decreased the water penetration rate due to its hydrophobicity. In case of 7:3 L:S loaded with PRO, the tablet completely eroded with constant its geometric shape because of the hydrophilicity of PRO and the effect of chloride ion as reported above. Chloride ion influenced the lowering of gel network strength. Moreover, PRO could easily dissolve and diffuse because of its hydrophilicity. The drug diffusion can enhance the void inside the gel network which promote the destruction of gel network and thereafter completely dissolved hence the release profile was best fitted with cube root law. Unlike the 7:3 L:S tablet loaded with HCT, this tablet did not completely erode but swelled. Moreover, the rate of drug release was slower than that of PRO. Because HCT could disperse into L it could not freely dissolve and diffuse. Its release depended on erosion of the matrix tablet and also its diffusivity from the polymer micelle or polymer structure. Therefore, HCT could promote more strength of gel network. Owing to the swelling of the tablet, the drug gradually dissolved and diffused out of that matrix and the concentration gradient of HCT was kept constant by the gel network hence its drug release was best described by Higuchi’s model. This result was similar to that of 8:2 L:S tablet in which both drug release profiles were best described by the same model. Increasing L amount could promote more concentration of the polymer resulted on the more compact of gel network which could overcome the hydrophilicity and salt effect of PRO therefore the tablet did not erode but swell and the drug released slowly with the constant of concentration gradient as described by Higuchi’s model. The tablets made from 10:0 L:S loaded with both HCT or PRO were completely eroded thus the cube root law which described the drug release from tablet erosion with constant geometric shape was the best fitted equation for these tablets.
The kinetic of drug release from combined formulation was similar to both HCT and PRO. However, some of them showed the different drug release kinetics when compared with its sole drug formulation. The total amount of drug in combined formulation was higher because they could influence on the gel strength. Therefore, the drug release was different from its single drug formulation especially for PRO formulation. The 7:3 L:S tablet loaded with both drugs did not completely erode because drug amount loaded was higher than the single drug formulation. The incorporation of HCT could overcome the hydrophilicity and there was the salt effect from PRO. Therefore, the tablet still remained in the dissolution medium. The drug release kinetic of 3:7 tablet was zero order for both drugs-loaded tablet since the drugs slowly released from the porous channel at the surface of matrix tablet. The release rate was controlled by the constant erosion, therefore the zero order drug release was attained. The drug release from tablet containing 5:5 was fitted well with Higuchi’s model from the reason as previously described for PRO release in 3:7 L:S sole drug loaded tablet. The drug release from 7:3 L:S was described by first order. The one of different factor between first order and Higuchi’s model was the concentration gradient which was the driving force of drug diffusion [36]. For the assumption of Higuchi’s model, the drug has the constant of diffusivity. If the matrix could keep the concentration gradient of drug inside matrix constancy, the drug released at the same diffusion rate, which depended on square root of time. In the other hand, if the concentration gradient could not keep constant, the drug release depended on its concentration which described well by first order kinetic. From the reason described above, the drug released from tablets containing 7:3 L:S could not keep the constant concentration gradient of drug inside the matrix tablet therefore both drugs released via the different concentration gradient with described by first order kinetic. The reason for the incapability to keep the constant concentration gradient in swollen gel for tablet comprising 7:3 L:S might describe by the higher initial drug loading moreover the hydrophilicity and the salt effect from PRO could disturb the gel strength resulting on the loosen of gel network. For 10:0 L:S tablet, both PRO and HCT release could fit well with cube root law as described previously.
Incorporation of hydrophilic L promoted higher drug release from S matrix tablet. The drug release and release kinetics varied depending on hydrophilicity of drug. Hydrophilic drug (PRO) released faster than that of hydrophobic drug (HCT). Increasing L content in tablet promoted faster drug release. However, for HCT loaded in 7:3 L:S and PRO loaded in 8:2 L:S tablets, the drug release profiles were apparently sustained because the gel formation occurred from these tablets. For combined formulation, the gel network occurred at the tablet made from 7:3 L:S, therefore, both drugs released slowly. The 3:7 L:S tablet showed the slowest drug release because the tablet composed of low content of L thus the tablet gradually eroded. Zero order release kinetic was obtained for both drugs at 3:7 L:S due to the balance between matrix erosion and drug diffusion. The first order kinetic was drug release behavior for 5:5 and 7:3 L:S tablets because of the more hydrophilic property for promoting more drug dissolution. Cube root law could be described the drug released from 10:0 L:S tablet which the drug released from matrix erosion with constant geometric shape. S which is natural product obtained as waste from shellac manufacturing process could be used as matrix base. The drug release from S matrix tablet could be tuned up by incorporation of hydrophilic polymer such as L.
Acknowledgements
This research work was supported by the Higher Education Research Promotion and National Research University (HERP and NRU), Office of the Higher Education Commission, Thailand, grant No. SURDI (57/01/02, HERP). We also thank for technical support from Research and Development Institute, Silpakorn University and the Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, Thailand.
References
- Pundir S, Badola1 A, Sharma D. Sustained release matrix technology and recent advance in matrix drug delivery system: A review. Int J Drug Res Tech 2013;3:12-20.
- Tiwari BM, Khare S, Mishra V, Bhargav, S. Matrix tablet: A potential drug carrier for oral drug delivery. J Pharm Res 2012;5:2448-56.
- Modi SA, Gaikwad PD, Bankar VH, Pawar SP. Sustained release drug delivery system: A review. Int J Pharm Res Dev 2011;2:147-60.
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:123-33.
- Kalam MA, Humayun M, Parvez N, Yadav S, Garg A, Amin S, et al.
- Release kinetics of modified pharmaceutical dosage forms: A review. Cont J Pharm Sci 2007;1:30-5.
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm Drug Res 2010;67:217-23.
- Siepmann J, Siepmann F. Mathematical modeling of drug release from lipid dosage form. Int J Pharm 2011;418:42-53.
- Chokshi R, Zia H. Hot-melt extrusion technique: A review. Iranian J Pharm Res 2004;3:3-16. Quinten T, Beer DT, Vervaet C, Remon JP. Evaluation of injection moulding as a pharmaceutical technology to produce matrix tablets. Eur J Pharm Biopharm 2009;71:145-54.
- Quinten T, Gonnissen Y, Adriaens E, Beer TD, Cnudde V, Masschaele B. et al. Development of injection moulded matrix tablets based on mixtures of ethyl cellulose and low-substituted hydroxyl propyl cellulose. Eur J Pharm Sci 2009;37:207-16.
- Mesnukul A, Mahadlek J, Phaechamud T. Formulation variables affecting physicochemical properties and drug release of matrix tablet fabricated with mold technique. Thai Pharm Health Sci J 2008;3:316-30.
- Edwards HG, Falk MJ. Fourier-transform raman spectroscopic study of unsaturated and saturated waxes. Spectrochim Acta A 1997;53:2685-94.
- Lau OL. Effect of growing season, harvest maturity, waxing, low O2 and elevated CO2 on flesh browning disorders in ‘braeburn’ apples. Postharvest Biol Tec 1998;18:131-41.
- Dou H, Ismail MA. Reduction of post-harvest pitting of citrus by changing wax by changing wax component and their concentration. P Fl St HorticSoc 1999;112:159-63.
- Mollohan K, Makhlouf JM. Wax-containing powder coatings. US Pat No. 3,872,040. 1975.
- Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res 2006;23:2709-28.
- Jannin V, Pochard E, Chambin O. Influence of poloxamers on the dissolution performance and stability of controlled-release formulations containing precirol® ATO5. Int J Pharm 2006;309:6-15.
- AHFS drug information. Wisconsin, USA: American soceity of health system pharmacists Inc; 2009. p. 1919-28; 2796-8.
- Akala EO, Adedoyin A, Ogunbona FA. Suppository formulation of amodiaquine: in vitro release characteristics. Drug DevInd Pharm 1991;17:303-7.
- Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersions: A review. Pakistan J Pharm Sci 2009;22:234-46.
- Chen N, Hong L. Surface phase morphology and composition of the casting films of PVDF–PVP blend. Polymer 2002;43:1429-36.
- MicroMath Scientist Handbook Rev. 7EEF. Salt Lake City, Utah, USA: MicroMath; 1995, p. 467.
- Ruguo Z, Hua Z, Hong Z, Ying F, Li K, Wenwen Z. Thermal analysis of four insect waxes based on differential scanning calorimetry (DSC). Procedia Eng 2011;18:101-6.
- Narine SS, Marangoni AG. Relating structure of fat crystal networks to mechanical properties: A review. Food Res Inter 1999;32:227-48.
- Sperati CA, Franta WA, Starkweather HW. The molecular structure of poly ethylene V. The effect of chain branching and molecular weight on physical properties. J Am ChemSoc 1953;75:6127-33.
- Lu C, Lu Y, Chen J, Zhang W, Wu W. Synchronized and sustained release of multiple components in silymarin from erodible glycerylmonostearate matrix system. Eur J Pharm Biopharm 2007;66:210-9.
- Gratieri T, Gelfuso GM, Rocha EM, Samento VC, Freitas OA, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm 2010;75:186-93.
- Kojarunchitt T, Hook S, Rizwan S, Rades T, Baldursdottir S. Development and characterization of modified poloxamer 407 thermoresponsive depot systems containing cubosomes. Int J Pharm 2011;408:20-6.
- Cohn D, Sosnik A, Levy A. Improved reverse thermo-responsive polymeric system. Biomaterials 2003;24:3707-14.
- Liu Y, Zhu YY, Wei G, Lu WY. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur J Pharm Sci 2009;37:306-12.
- Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev 2011;48:229-47.
- Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov Today 2008;13:606-12.
- Ivanova R, Lindman B, Alexandridis P. Effect of pharmaceutically acceptable glycols on the stability of the liquid crystalline gels formed by poloxamer 407 in water. J Colloid Interface Sci 2002;252:226-35.
- Müller-Goymann CC. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur J Pharm Biopharm 2004;58:343-56.
- Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm 2008;364:328-43.
- Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxyl propyl methylcellulose (HPMC). Adv Drug Deliv Rev 2011;48:139-57.