- Corresponding Author:
- N. Udupa
Manipal College of Pharmaceutical Sciences, Manipal University, Manipal-576 104, India
E-mail: n.udupa@manipal.edu
| Date of Submission | 05 June 2014 |
| Date of Revision | 12 January 2015 |
| Date of Acceptance | 21 July 2015 |
| Indian J Pharm Sci 2015;77(4):367-375 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Nasal drug delivery has now been recognized as a promising route for drug delivery due to its capability of transporting a drug to systemic circulation and central nervous system. Though nasal mucosa offers improved bioavailability and quick onset of action of the drug, main disadvantage associated with nasal drug delivery is mucocilliary clearance due to which drug particles get cleared from the nose before complete absorption through nasal mucosa. Therefore, mucoadhesive polymeric approach can be successfully used to enhance the retention of the drug on nasal mucosal surface. Here, some of the aspects of the stimuli responsive polymers have been discussed which possess liquid state at the room temperature and in response to nasal temperature, pH and ions present in mucous, can undergo in situ gelation in nasal cavity. In this review, several temperature responsive, pH responsive and ion responsive polymers used in nasal delivery, their gelling mechanisms have been discussed. Smart polymers not only able to enhance the retention of the drug in nasal cavity but also provide controlled release, ease of administration, enhanced permeation of the drug and protection of the drug from mucosal enzymes. Thus smart polymeric approach can be effectively used for nasal delivery of peptide drugs, central nervous system dugs and hormones.
Keywords
Nasal drug delivery, smart polymers, temperature responsive, pH responsive, ion responsive
Now days, there has been significant interest and developments in transdermal and transmucosal routes of drug administration because these routes of drug administration are noninvasive, self-administrable and can decipher the problems associated with oral route of drug administration such as first pass metabolism, drug degradation in variable pH condition in gastrointestinal tract, inadequate absorption and slow onset of action [1,2]. Transdermal delivery is the most widely considered route for topical drug delivery. But keratinized outermost layer of the skin i.e. stratum corneum can act as a permeability barrier for the transportation of the drug to the systemic circulation. Several mucosal surfaces such as nasal, rectal, vaginal, ocular and oral have been investigated as delivery routes and proved efficient due to low level of keratinization compared to skin. But rectal, vaginal and ocular route for systemic drug delivery possess lack of patient compliance and are more suitable for local drug delivery. Nasal and oral transmucosal routes have proved to be attractive for systemic drug delivery [1]. Several nasal and oral transmucosal products have been successfully marketed and various products are in developmental stage [1,3]. For some drugs, with intranasal administration it is possible to obtain pharmacokinetic profiles similar to those obtained after an intravenous injection [4]. Some of physiological features of the nasal and oral mucosa affecting permeability of drug molecules are discussed in Table 1 [1,3,5-8].
| Parameters | Nasal mucosa | Oral mucosa |
|---|---|---|
| Thickness | 1-2 mm | 500–600 µm in buccal; 100–200 µm in sublingual |
| Surface area | 150–180 cm2 Presence of microvilli enhances effective surface area |
100 cm2 |
| Permeability: Epithelium | The nasal mucosa possesses porous epithelium | Membrane coating granules release lipophilic materialinto the intercellular spaces to maintain epithelialcohesion, this lipophilic material slow down thepassage of hydrophilic materials across the epithelium |
| Endothelial basement membrane | Porous and thin structure of endothelial basement membrane possesses no restriction to the entry of the drug molecule to the systemic circulation | The charge on the constituents of the basal lamina and high level of hydration of connective tissues may limit the rate of penetration of lipophilic compounds |
| Vascularity | Arterial supply: External and internal carotid artery, maxillary artery via sphenopalatine artery, ophthalmic artery via ethemoid branches, facial artery, palatine artery Venous supply: Pterygoid plexus, Ophthalmic vein, facial vein | Arterial supply: External carotid artery, buccal artery, facial artery, infra orbital artery, posterior alveolar artery, sublingual artery |
Table 1: Physiological features of the nasal and oral mucosa affecting permeability of drug
Nasal cavity has been used from ancient time as a drug delivery route to alleviate diseases and disorders. One of the ‘panchakarmas’ stated in Ayurveda is ‘nasyakarma’, a practice in which a drug is administered through the nostrils [9]. Nasal cavity is an excellent doorway for the entry of the drug moiety to systemic circulation and central nervous system. As mentioned above nasal cavity is lined with mucous membrane with large absorptive surface area, low thickness, high vascularity, porous and thin endothelial basement membrane below the epithelium leads to high permeability of nasal mucosa. Therefore, after absorption through nasal mucous membrane, drug can be directly reach to the systemic circulation with enhanced bioavailability and improved onset of action [3,5,6]. Also it has the potential to target the drug directly across the blood brain barrier via olfactory and trigeminal nerve cells [10].
Nasal Anatomy and Physiology
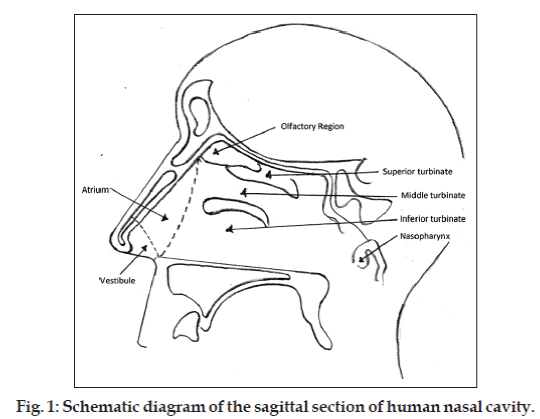
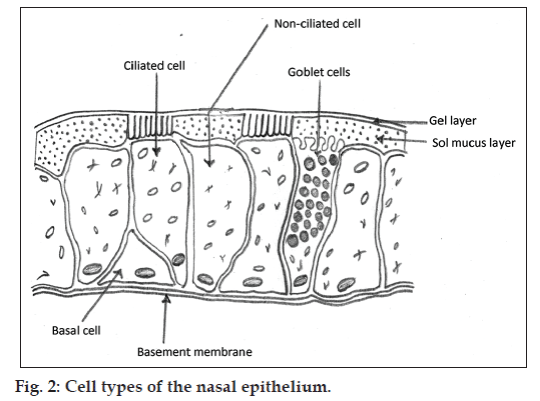
The human nasal cavity is separated into 2 halves by nasal septum and has the total volume of 15-20 ml. Nasal cavity is divided into 5 regions, nasal vestibule, atrium, respiratory region, olfactory region and extends posteriorly to nasopharynx. Vestibule is the most anterior part of the nasal cavity (fig. 1). Respiratory region occupies most of the volume of the nasal cavity and lined by respiratory epithelium. If we rank the permeability, vestibule is least, atrium is less and respiratory region is most permeable area of the nasal cavity. Beneath the respiratory epithelium, there is a thin and porous basement membrane. Respiratory mucosa comprises of several types of cells, ciliated pseudostratified columnar epithelial cells, goblet cells, basal cells and nonciliated cells (fig. 2). Basal cells are thought to be precursors of columnar and goblet cells. Goblet cells are mucosecretory cells. Ciliated pseudostratified columnar cells are the tall columnar cells which bear 4-6 μm long and 0.3 μm wide hairs like projections called cilia. There are approximately 100 cilia per cell also nonciliated and ciliated cells possess about 300 microvilli each. Cilia are responsible for mucocilliary clearance (MCC), the protective mechanism of respiratory system [6,11].
Mucosa of the nasal cavity is covered with mucus; Mucus is a complex submucosal secretion comprises of about 95% water, 2% mucin, 1% salts, 1% of other proteins for example albumin, immunoglobulins, lysozyme and lactoferrin, and 1% lipids. Nasal mucosa covered with the blanket of mucus which is 5 μm thick comprises of 2 layers, lower sol layer and upper gel layer. The cilia provide sweeping motion into the sol layer by moving back and forth. The entrapped foreign particles along with the gel layer get transported to the nasopharyngeal area for ingestion. The beating action exhibited by cilia at a frequency of 10 to 13 Hz results in the movement of mucus. As mucus moves at a rate of 5 to 6 mm per min, the particles get cleared within 20 min from nose. New mucus layer occupy the epithelium about every 10 min. The MCC can be influenced by environmental and pathological conditions. It specifies that the area of the high permeability characteristics in the nasal cavity where drug should be retained for its higher absorption, from that region only it is clearing rapidly due presence of ciliated cells [6,12]. However problems associated with the nasal drug administration are anterior leakage if dose volume is not maintained in between 25-200 μl and post nasal drip due to MCC [12]. Therefore it is necessary to enhance the contact time of the drug with nasal mucosal surface or prolong the retention of the drug on mucosal surface.
Now a days, various products are available in market to treat local and systemic conditions include nasal sprays and nasal drops [3]. Nasal drops have the problem of anterior leakage from nasal cavity. Liquid nasal sprays get spread on the nasal mucosa finely to provide prompt drug absorption but it may get cleared from plasma shortly. Extended or prolonged drug release hardly expected from such systems and formulations with the viscosity more than 0.5 Pa.s are difficult to be sprayed in nasal cavity [13]. Therefore in situ nasal gelling systems with smart polymers (Stimuli responsive polymers) came into the picture, which are liquid at a room temperature, and can be instilled easily or sprayed in nasal cavity and where they attain semisolid or gel form to get retained in nasal cavity. Nasal cavity has the temperature of about 32±2° and pH 5.5-6.5 also mucous secreted by nasal submucosal glands comprises of sodium, calcium and potassium ions. In response to these conditions certain temperature, pH and ion responsive polymers can undergo reversible gelation upon exposure to nasal cavity and can be used in delivering drug in controlled manner [12,14-16].
Advantages of smart polymers in nasal drug delivery [17]
Along with ease of administration, prolonged retention in nasal cavity and sustainable drug delivery, these systems possess some additional advantages such as, polymers used in stimulus responsive in situ nasal gel may have absorption enhancement effect on drug e.g. chitosan derivatives like trimethyl chitosan enable the paracellular transport of large molecules across the mucosal surface by opening tight junctions. Isoforms of the P450 enzyme, rhodanase, glutathione S-transferases, and carboxylesterases have been detected in the human nasal mucosa. Entrapment of the drug in viscous gel matrix can protect the drug from enzymatic degradation.
Temperature responsive polymers in nasal drug delivery
This type of polymers exhibit sol to gel transition upon exposure to the nasal temperature, for example poloxamer 407, which is a thermosensitive polymer frequently used for in situ gelation. It is a nonionic surfactant consists of polyoxyethylene-polyoxypropylene copolymers. At a given temperature when poloxamer is dispersed in aqueous phase above the critical micellar concentration, there is formation of micelles with hydrophilic shell and hydrophobic core. Micellization is mainly the function of hydrophobic block. At higher concentrations these micelles start arranging themselves in various structures (liquid crystalline phases) like lamellar, cubic and hexagonal. At higher temperature the hydrophilic chains of the copolymer (polyoxyethylene) become desolvated due to the rupture of the hydrogen bonds present between the chains and solvent. This leads to enhanced hydrophobic interactions among the polyoxypropylene chains, and leads to gel formation. At low temperatures a liquid micellar phase is stable however at high temperature it transforms into the cubic structure. Thermoreversible gelling properties of various poloxamer grades depends upon the molecular weight and ratio of molecular weight of hydrophilic core to molecular weight of hydrophobic core [18,19]. Poloxamer 407 aqueous solution 16-18% exhibited thermoresponsive gelling at 32±2°, which is closer to nasal temperature [20-22].
Though poloxamer is responsible for in situ gelling comparatively low molecular weight and nonionic nature makes the poloxamer weak mucoadhesive agent. Therefore to enhance the retention, mucoadhesive polymers like carbopol 934P, chitosan, sodium carboxymethyl cellulose (NaCMC), hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose and methylcellulose can be added to the poloxamer gel in the concentration range 0.2-0.5%. As mentioned above mucous comprises of mucin which is anionic polyelectrolyte rich in sulphate groups therefore polymers having ability to interact electronically or to form hydrogen bonds can act as good candidates for mucoadhesion. In general, it has been shown that the bioadhesive strength of a polymer increases with molecular weights above 1 00 000 D. Therefore anionic polymers like Carbopol 934P, NaCMC, HPMC K-15 due to their hydrogen bond forming ability with mucin and cationic polymers like chitosan and its derivatives, aminated gelatin due to their ability to form ionic interaction have proved efficient bioadhesives in nasal drug delivery [23]. Addition of mucoadhesive agents reduces the gelling temperature of the poloxamer [22]. Also other formulation additives can influence gelling time and temperature of poloxamer. Jadhav et al. formulated thermoresponsive nasal gel of Nardostachys jatamansi with poloxamer 407-polyethylene glycol (PEG) 400-PEG 4000 [24]. It is reported that as the concentration of the PEG 4000 increases there is increase in the gelling temperature. This can be predicted as; PEG is nonionic, hydrophilic compound, which may establish intermolecular hydrogen bonding with poloxamer chains and water. At the elevated temperature, this hydrophilic interaction has to be weakened and hydrophobic interaction between poloxamer chains should become dominant for gelling. Therefore with increasing concentration of PEG, there is delayed gelation time and temperature [24].
Hydrophobically modified polyelectrolytes
These are the alternatives for above discussed systems. Mucoadhesion of the poloxamers can be enhanced by using copolymers containing both hydrophobic segment (assist the copolymer to aggregate and gel) and a polyelectrolyte segment (provide mucoadhesiveness). Hydrophobically modified polyelectrolytes are the class of polymers where polymers having ionizable groups are attached to hydrophobic backbone. One of the example of this is copolymer of pluronic and poly(acrylic acid), which at low concentrations has shown thermogelling property and enhanced mucoadhesion. poly(acrylic acid) is the standard polymer used regularly as a benchmark for providing mucoadhesiveness. Bromberg worked with different polymers such as Pluronic-poly(acrylic acid) copolymers, Carbomer and Pluronic F127. The study reported enhancement of the residence time of fluorescent labels by the Pluronic-poly(acrylic acid) copolymers compared to other polymers in rat nasal cavity [25,26].
Actually poloxamer F127 is thermosensitive polymer and poly(acrylic acid) is pH-sensitive polymer. Due to presence of carboxylic acid in poly(acrylic acid), which get deprotonated at the basic pH and acquire negative charge. Thus polymers possessing similar charged group causes repulsion and the material expand in dimensions leading to gelation. In hydrophobically modified system, in order to achieve adequate mucoadhesion to the polymer, high concentration of the pH sensitive polymer i.e. poly(acrylic acid) has to be used. This makes physical mixture and/or random copolymer of thermosensitive and pH-sensitive polymer only pH-sensitive. It loses its thermosensitivity. But 1–5% w/v Pluronic-poly(acrylic acid) graft-copolymer (1:1) solution shows 10–103 fold increase in viscosity at the nasal temperature. Graft-copolymerization retains the thermosensitivity of Pluronic-poly(acrylic acid) copolymer at physiological pH [27].
Chitosan-based temperature responsive systems in nasal drug delivery
Aqueous chitosan solutions are pH-dependent gelling systems. Amino groups present on chitosan get protonated towards acidic pH (below the pKa value of chitosan 6.2) and repulsion between them causes expansion/swelling of the system thus pH-dependent gelling phenomenon is observed with chitosan [28]. But Nazar et al. synthesized thermosensitive in situ nasal gel from N-trimethyl chitosan chloride with PEG and glycerophosphate for drug delivery [29]. Basics of this thermosensitive gelling of chitosan found in U.S. patent 6,344,488 by Chenite et al. [30]
Chenite et al. mixed chitosan acidic solution with glycerophosphate aqueous solution, which can undergo gelling at 37° and pH above 6.5 [30]. Mechanism of this gelling was predicted as, at lower temperature strong interaction between chitosan and water prevents the aggregation of hydrated chitosan molecules. But at elevated temperature, (a) chitosan/chitosan inter chain hydrogen bonding; (b) chitosan/organophosphate electrostatic attractions (between ammonium group of chitosan and phosphate group of glycerophosphate; (c) structuring action of the polyol parts on water molecules facilitates hydrophobic interactions between chitosan chains. Thus chitosan-chitosan and chitosan-phosphate interaction at elevated temperature responsible for gelling [29].
Any solution of chitosan-glycerophosphate cannot undergo gelation as long as pH of the solution maintained below 6.45. Thermoreversible gelation property exhibited by chitosan-glycerophosphate solution in the pH range 6.5-6.9 and above pH 6.9 irreversible gelation occurs [29]. But drawback with this system is chitosan-based thermosensitive gel undergo a slow transition from sol to gel at body temperature because chitosan is soluble in low pH in its protonated form especially in the acidic environments. Therefore, Nazar et al. substituted chitosan with N-trimethyl chitosan chloride, a positively charged, water soluble chitosan derivative and added PEG 4000 which provides additional sites for hydrogen bonding and allows the formation of more extensive gel network [29]. N-trimethyl chitosan of medium average molecular weight and low degree of quaternisation (3.6% w/v) with 5.8% w/v PEG 4000 and 2.5% w/v glycerophosphate undergo thermal gelation at 32.5° within 7 min and exhibit good mucoadhesive properties [30]. Wu et al. formulated temperature sensitive hydrogel using N- [(2-hydroxy-3-trimethylammonium)propyl] chitosan chloride (HTCC) and PEG with a small amount of α ,β -glycerophosphate [31]. HTCC is water soluble, mucoadhesive derivative of chitosan which has absorption enhancement effect on nasal mucosa. Role performed by α,β-glycerophosphate and PEG are same as discussed earlier. Insulin loaded this thermogel delivered through nasal route showed enhanced retention, absorption and decreased blood glucose concentration drastically almost 40–50% of initial blood glucose concentration for at least 4 to 5 h after administration in rats [31]. Incorporation of glycerophosphate responsible for turbid nature of the gel and negatively charged moieties of glycerophosphate may interact with various bioactive components [32].
Chitosan-polyvinyl alcohol
Polyvinyl alcohol (PVA) is water-soluble polyhydroxy polymer. At the lower temperature PVA-chitosan is a liquid solution. At the room temperature there is existence of intermolecular H-bonds between –OH and –NH2 groups of chitosan and –OH groups of PVA, also H bonding between water and PVA due to hydrophilic nature of PVA. These hydrophilic interactions lead to dissolution of chitosan chains. At lower temperature low mobility of the chitosan chains prevent association of junction chains. At the higher temperature intermolecular H bonding gets ruptured, mobility of chitosan chains enhances which removes surrounding water molecules and increases association of hydrophobic chitosan chains with each-other. Thus, hydrophobic interaction between chitosan chains is dominant at higher temperature responsible for thermo responsible gelling while at lower temperature hydrophilic interactions of PVA with water and chitosan are dominant. If ratio of PVA to chitosan is exceeded than 10:1, temperature sensitive in situ gelling property vanishes [33].
Agrawal et al. [32] incorporated insulin in chitosan-PVA thermosensitive gel and evaluated in vitro and in vivo. Formulation containing 3% chitosan and 2% PVA showed thermo responsive gelling, high swelling index and the potential of controlling the blood sugar level for 6 h [32].
Poly(N-isopropylacrylamide) (PNiPAAm)
Some copolymers can be considered by their critical solution temperature around which their solubility behavior gets changed. In other words, hydrophobic and hydrophilic interactions between the polymeric chains and the aqueous media sharply get altered. Polymer is soluble in water when the temperature is lower than the lower critical solution temperature (LCST) and hydrophilic interactions between polymer chains and water are dominant, but as the temperature increases above the LCST hydrophobic interactions between polymer chains become stronger [34]. LCST of (PNiPAAm) is 32° therefore it can be effectively used for localization of drug in nasal cavity. Ryden and Edman [35] observed influence of intranasal administration of insulin on plasma glucose levels in rats by incorporating insulin in particulate systems based on solid epichlorohydrine cross-linked dextran spheres and 2 thermogels namely ethyl (hydroxyethyl) cellulose and (PNiPAAm)-co-polyacrylamide. Ethyl (hydroxyethyl) cellulose also possessed thermogelling characteristics like (PNiPAAm) and having LCST at 30-32°. Addition of small amount of ionic surfactant to ethyl(hydroxyethyl) cellulose leads to the formation of micelle aggregates, which interact with polymer chains at elevated temperature to form stiff gel [35]. Examples of thermresponsible nasal gels are shown in Table 2.
| Drug | Category | Composition of thermo-responsive gel | References |
|---|---|---|---|
| Venlafaxine hydrochloride | Dual acting antidepressant | 17% poloxamer 407, | [21] |
| 1% methocel A4M (mucoadhesive) | |||
| Fexofenadine hydrochloride | Antihistaminic | Poloxamer 407, chitosan (mucoadhesive) | [36] |
| Hydroxypropyl β-cyclodextrin | Antimalarial | Poloxamer 407 (18%), HPMC K4M (0.5–1.5%) | [37] |
| inclusion complex of artemether | |||
| Midazolam hydrochloride | Antiepileptic | 16% poloxamer 407, carbopol 934P, HPMC, mucilage | [20] |
| extracted from Ficuscarica (mucoadhesive agent) | |||
| Ondansetron hydrochloride | Management of nausea | 18% poloxamer 407, hydroxypropyl cellulose | [22] |
| and vomiting associated | |||
| with cancer chemotherapy | |||
| Metoclopramide | Antiemetic | Poloxamer 407, carbopol, polyethelene glycol 6000, | [38] |
| hydroxypropyl cellulose, PVA, chitosan | |||
| Sumatriptan | Antimigraine | Poloxamer 407, carbopol 934P | [39] |
| Plasmid DNA | - | Poloxamer 407 (12% w/w), poloxamer 188 (20% w/w), | [40] |
| polycarbophil, polyethelene oxide | |||
| Ropinirole | Dopamine agonist | Chitosan/β-glycerophosphate, HPMC for mucoadhesion | [41] |
| Ellagic acid | Treatment of brain cancer | Chitosan/β-glycerophosphate | [42] |
| Insulin | Peptide hormone | 3.6% HTCC, 5.4% PEG 4000, 3% α,β-glycerophosphate | [31] |
| Insulin | Peptide hormone | 3% chitosan and 2% PVA | [32] |
| Insulin | Peptide hormone | Poly(N-isopropylacrylamide) | [35] |
Table 2: Examples of thermoresponsible nasal gels
pH-responsive polymers in nasal drug delivery
Generally pH sensitive polymers are the ionisable moieties (weakly basic/weakly acidic) on the hydrophobic backbone. Polymers having acidic groups e.g. carboxylic acid group get deprotonated at the (basic) pH and acquire negative charge. Thus polymers possessing similar charged group causes repulsion and the material expand in dimensions. When pH becomes normal the functional groups lose their charge hence the repulsion disappears and the material regains its original shape. Same mechanism expected with the polymers having basic groups which get protonated in acidic pH and causes electrostatic repulsion [28]. Carbopol 934, an acrylic acid derivative showed in situ gelling by deprotonation at nasal pH. Nandgude et al. formulated pH induced in situ nasal gel of salbutamol sulphate using 0.4-0.5% w/v carbopol 934 for sustained release and enhanced bioavailability whereas chitosan exhibit acidic pH-responsive gelation [43]. Amino groups present on chitosan get protonated towards acidic pH and repulsion between them causes expansion/swelling of the system thus pH dependent gelling [28].
Hornof et al. evaluated viscoelastic properties of chitosan-thioglycolic acid conjugates in vitro [44]. Chitosan-thioglycolic acid also called as thiolated chitosan. This polymer is formed by amide linkage between amino group of chitosan and carboxylic group of thioglycolic acid. In situ pH dependent gelation due to formation of intermolecular and intramolecular disulphide bonds at physiological pH was reported with this polymer. At the physiological pH, concentration of the H+ ions decreases due to which thiol groups (-SH) present on the chitosan get converted to thiolated ions (S−) which represents active form for oxidation to form intermolecular and intramolecular disulphide bonds. Also thiolated chitosan has superior mucoadhesive properties over unmodified chitosan. This new excipient found promising for gelation at physiological pH because the elastic properties of this gel were found to increase significantly with the degree of thiolation at pH 5.5 [44].
Polymethacrylic acid and polyethylene glycol (P(MAA-g-EG))
Nakamura et al. formulated mucoadhesive pH sensitive budesonide micro particles of polymethacrylic acid and PEG for nasal delivery [45]. Mechanism behind its swelling at nasal pH is same as that of carbopol i.e. deprotonation at nasal pH and deswelling in acidic pH due to its strong intermolecular interaction with PEG. Following intravenous administration of budesonide, the plasma concentration peaked immediately and decreased rapidly over the next 4 h but with nasal administration of the polymeric formulations, the peak plasma concentration was reached in about 45 min, and the concentration in plasma remained constant for a minimum of 8 h. Thus intranasally administered budesonide-polymer possess enhanced durability of the drug concentration in plasma [45].
Polyvinylacetal diethylamino acetate
Aikawa et al. [46] incorporated chlorpheniramine maleate and tetrahydrozoline hydrochloride in polyvinylacetal diethylamino acetate pH sensitive gel. Polyvinylacetal diethylamino acetate forms transparent solution at pH 4, which shows abrupt changes at pH 7 by turbidometry studies. This change appeared to be due to precipitation or hydrogel formation. The hydrogel formation on the mucous membranes in the rat nasal cavity was visually confirmed. Dynamic light scattering and scanning electron microscopy indicate there is pore shrinkage of the hydrogel with increase in the temperature from 25-37° at pH 7.4, which potentially responsible for controlling the drug release [46,47]. Examples of pH responsive nasal gels are listed in Table 3.
| Drug | Category | Composition of pH-sensitive gel | References |
|---|---|---|---|
| Salbutamol sulphate | β2-adrenergic agonists used in treatment of bronchospasm | 0.4–0.5% carbopol934P, HPMC | [43] |
| Budesonide | Glucocorticoid steroid used for the treatment of asthma, rhinitis | Polymethacrylic acid and polyethylene glycol | [45] |
| Chlorpheniramine maleate, tetrahydrozoline hydrochloride | Antihistaminic | Polyvinylacetal diethylamino acetate 3–7%3–7% | [46] |
Table 3: Examples of ph-responsive nasal gel
Ion responsive polymers in nasal drug delivery
Ion responsive polymers generally have ionisable groups. These polymeric systems exhibit uncommon rheological behavior upon coulombic interaction with appositely charged species. Gellan gum is anionic polysaccharide composed of 1,3-β-D-glucose, 1,4-β-D-glucoronic acid, 1,4-β-D-glucose and 1,4-α-Lrhamnose repeat units. It has the characteristic property of temperature-dependent and cation-induced gelation. Upon complexation with cations and hydrogen bonding with water, there is formation of double helical junction zones and a three-dimensional network responsible for in situ gelling [48].
Cao et al. developed ion-activated in situ gel of mometasone furoate with 0.2-0.5% w/v gellan gum and 0.15% xylan gum [49]. Sodium, potassium and calcium ions present in nasal mucous interact with anionic groups of the gellan gum which results into sol to gel transition to produce prolonged release of mometasone furoate [15]. Also in situ nasal gel formulation of scopolamine hydrobromide with gellan gum was compared with subcutaneous and oral administration in rats, the study exhibited decreased symptoms of motion sickness with nasal gel formulation. Prolonged radioactivity of 99mTc in the rabbit nasal cavity indicated prolonged retention of gel in nasal cavity [49].
Krauland et al. modified gellan gum by linking covalently l-cysteine to deacetylated gellan gum [50]. The deacetylated gellan gum-cysteine conjugate displayed superior in situ gelling properties in vitro compared to unmodified polymer [50].
Another famous example of ion responsive in situ gelling nasal drug delivery is PecFent® (fentanyl citrate) nasal spray which uses Archimedes Pharma’s patented drug delivery system, PecSys™. PecSys™ is a proprietary pectin-based drug delivery system which delivers fentanyl in controlled manner with fast onset of action and is designed as fine mist spray which forms a gel when it comes in contact with the nasal mucosa. In PecFent® gelling agent is a mixture of sucrose and low methoxyl (LM) pectin. Pectins are heterogeneous polysaccharides comprising a backbone of galacturonic acids units linked by α-1,4 bonds, with a component of neutral sugars such as galactose, xylose, rhamnose and arabinose either in the backbone or as side chains. LM pectin has the degree of esterification ≤50%. High methoxyl (HM) pectins have a degree of esterification (DE) of >50%. Gelling properties of pectin are highly affected by degree of esterification. LM pectins can form gel in the presence of divalent cations, such as calcium following similar mechanism of gellan gum due to the formation of intermolecular junction zones by side by side association of homogalacturonic smooth regions of different chains. Unlike LM pectin HM-pectin forms gel with sugar and acid but in PecFent® sucrose may be added to stabilize the structure of junction zones [51-54].
When nasal fentanyl-pectin spray compared with fentanyl chitosan and fentanyl in chitosan-poloxamer 188 in phase I studies, it exhibited the most favorable tolerability profile and lowest nasal reactogenicity symptom incidence compared to fentanyl chitosan and fentanyl in chitosan-poloxamer 188. In pharmacokinetic studies, PecFent showed superior pharmacokinetic profile compared to transmucosal oral fentanyl citrate [55]. Ion sensitive nasal in situ gels are listed in Table 4.
| Drug | Category | Gelling agents | References |
|---|---|---|---|
| Fentanyl citrate | Management of break through pain in cancer | 1% w/v pectin LM | [56] |
| Gastrodin | CNS diseases such as vertigo, headache, insomnia, neuralgia, neurasthenia and epilepsy | 0.5% w/v deacetylated gellan gum | [57] |
| Mometasone furoate | Antiinflammatory in the treatment of allergic rhinitis | Gellan gum (0.2–0.5%), xylan gum (0.15%) | [15] |
| Dimenhydrinate | Antiemetic | Gellan gum and carbopol 934P | [58] |
| Radix bupleuri | Antipyretic, antiinflammatory | 0.5% w/v gellan gum | [59] |
| Scopolamine hydrobromide | Antiemetic | 0.2–1% w/v gellan gum | [49] |
LM: Low methoxyl, CNS: central nervous system
Table 4: Ion sensitive nasal in-situ gels
Conclusion
Era of the nasal drug delivery has already been started and we can expect more nasal products in upcoming years in market. Nasal drug delivery has the great future with peptides/protein drugs, CNS drugs, drugs in crisis treatment as well as in long term treatment. Problems associated with retention of the drug, permeability and physicochemical properties of the drug can be deciphered with development of stimuli responsive polymeric approaches. Still in situ gel forming nasal drug delivery limited to very few stimuli responsive polymers. Stimuli responsive polymers like poly(N,N-diethyl acrylamide), poly(N-vinyl caprolactum) having LCST which can be modulated nearer to the nasal temperature, grafted copolymers can also be used in in situ gelling nasal drug delivery. Stimuli responsive vesicular, nanoparticulate systems can be expected of great potential towards efficient nasal drug delivery systems.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hearnden V, Sankar V, Hull K, Juras DV, Greenberg M, Kerr AR, et al. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deliv Rev 2012;64:16-28.
- Jain KK, editor. Drug delivery systems – An overview. In: Drug Delivery Systems. 1st ed. Basel, Switzerland: Humana Press; 2008. p. 3.
- Behl CR, Pimplaskar HK, Silenoy AP, deMeireles J, Romeo VDEffects of physicochemical properties and other factors on systemic nasal drug delivery. Adv Drug Deliv Rev 1998;29:89-116.
- Illum L. Nasal drug delivery: New developments and strategies. Drug Discov Today 2002;7:1184-9.
- Sarkar MA. Drug metabolism in the nasal mucosa. Pharm Res 1992;9:1-9.
- Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv Drug Deliv Rev 2005;57:1640-65.
- Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release 2011;153:106-16.
- Verma S, Kaul M, Rawat A, Saini S. An overview on buccal drug delivery system. Int J Pharm Sci Res 2011;2:1303-21.
- Patwardhan S. Ayurveda-foryou.com; cc2000-14. Available from: http://ayurveda-foryou.com/panchakarma/nasya.html. [Last cited on 2014 Jan 10].
- Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 2008;9Suppl 3:S5.
- Kim DD. In vitro cellular models for nasal drug absorption studies. In: Ehrhardt C, Kim KJ, editors. Drug Absorption Studies: In Situ, InVitro, In Silico Models. 1st ed. New York: Springer; 2008. p. 216-34.
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today 2002;7:967-75.
- Nakamura F, Ohta R, Machida Y, Nagai T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int J Pharm 1996;134:173-81.
- Lindemann J, Leiacker R, Rettinger G, Keck T. Nasal mucosal temperature during respiration. ClinOtolaryngol Allied Sci 2002;27:135-9.
- Cao SL, Ren XW, Zhang QZ, Chen E, Xu F, Chen J, et al. In situ gel based on gellan gum as new carrier for nasal administration of mometasonefuroate. Int J Pharm 2009;365:109-15.
- Madan M, Bajaj A, Lewis S, Udupa N, Baig JA. In situ forming polymeric drug delivery systems. Indian J Pharm Sci 2009;71:242-51.
- Rahisuddin, Sharma PK, Garg G, Salim M. Review on nasal drug delivery system with recent advancement. Int J Pharm PharmSci 2011;3:6-11.
- Escobar-Chávez JJ, López-Cervantes M, Naïk A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm PharmSci 2006;9:339-58.
- Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 2007;66:303-17.
- Basu S, Bandyopadhyay AK. Development and characterization of mucoadhesivein situ nasal gel of midazolam prepared with Ficuscarica mucilage. AAPS PharmSciTech 2010;11:1223-31.
- Pund S, Rasve G, Borade G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur J Pharm Sci 2013;48:195-201.
- Bhalerao AV, Lonkar SL, Deshkar SS, Shirolkar SV, Deshpande AD. Nasal mucoadhesivein situ gel of ondransetron hydrochloride. Indian J Pharm Sci 2009;71:711-3.
- Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm 2009;71:505-18.
- Jadhav PP, Jadhav NR, Hosmani AH, Patil S. Development and evaluation of in situthermoresponsive nasal gel system for Nardostachysjatamansi. Pharm Lett 2013;5:113-25.
- Bromberg L. Poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide)-g-poly(acrylic acid) copolymers as in-situ vehicle for nasal delivery. In: Rathbene MJ, Hadgraft J, Roberts MS, editors. Modified Release Drug Technology. 1st ed. New York: Marcel Dekker; 2002. p. 749-58.
- Bromberg LE. Enhanced nasal retention of hydrophobically modified polyelectrolytes. J Pharm Pharmacol 2001;53:109-14.
- Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels – Review of temperature-sensitive systems. Eur J Pharm Biopharm 2004;58:409-26.
- Kun N, Bae YH. pH sensitive polymers for drug delivery. In: Kwon GS, editors. Polymeric Drug Delivery Systems. 1st ed. Florida, USA: Talor and Francis Group; 2005. p. 129-94.
- Nazar H, Fatouros DG, van der Merwe SM, Bouropoulos N, Avgouropoulos G, Tsibouklis J, et al.Thermosensitive hydrogels for nasal drug delivery: The formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm 2011;77:225-32.
- Chenite A, Chaput C, Cpmbes C, Selmani A, Jalal F. Temperature-Controlled pH-Dependant Formation of Ionic Polysaccharide Gels. US Patent 6344488; 2002.
- Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials 2007;28:2220-32.
- Agrawal AK, Gupta PN, Khanna A, Sharma RK, Chandrawanshi HK, Gupta N, et al. Development and characterization of in situ gel system for nasal insulin delivery. Pharmazie 2010;65:188-93.
- Zao J. Chitosan-based gels for the drug delivery system. In: Yao K, Li J, Yao F, Yin Y, editors. Chitosan-Based Hydrogels: Functions and Applications. 1st ed. USA: CRC Press; 2011. p. 263-314.
- Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev 2002;54:37-51.
- Rydén L, Edman P. Effect of polymers and microspheres on the nasal absorption of insulin in rats. Int J Pharm 1982;83:1-10.
- Cho HJ, Balakrishnan P, Park EK, Song KW, Hong SS, Jang TY, et al.Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J Pharm Sci 2011;100:681-91.
- Mahajan HS, Shah SK, Surana SJ. Nasal in situ gel containing hydroxypropyl β-cyclodextrin inclusion complex of artemether: Development and in vitro evaluation. J InclPhenomMacrocyclChem 2011;70:49-58.
- Zaki NM, Awad GA, Mortada ND, AbdElhady SS. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesivein situ gel with modulated rheological and mucociliary transport properties. Eur J Pharm Sci 2007;32:296-307.
- Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech 2006;7:67.
- Park JS, Oh YK, Yoon H, Kim JM, Kim CK. In situ gelling and mucoadhesive polymer vehicles for controlled intranasal delivery of plasmid DNA. J Biomed Mater Res 2002;59:144-51.
- Khan S, Patil K, Bobade N, Yeole P, Gaikwad R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J Drug Target 2010;18:223-34.
- Kim S, Nishimoto SK, Bumgardner JD, Haggard WO, Gaber MW, Yang Y. A chitosan/beta-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010;31:4157-66.
- Nandgude T, Thube R, Jaiswal N, Deshmukh P, Chatap V, Hire N.Formulation and evaluation pH induced in-situ nasal gel of salbutamol sulphate. Int J Pharm SciNanotechnol 2008;1:177-83.
- Hornof MD, Kast CE, Bernkop-Schnürch A. In vitro evaluation of the viscoelastic properties of chitosan-thioglycolic acid conjugates. Eur J Pharm Biopharm 2003;55:185-90.
- Nakamura K, Maitani Y, Lowman AM, Takayama K, Peppas NA, Nagai T. Uptake and release of budesonide from mucoadhesive, pH-sensitive copolymers and their application to nasal delivery. J Control Release 1999;61:329-35.
- Aikawa K, Mitsutake N, Uda H, Tanaka S, Shimamura H, Aramaki Y, et al. Drug release from pH-response polyvinylacetaldiethylaminoacetate hydrogel, and application to nasal delivery. Int J Pharm 1998;168:181-8.
- Aikawa K, Matsumoto K, Uda H, Tanaka S, Shimamura H, Aramaki Y. Hydrogel formation of the pH response polymer polyvinylacetaldiethylamino acetate (AEA). Int J Pharm 1998;167:97-104.
- Yuguchi Y, Urakawa H, Kitamura S, Wataoka I, Kajiwara K. The sol-gel transition of gellan gum aqueous solutions in the presence of various metal salts. Prog Colloid PolymSci 1999;114:41-7.
- Cao SL, Zhang QZ, Jiang XG. Preparation of ion-activated in situ gel systems of scopolamine hydrobromide and evaluation of its antimotion sickness efficacy. ActaPharmacol Sin 2007;28:584-90.
- Krauland AH, Leitner VM, Bernkop-Schnürch A. Improvement in the insitu gelling properties of deacetylatedgellan gum by the immobilizationof thiol groups. J Pharm Sci 2003;92:1234-41.
- Illum L. Nasal drug delivery – Recent developments and future prospects. J Control Release 2012;161:254-63.
- Watts P, Smith A. PecSys: In situ gelling system for optimised nasal drug delivery. Expert Opin Drug Deliv 2009;6:543-52.
- www.medicines.org.uk. Datapharm Communications Ltd.; c2014. Available from: http://www.medicines.org.uk/emc/medicine/23962/SPC/ pecfent. [Last updated on 2014 Feb 28; Last cited on 2014 Mar 08].
- Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: A review. SilpakornUniv J SocSciHumanit Arts 2003;3:206-23.
- Fisher T, Knight A, Watling M, Smith A. Fentanyl pectin nasal spray (FPNS) with PecSys® provides most favorable pharmacokinetic/ tolerability profile compared with nasal chitosan-based fentanyl and oral transmucosal fentanyl citrate (OTFC). J Pain 2009;10 Suppl:S45.
- Castile J, Cheng YH, Simmons B, Perelman M, Smith A, Watts P. Development of in vitro models to demonstrate the ability of PecSys®, an in situ nasal gelling technology, to reduce nasal run-off and drip. Drug DevInd Pharm 2013;39:816-24.
- Cai Z, Hou SX, Yang ZX, Song XR, Chen QH, Li YB, et al. Brain targeting of gastrodin nasal in situ gel. Sichuan Da XueXueBao Yi Xue Ban 2008;39:438-40.
- Belgamwar VS, Chauk DS, Mahajan HS, Jain SA, Gattani SG, Surana SJ. Formulation and evaluation of in situ gelling system of dimenhydrinate for nasal administration. Pharm DevTechnol 2009;14:240-8.
- Chen E, Chen J, Cao SL, Zhang QZ, Jiang XG. Preparation of nasal temperature-sensitive in situ gel of Radix bupleuri and evaluation of the febrile response mechanism. Drug DevInd Pharm 2010;36:490-6.