- Corresponding Author:
- M. Shah
Gujarat Forensic Sciences University, Institute of Research and Development, Near Police Bhavan, Sector 18A, Gandhinagar-382 007
E-mail: malay2512@yahoo.co.in
| Date of Submission | 21 August 2011 |
| Date of Revision | 24 October 2012 |
| Date of Acceptance | 26 October 2012 |
| Indian J Pharm Sci, 2012, 74 (5): 434-442 |
Abstract
The aim of this study was to understand and investigate the relationship between experimental factors and their responses in the preparation of ciprofloxacin hydrochloride based solid lipid nanoparticles. A quadratic relationship was studied by developing central composite rotatable design. Amount of lipid and drug, stirring speed and stirring time were selected as experimental factors while particle size, zeta potential and drug entrapment were used as responses. Prior to the experimental design, a qualitative prescreening study was performed to check the effect of various solid lipids and their combinations. Results showed that changing the amount of lipid, stirring speed and stirring time had a noticeable influence on the entrapment efficiencies and particle size of the prepared solid lipid nanoparticles. The particle size of a solid lipid nanoparticle was in the range of 159â??246 nm and drug encapsulation efficiencies were marginally improved by choosing a binary mixture of physically incompatible solid lipids. Release of ciprofloxacin hydrochloride from solid lipid nanoparticle was considerably slow, and it shows Higuchi matrix model as the best fitted model. Study of solid lipid nanoparticle suggested that the lipid based carrier system could potentially be exploited as a delivery system with improved drug entrapment efficiency and controlled drug release for water soluble actives.
Keywords
Central composite design, ciprofloxacin HCl, encapsulation, particle size, solid lipid nanoparticle
Ciprofloxacin hydrochloride (ciprofloxacin HCl) is a broad spectrum fluoroquinolone antimicrobial agent, frequently used in external ocular infection, such as conjunctivitis, bacterial keratitis and kerato conjunctivitis [1,2]. Ciprofloxacin was found superior to other antibiotics against Gram‑negative as well as Gram‑positive ocular pathogens tested in vitro [3,4]; however, efficacy of the marketed aqueous solutions of ophthalmic products is limited by poor ocular bioavailability. A number of approaches have been developed to increase the ocular bioavailability of the drugs. In order to improve ophthalmologic bioavailability of drugs and the therapeutic action, we attempted to prepare and optimize the solid lipid nanoparticles (SLNs) containing ciprofloxacin HCl.
Since last 10 years SLN has received significant attention from academia and industry because of its fascinating properties [5‑7]. SLNs are, made up of physiological lipids which are excipients generally recognized as safe (GRAS), nontoxic and biocompatible material [8,9]. Exceptional admissibility, large‑scale industrial production feasibility, and sterilizable is the distinct advantages of SLNs [10,11]. SLN is found to be a most promising carrier for hydrophobic drugs because of its high compatibility and easy incorporation efficiency. However, SLN as a carrier for hydrophilic water soluble molecule is remained a subject of an exhaustive research and discussion due to its incompatibility with hydrophilic molecule. Hydrophilic drug has a maximum tendency to rapid migration into the external aqueous phase during the fabrication process because of low affinity and weak interaction between drug and lipid. Till date there are very few papers reported on lipids as a carrier for water soluble drug molecule, i.e. zidovudine, insulin, diminazene, thymocratin [12‑18]. To overcome this downside of the system, a combination of solid lipid was used in this study which leads to an imperfect crystal lattice giving more flexibility for drug loading, prevents drug leakage and modulates the drug release profile. [19,20]
Materials and Methods
Ciprofloxacin HCl was generously supplied as a gift sample by Aarti Drugs Ltd., Mumbai, India. Solid lipids such as Monecol PC (cetyl palmitate, melting point (MP) 45°) and Softemul 165 (PEG 100 glyceryl stearate, MP 54°) was obtained as a gift sample from Mohini Organics Pvt. Ltd., Mumbai, India. Dynasan 114 (glyceryl trimyristate, MP 57°) and Imwitor 900 (monoglycerides and diglycerides of stearate, MP 59°) were received as a gift sample by Sasol GmbH, Witten, Germany. Distilled and deionized (DDI) water was prepared with a Milli‑Q water system (Milli‑Pore, India). Acetone was of analytical grade and purchased from Finar Chem., India. All the excipients and solvents were used as received.
Experimental design
Before the experimental model was developed, a prescreening study was performed to check the effect of various solid lipids and their combination on SLN. The experiment was carried out by taking surfactant amount 270 mg, lipid amount 243 mg (1:1 ratio of binary mixtures), and stirring speed at 8500 rpm. Few solid lipids, Dynasan 114, Softemul 165, Imwitor 900 and Monecol PC were tested for particle size and polydispersity index (PDI) as a characterization tool.
Central composite response surface design was constructed using four quantitative preparation factors such as amount of lipid, amount of drug, stirring speed and stirring time with 30 experiments. The responses studied were particle size (Y1), zeta potential (Y2) and entrapment efficiency (EE) (Y3). A statistical software ‘Design Expert’ was used to generate the combinations of these factors at different levels.
This model is described by following equation:
Y=b0+b1X1+b2X2+b3X3+b4X4+b11X12+b22X22+b33X32+b44X42+b12X1X2+b12X1X3+b14X1X4+b23X2X3+b24X2X4+b34X3X4 ...(1)
Where, Y is the response, b0 is the intercept and b1, b2, b3 and b4 are the regression coefficient, X1, X2, X3 and X4 are coded level of independent variables, X1 X2 and X2 are interaction and quadratic terms, respectively.
Preparation of solid lipid nanoparticles
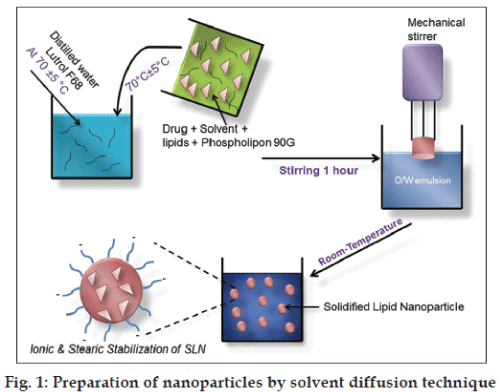
The surface modified SLN were fabricated using solvent diffusion method called as Ouzo effect because of the rapid diffusion of the organic solvent through oily phase to aqueous phase [21]. As shown in fig. 1 solid lipid, drug and surfactant were dissolved in organic solvent at 70±5°. An aqueous phase was prepared by dissolving water soluble surfactant in double distilled water and heated to the same temperature of the oil phase. The resultant organic solution was quickly dispersed into aqueous phase under mechanical agitate with 8500 rpm for 1 h. The obtained preemulsion (melted lipid droplet) was then cooled at room temperature till drug‑loaded nanoparticle dispersion was obtained. The resulting dispersion was then filtered with a paper filter (0.2 μm) to remove any excess lipid. In these experiments, the stirring rate (8500 rpm) and drug amount (50 mg) was kept constant.
Photon correlation spectroscopy
The size and the poly dipsersity index (PDI) of the particles were measured on a Zetasizer-NanoZS, ZEN3600, Malvern Instruments Ltd., U.K. with, He-Ne laser (633 nm). All samples were diluted with deionized particle‑free water to an adequate scattering intensity prior to the measurement. Photon correlations of spectroscopic measurements were carried out at a scattering angle of 173° in 10 mm diameter cells. The system was thermostated at 25°. Particle analysis was carried out using the Malvern software package using multiple mode analysis. Accordingly, the results are expressed as the z‑average diameter and the PDI, for an ideally monodisperse system, the theoretical PDI would be 0.
Zeta potential of nanoparticles
Zeta potential is one of the important parameters of the colloidal system which measures the surface charge of the particle based on their electrophoretic mobility and indicates the stability of colloidal particles. The surface charge of the ciprofloxacin hydrochloride‑loaded nanoparticles was determined by same instruments Zetasizer-NanoZS, ZEN3600, Malvern Instruments Ltd., UK, and He-Ne laser (633 nm). The measurements were carried out using a suspension of the nanoparticles in deionized and distilled water, at 25°. The zeta potential was determined in triplicates and the average values were calculated. The electrophoretic mobility was determined in an aqueous medium, thus the Henry’s function in this case is 1.5 and is referred to as a Smoluchowski approximation.
Yield
The yield, which refers to the quantity of nanoparticles recovered from the preparation process, was determined gravimetrically after drying 5 ml of suspension in an oven at 30° until constant weight was obtained.
Total drug content
With the aim to quantify the total drug content (TDC) (free plus bonded), 1 ml of suspension was added in 10 ml of acetone and water mixture (1:1) and stirred for 3 h to extract drug completely. Afterwards, the sample was filtered with filters of 0.2 μm and the total amount of ciprofloxacin HCl in solution was determined spectrophotometrically by measuring the absorbance of the clear supernatant at λmax of 275 nm. A standard calibration curve of concentration versus absorbance was plotted for this purpose. The TDC was calculated by using Eqn. 2, which is, TDC=concentration dilution factorxvolume of fraction. Possible lipid interferences during UV determination of drug were also investigated by comparing the two standard curves of each drug alone and in the presence of lipids. The differences observed between the standard curves were within the experimental error, thus implying that no lipid interference occurred (data not shown).
Entrapment efficiency
The entrapment efficiency (EE) of the prepared systems were determined by measuring the concentration of free drug in the dispersion medium. This determination was carried out at least 2 weeks after preparation to allow for complete crystallization of the nanoparticles. The free drug was determined by adding 1 ml of the nanosuspension to 4 ml water in order to dissolve the free drug; the obtained suspension was centrifuged for 90 min at 15,000 rpm. The supernatants were examined by UV/Vis spectrophotometer with further dilution in water. The amount of free drug was detected in the filtrate and the amount of incorporated drug was determined as a result of the initial drug minus the free drug. EE was calculated using the formula, % entrapment=(Wtotal drug added-Wfree drug)/(Wtotal drug added ×100
Stability studies
Stability studies of all formulations were performed for 90 days. The samples were kept at room temperature and stability of the all formulations was periodically monitored, evaluating the organoleptic changes, aggregation, gelation, sedimentation, particle size, drug entrapment and zeta potential.
In vitro release studies
In vitro release study was performed in pH 6.8 phosphate buffer by dialysis bag method using dialysis membrane of 12,000-14,000 molecular weight cut-off (Fisher brand). Prior to the experiment, the membrane was washed with warm Milli-Q double distilled water (70°) for 1 h and then rinsed thrice with Milli-Q water to remove the glycerin. Five milliliters of suspension were placed inside the dialysis bag, tied at both ends and dipped in the dissolution medium. Stirring of the medium was maintained at 100 rpm using a magnetic bead and the temperature at 37±0.2°. Two milliliters aliquot were withdrawn at preset time intervals and replaced by an equal volume of a fresh dissolution medium. After suitable dilution, the samples were analysed spectrophotometrically at 275 nm. The concentration of ciprofloxacin HCl in test samples was corrected and calculated by using the regression equation of the calibration curve.
Statistical evaluation
Results were expressed as means±standard deviation. Data obtained from the study were treated statistically using t-test and one-way analysis of variance to check the existence of significance by Graphpad Instat Version 3.05. The significance level was set at P<0.05.
The in vitro release data were fitted to the various kinetic models using PCP Disso 2.0v software, Pune, India which describes the release kinetics of the drug [22-27].
Results and Discussion
The results of the various binary mixtures of solid lipids prescreening study are presented in Table 1. For the chosen conditions, the particle size of the SLN was lowest when a PEGylated glyceryl monostearate (Softemul 165) and triglycerides of palmitic acid (Dynasan 114) was used. The PDI for batch A and B of the SLN formulations was within a considerable range (0.15-0.25), indicating homogeneous particle size distribution [28]. While batch C containing Softemul 165 and Monecol PC had presented high PDI with higher particle size. The higher particle size and PDI is assumed to be due to the poor solubility of the Monecol PC in acetone, which created improper diffusion of the lipids at the interface, thus producing poor and highly polydispersed SLN. The obtained results of the qualitative screening study was in agreement with the solubility parameter theory which states that the best miscibility of a solute and solvent is expected when intermolecular forces (dispersion, polar and hydrogenbonding forces) between the molecules of the solvent and between the molecules of a solute are of a similar strength. The Monecol PC being a wax seems to be a poor choice for preparation of SLN because of its very low polarities and hydrogen bonding abilities as shown in Table 2 [29-36].
| Batch | Surfactant | Particle size (nm) | PDI |
|---|---|---|---|
| A | Softemul 165+Imwitor 900 | 191.3±6 | 0.243 |
| B | Softemul 165+Dynasan 114 | 177.6±3 | 0.168 |
| C | Softemul 165+Monecol PC | 233.1±7 | 0.343 |
n=3, PDI=Polydispersity index
Table 1: Influence Of Various Binary Mixtures Of Solid Lipids On Solid Lipid Nanoparticles
| Compound | δd(J/cm3)1/2 | δp(J/cm3)1/2 | δh(J/cm3)1/2 | δt(J/cm3)1/2 |
|---|---|---|---|---|
| Dynasan 114 | 16.7363 | 1.0943 | 5.2035 | 17.5607 |
| Imwitor 900 | 16.767 | 1.4994 | 9.1841 | 19.2619 |
| Monecol PC | 16.3114 | 0.8754 | 3.5363 | 16.7133 |
| Softemul 165 | 16.9278 | 2.0024 | 10.92227 | 20.2451 |
| Acetone | 14.5244 | 9.8971 | 5.0702 | 18.2926 |
Group contributions as a methods of Van Krevelen/Hoftyzer
Table 2: Hansen Solubility Parameters Of Lipid And Solvent Determined By Software Molecular Modeling Pro
The use of design of experiment (DOE) is nowadays a common method of exploring the potential interaction between variables in developing the effective drug delivery systems [37-39]. The quadratic polynomial equation and surface response plot was obtained by using design expert software and it was further evaluated to study the relationship between variables and responses.
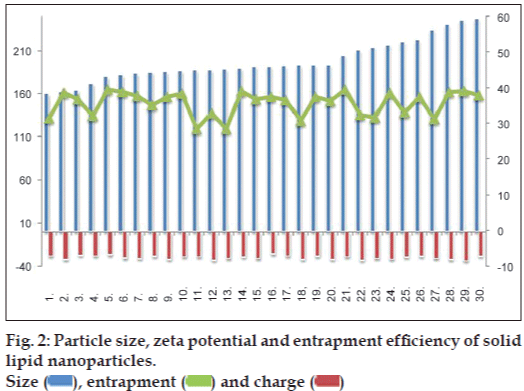
Table 3 displays the mean particle size, zeta potential and EE for all the prepared 30 batches. The particle size of the fabricated batches was in the range of 150-250 nm, EE in the range of 28-39% and ZP in the range of −26 to −34 mV, as presented in fig. 2. With regard to yield as the parameter for the efficiency of SLN formation, total yield of nanoparticles was found 93%.
| Batch | Lipid | Drug | Stirring | Stirring Size Charge EE (%) | |||
|---|---|---|---|---|---|---|---|
| amount | amount | speed | time | (nm) | (mV) | ||
| (mg) | (mg) | (rpm) | (min) | ||||
| 1 | 200 | 50 | 8000 | 40 | 159.5 | −28.4 | 31.4 |
| 2 | 300 | 65 | 9000 | 30 | 161.2 | −32.6 | 38.6 |
| 3 | 200 | 80 | 8000 | 40 | 163.7 | −27.7 | 36.8 |
| 4 | 200 | 50 | 8000 | 20 | 171.5 | −28.1 | 32.1 |
| 5 | 200 | 80 | 8000 | 20 | 179.3 | −27.1 | 39.5 |
| 6 | 300 | 95 | 7000 | 30 | 181.3 | −30.9 | 38.8 |
| 7 | 400 | 80 | 8000 | 40 | 183.6 | −31.5 | 37.7 |
| 8 | 300 | 65 | 7000 | 30 | 184.9 | −28.8 | 35.1 |
| 9 | 300 | 65 | 7000 | 30 | 185.1 | −32.5 | 37.4 |
| 10 | 300 | 65 | 7000 | 30 | 186.4 | −29.1 | 38.4 |
| 11 | 100 | 65 | 7000 | 30 | 187.2 | −29.4 | 28.4 |
| 12 | 400 | 50 | 8000 | 40 | 187.4 | −33.4 | 32.7 |
| 13 | 300 | 35 | 7000 | 30 | 188.4 | −31.4 | 28.4 |
| 14 | 300 | 65 | 7000 | 30 | 189.2 | −29.6 | 38.9 |
| 15 | 300 | 65 | 7000 | 30 | 191.3 | −31.4 | 36.6 |
| 16 | 200 | 80 | 6000 | 40 | 191.6 | −26.1 | 37.4 |
| 17 | 300 | 65 | 7000 | 10 | 192 | −28.3 | 36.4 |
| 18 | 200 | 50 | 6000 | 40 | 192.8 | −32.4 | 30.6 |
| 19 | 300 | 65 | 7000 | 50 | 193.1 | −28.8 | 37.3 |
| 20 | 300 | 65 | 7000 | 30 | 193.1 | −32.4 | 36.3 |
| 21 | 400 | 80 | 8000 | 20 | 204.1 | −29.3 | 39.5 |
| 22 | 400 | 50 | 8000 | 20 | 210.2 | −33.4 | 32.4 |
| 23 | 200 | 50 | 6000 | 20 | 213.6 | −31.1 | 31.7 |
| 24 | 400 | 80 | 6000 | 40 | 216.5 | −32.1 | 38.6 |
| 25 | 400 | 50 | 6000 | 40 | 219.7 | −29.7 | 33.1 |
| 26 | 200 | 80 | 6000 | 20 | 221.9 | −28.7 | 37.5 |
| 27 | 400 | 50 | 6000 | 20 | 233.2 | −31.2 | 31.1 |
| 28 | 400 | 80 | 6000 | 20 | 239.6 | −32.7 | 38.8 |
| 29 | 500 | 65 | 7000 | 30 | 244.5 | −33.9 | 39.1 |
| 30 | 300 | 65 | 5000 | 30 | 246.7 | −28.6 | 37.9 |
EE=Entrapment efficiency
Table 3: Factorial Design Operating Variable And Their Responses
Batches of SLN were prepared using a central composite rotatable design which includes great precision and allows studying the effect of one variable by keeping the other variable constant or at different level. In this design a statistical model utilizing polynomial equation was used to study the response of a variable. The whole factorial analysis was performed using Design Expert software.
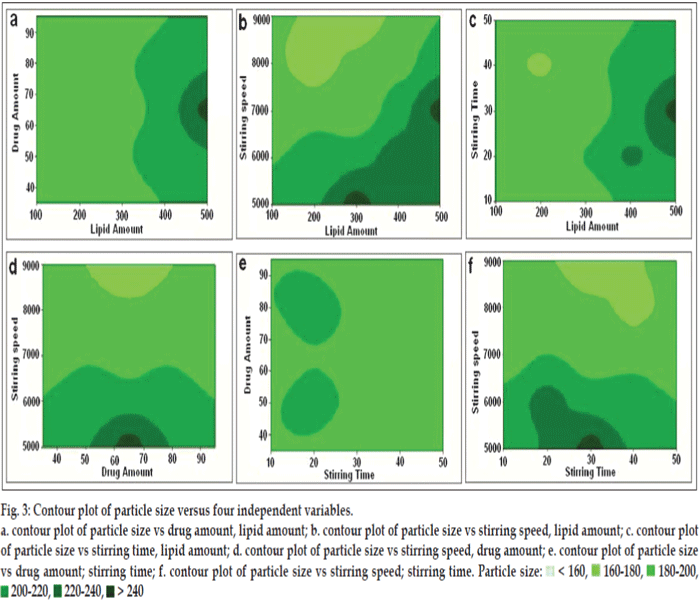
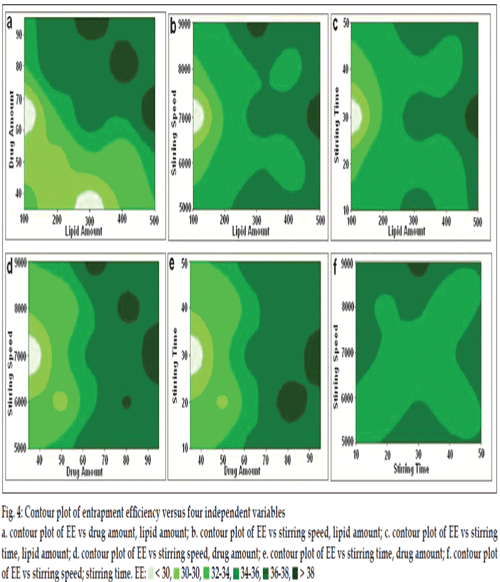
The effects of A, B, C and D and their interaction on Y1 are presented in figs. 3 and 4.
Fig. 3: Contour plot of particle size versus four independent variables.
a. contour plot of particle size vs drug amount, lipid amount; b. contour plot of particle size vs stirring speed, lipid amount; c. contour plot of particle size vs stirring time, lipid amount; d. contour plot of particle size vs stirring speed, drug amount; e. contour plot of particle size
vs drug amount; stirring time; f. contour plot of particle size vs stirring speed; stirring time. Particle size: < 160, 160-180, 180-200,
200-220, 220-240, > 240
Fig. 4: Contour plot of entrapment efficiency versus four independent variables a. contour plot of EE vs drug amount, lipid amount; b. contour plot of EE vs stirring speed, lipid amount; c. contour plot of EE vs stirring
time, lipid amount; d. contour plot of EE vs stirring speed, drug amount; e. contour plot of EE vs stirring time, drug amount; f. contour plot
of EE vs stirring speed; stirring time. EE: < 30, 30-30, 32-34, 34-36, 36-38, > 38
The polynomial equation obtained for particle size Y1 is as follows:
Y 1= + 1 8 8 . 3 3 + 1 3 . 1 2 A - 0 . 0 7 5 B - 1 8 . 3 6 C - 6.52D-1.61AB+1.39AC-0.075AD-0.051BC- 1.28BD+1.05CD+6.87A2-0.88B2+3.90C2+1.05D2…..(4)
The particle size measured for the different batches showed wide variation. The high negative coefficients for independent variables C indicated reduction in particle size is prominent with the increasing the stirring speed. While the high positive coefficients for the variables A indicated an increase in size of the particle with the increasing the amount of lipid. The quadratic model was found to be significant with an F value of 7.18, which indicated that response Y1 and the set of variables were significantly related. There were significant changes found when the level of lipid amount, stirring time and stirring speed was changed. Simple explanation of the obtained result was, increased amount of lipid causes increased viscosity and reduced homogenization efficiency which further requires an ideal stirring speed and stirring time for the chosen properties of SLN. Increasing the stirring speed and time will increases the diffusion rate of the solute molecules in the outer phase which further provides rapid solvent evaporation and solidification of lipids. It was found that increasing the stirring speed from 5000 to 8000 rpm in the SLN preparation, particle size was substantially reduced. Optimum particle size was found when amount of lipid was 300 mg, drug amount 65 mg, stirring speed 9000 rpm and stirring time was 30 min. On the other hand the amount of drug was found insignificant in particle size response of the SLN preparation study.
Zeta potential is the measure of overall charges acquired by the particles in a particular medium and is considered second major characterization tool in understanding the dispersion system and its stability. It was reported that if the absolute value of zeta potential is above 30 mV, the dispersion system presents good stability. Instability may occur if electrostatic repulsion in particles is not strong enough or in another sense absolute value of zeta potential is below 30 mV [40,41].
The polynomial equation for zeta potential Y2 is as follows:
Y2=-30.63-1.36A+0.56B-0.12C-0.029D- 0.52AB0.56AC-0.031AD+0.18BC-0.31BD-0.41CD 0.20A2-0.078B2+0.059C2+0.57D2 …..(5)
The quadratic regression coefficients were all statistically insignificant with an F value 1.64, which may indicate that zeta potential did not have quadratic relationships with the preparation factors. This suggests that the zeta potential was not significantly affected by the experimental conditions employed in this study. The study indicated that the zeta potential, as an indication of the physical stability of the SLN, was not change considerably upon changing the level of independent variables. All the batches of SLN showed very good stability despite of its very low magnitude of charges because of the combination of electrostatic and steric stabilization which can avoid the particles aggregation.
The EE of the SLN was in the range of 28‑39% for various level combinations. The effect of four independent variables could be explained by following quadratic equation:
Y3=+37.12+1.18A+2.98B+0.20C-0.10D-6.250AB- 0.12AC+0.31AD-0.056BC-0.33BD-0.34CD-0.93A2 0.97B2+0.19C2-0.16D2
The positive value of a factor in the regression equation indicates the enhancement of that response and vice versa. The value of correlation coefficient, (R2) of 0.8742 indicated poor correlation between observed and predicted value of EE (Table 3).
Unlike particle size, as shown in figs. 3 and 4, the EE was insignificantly affected by both stirring time and stirring speed at every level studied. With increasing the amount of lipid, %EE is bound to increase marginally from our other studies (glyceryl monostearate based SLN with 34% entrapment of ciprofloxacin HCl, data not shown) because of the binary incompatible mixtures that act as solubilizing agents for drug and provides additional space to accommodate the more amount of drug [42‑44]. However, this formulation showed high entrapment when the amount of drug is at 65‑80 mg and the amount of lipid was 300 mg.
The Design Expert software had suggested some formulations and predicted their responses by taking the desirability values 0‑1. Based on this factor best composition selected was 300 mg lipid, 65 mg drug, 9000 rpm stirring speed and 30 min stirring time. A verification test was conducted to prove the accuracy and usefulness of this model. The particle size of an optimized formulation was found to be 164 nm that displayed an EE of 38.6% and zeta potential - 32.6 mV which were in good agreement with the predicted values.
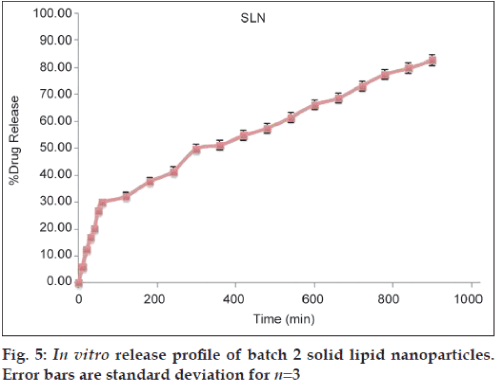
A key issue investigated in this study was the feasibility of SLN formulation delivery of hydrophilic drug molecules. The prepared SLN batch 2 was evaluated for drug release study and a result was presented in fig. 5. The binary mixture of lipid significantly influenced the physicochemical characteristics of the lipid nanoparticles. SLN showed sustained release pattern, with ~80% of total drug amount released within 15 h. SLN showed the slowest release rate because of the reduced mobility of the drug in solidified state of the binary lipids. Sustained release pattern was found more effective in comparison with our previous study employing single lipid (almost completely released in 15 h). A result was an explanation of the drug accommodation in the binary mixture of lipids. Because of the disrupted polymorphism and crystallinity of lipid, drug was accommodated in higher amount with depressed drug expulsion to the outer surface of the nanoparticles causing the very low burst release, drug leaking and controlled release. The release kinetics of the system was best fitted for Higuchi matrix model (Table 4).
| Batch | Zero order | First order | Higuchi | |||
|---|---|---|---|---|---|---|
| K0 | r | K1 | r | Kh | r | |
| SLN | 0.108 | 0.7273 | 0.002 | 0.8982 | 3.22 | 0.9822 |
Table 4: Regression Analysis Data for Release Study of Solid Lipid Nanoparticles
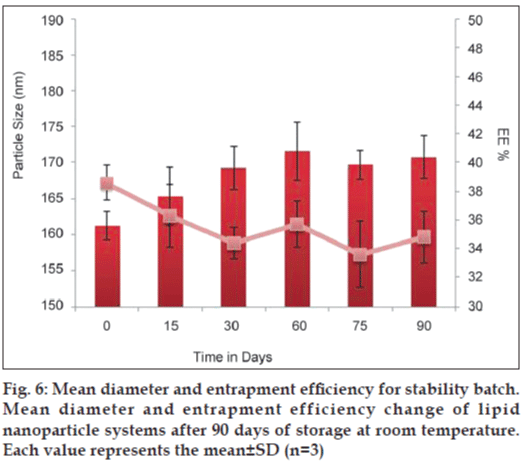
SLN batch 2 was stored in amber colored bottle at room temperature and analyzed for change in size and drug entrapment for 90 days. Result was presented in fig. 6 and indicated that the particle sizes of the all formulations were maintained during the storage period. Concerning the EE of the preparation, it was slightly changed from 38 to 34%. The experimental results of the stability study demonstrated that particle size was not influenced by the time of crystallization but %EE was affected marginally. Result of the EE indicated that binary mixture of two incompatible lipids could be efficient in protecting the drug within the nanoparticles for a longer period. During the period of storage, the formulation showed no change in colour and creaming and phase separation in SLN. According to the study conducted, SLN made up of two incompatible lipids can be considered as more suitable carriers rather than using single lipid for protecting the drug into the nanoparticles for a longer period.
In conclusion, SLN dispersions containing ciprofloxacin HCl having low particle size and long‑term physical stability are prepared successfully using solvent diffusion technique. The main and interaction effects of selected variables (amount of lipid, amount of drug, stirring speed, stirring time) on the critical quality attributes of solid lipid nanoparticles determined by central composite DOE strategy. Particle size and EE of the nanoparticles can be controlled by varying process variables such as stirring time and stirring speed. The promising results reported above suggest that SLN based on binary mixtures of two lipids could be proposed as carrier system to administer ciprofloxacin HCl. The results here provide the framework for further study involving the SLN formulation design for the delivery of hydrophilic drugs.
References
- Charoo NA, Kohli K, Ali A, Anwer A. Ophthalmic delivery of ciprofloxacin hydrochloride from different polymer formulations: In vitro and in vivo studies. Drug DevInd Pharm 2003;29:215-21.
- Mishra GP, Bagui M, Tamboli V, Mitra AK. Recent applications of liposomes in ophthalmic drug delivery. J Drug Deliv 2011;2011:863734.
- Egger SF, Ruckhofer J, Alzner E, Hell M, Hitzl W, Huber-Spitzy V, et al. In vitro susceptibilities to topical antibiotics of bacteria isolatedfrom the surface of clinically symptomatic eyes. Ophthalmic Res 2001;33:117-20.
- Dillen K, Vandervoort J, Van den Mooter G, Verheyden L, Ludwig A. Factorial design, physicochemical characterisation and activity of ciprofloxacin-PLGA nanoparticles. Int J Pharm 2004;275:171-87.
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - A review of the state of the art. Eur J Pharm Biopharm 2000;50:161-77.
- Schwarz C, Mehnert W, Lucks J, Müller R. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Release 1994;30:83-96.
- Mehnert W, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev 2001;47:165-96.
- Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev 1995;17:31-48.
- Shegokar R, Singh KK, Müller RH. Production and stability of stavudine solid lipid nanoparticles - From lab to industrial scale. Int J Pharm 2011;416:461-70.
- Gokce EH, Ozyazici M, Souto EB. Nanoparticulate strategies for effective delivery of poorly soluble therapeutics. TherDeliv 2010;1:149-67.
- Torchilin VP. editor. Nanoparticulates as Drug Carriers. London: Imperial College Press; 2006.
- Gasco MR, Morel S, Ugazio E, Cavalli R. Thymopentin in solid lipid nanoparticles. Int J Pharm 1996;132:259-61.
- Reithmeier H, Herrmann J, Gӧpferich A. Lipid microparticles as a parenteral controlled release device for peptides. J Control Release 2001;73:339-50.
- Olbrich C, Gessner A, Schröder W, Kayser O, Müller RH. Lipid-drug conjugate nanoparticles of the hydrophilic drug diminazene-cytotoxicity testing and mouse serum adsorption. J Control Release 2004;96:425-35.
- Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev 2007;59:491-504.
- Yuan H, Jiang SP, Du YZ, Miao J, Zhang XG, Hu FQ. Strategic approaches for improving entrapment of hydrophilic peptide drugs by lipid nanoparticles. Colloids Surf B Biointerfaces 2009;70:248-53.
- Yassin AE, Anwer MK, Mowafy HA, El-Bagory IM, Bayomi MA, Alsarra IA. Optimization of 5-fluorouracil solid-lipid nanoparticles: A preliminary study to treat colon cancer. Int J Med Sci 2010;7:398-408.
- Singh S, Dobhal AK, Jain A, Pandit JK, Chakraborty S. Formulation and evaluation of solid lipid nanoparticles of a water soluble drug: Zidovudine. Chem Pharm Bull 2010;58:650-5.
- Trotta M, Gallarate M, Battaglia L, Chirio D. Cisplatin loaded SLN produced by coacervation technique. J Drug DelivSciTechnol 2010;20:343-7.
- Trotta M, Gallarate M, Battaglia L, Peira E. Peptide loaded solid lipid nanoparticles prepared through coacervation technique. Int J ChemEng 2011;2011:1-6.
- François G, Katz JL. Nanoparticles and nanocapsules created using the
- Ouzo effect: Spontaneous emulsification as an alternative to ultrasonic and high-shear devices. ChemPhysChem 2005;6:209-16.
- Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Themistocles P, Gary D, Michael A, Panayotis E. Quantitative calculations in Pharmaceutical Practice and Research. New York: VCH Publishers; 1993. p. 189-209.
- Gohel MC, Panchal MK, Jogani VV. Novel mathematical method for quantitative expression of deviation from the Higuchi model. AAPS PharmSciTech 2000;1:E31.
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:123-33.
- Banker GS, Rhodes CT. Modern pharmaceutics. Basel: Macel Dekker Inc; 2002.
- Reza M, Quadir MA, Haider SS. Development of theophylline sustained release dosage form based on kollidon SR. Pak J Pharm Sci 2002;15:63-70.
- Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int J Pharm 2003;257:153-60.
- Allan FM, Barton PD. CRC Handbook of Solubility Parameters and Other Cohesion Parameters. Boca Raton, Florida: CRC; 1983.
- Barton AF. Applications of solubility parameters and other cohesion parameters in polymer science and technology. Pure ApplChem 1985;57:905-12.
- Savova M, Kolusheva T, Stourza A, Seikova I. The use of group contribution method for predicting the solubility of seed polyphenols of Vitisvinifera L. within a wide polarity range in solvent mixtures. J UnivChemTechnol Metallurgy 2007;42:295-300.
- Srinivas K, King JW, Monrad JK, Howard LR, Hansen CM. Optimization of subcritical fluid extraction of bioactive compounds using Hansen solubility parameters. J Food Sci 2009;74:E342-54.
- Li Y, Taulier N, Rauth AM, Wu XY. Screening of lipid carriers and characterization of drug-polymer-lipid interactions for the rational design of polymer-lipid hybrid nanoparticles (PLN). Pharm Res 2006;23:1877-87.
- Long C, Zhang L, Qian Y. Preparation and crystal modification of ibuprofen loaded solid lipid microparticles. Chin J ChemEng 2006;14:518-25.
- Hansen CM. Hansen Solubility Parameters: A User’s Handbook. Florida: CRC; 2007.
- Vaughan CD. Using solubility parameters in cosmetics formulation. J SocCosmetChem 1985;36:319-33.
- Ledolter J. Designed experiments in nanotechnology: Reviewing the statistical analyses of five studies. QualTechnol Quant Manag 2011;8:183-209.
- Bhavsar MD, Tiwari SB, Amiji MM. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Control Release 2006;110:422-30.
- Vandervoort J, Ludwig A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: A factorial design study. Int J Pharm 2002;238:77-92.
- Komatsu H, Kitajima A, Okada S. Pharmaceutical characterization of commercially available intravenous fat emulsions: Estimation of average particle size, size distribution and surface potential using photon correlation spectroscopy. Chem Pharm Bull 1995;43:1412-5.
- Shi L, Li Z, Yu L, Jia H, Zheng L. Effects of surfactants and lipids on the preparation of solid lipid nanoparticles using double emulsion method. J DispersSciTechnol 2011;32:1-9.