- *Corresponding Author:
- G. K. Kapse
Department of Pharmaceutical Sciences, College of Engineering, Andhra University, Visakhapatnam-530 003, 1H. K. E. Society’s College of Pharmacy, Gulbarga - 585105, India
E-mail: kapse.gourishankar@ rediffmail.com
| Date of Submission | 13 April 2005 |
| Date of Revision | 11 August 2005 |
| Date of Acceptance | 20 June 2006 |
| Indian J Pharm Sci,2006, 68 (3): 403-406 |
Abstract
Three simple and sensitive spectrophotometric methods (I, II, and III) in the visible region have been developed for the quantitative estimation of nitazoxanide in bulk drug and pharmaceutical formulations. These methods are based on the reaction of reduced nitazoxanide with p-dimethylaminobenzaldehyde, p-dimethylaminocinnamaldehyde and vanillin in acidic conditions to form pink, orange red, and orange yellow coloured chromogens with absorption maxima at 559 nm, 534.5 nm, and 475 nm respectively. The reduction of nitazoxanide was carried out with zinc granules and 5 N hydrochloric acid at room temperature in methanol. Beer's law is obeyed in the concentration range of 5-25 mg/ml, 5-25 mg/ml, and 10-50 mg/ml respectively. The results of analysis have been validated statistically and by recovery studies. The methods were found to be accurate, precise, rapid, and economic. The results are comparable with those obtained with visible spectrophotometric method in methanol at 402 nm.
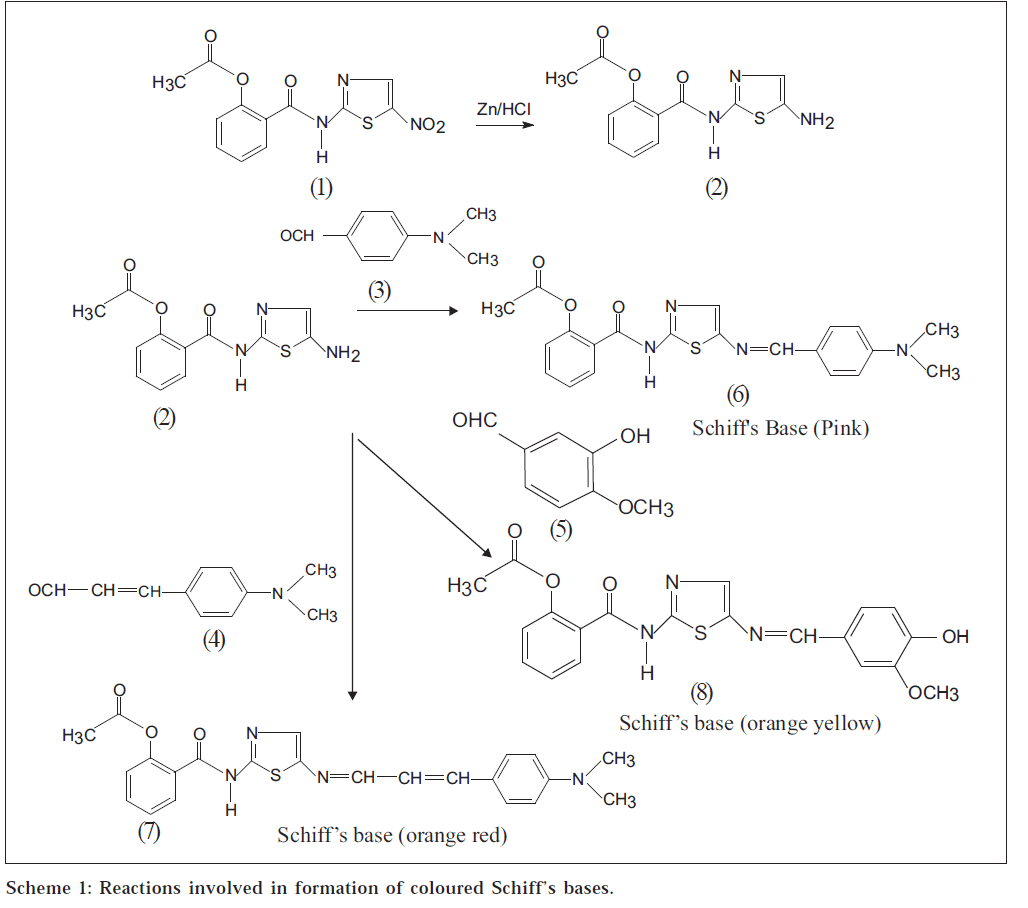
Nitazoxanide (1) [1-5], N-(5-nitro-2-thiazolyl) salicylamide acetate, is used as antiprotozoal and anthelmintic agent. It is used in the treatment of giardiasis [2] and cryptosporidiasis [2]. It is not official in any pharmacopoeia, and analytical reports are not found in literature for its quantitative estimation in bulk drug and pharmaceutical formulations. Three simple and sensitive spectrophotometric methods for the quantitative estimation of nitazoxanide have been developed after converting it to its reduced form, N-(5- amino-2-thiazolyl) salicylamide acetate (2), by using zinc granules and 5 N hydrochloric acid in methanol at room temperature.
These methods (I, II, and III) are based on the condensation reaction of reduced nitazoxanide (2) as shown in Scheme 1 with p-dimethylaminobenzaldehyde (PDAB) (3), p-dimethylaminocinnamaldehyde (PDAC) (4), and vanillin (5) to form pink (6), orange red (7), and orange yellow (8) coloured chromogens with absorption maxima at 559, 534.5 and 475 nm, respectively. Beer’s law is obeyed in the concentration range of 5-25 μg/ml for methods I and II, and 10-50 μg/ml for method III. These methods have been successfully extended to the pharmaceutical preparations (tablets) containing nitazoxanide.
A Shimadzu UV/Vis double beam spectrophotometer A Shimadzu UV/Vis double beam spectrophotometer all spectral measurements. p-dimethylaminobenzaldehyde, p-dimethylaminocinnamaldehyde and vanillin, AR grade, were obtained from Qualigens, Mumbai. Nitazoxanide sample was obtained from M/s Lupin Ltd., Mumbai. Methanol obtained from Qualigens, Mumbai, was used.
About 100 mg of nitazoxanide (pure or equivalent tablet powder) was accurately weighed and dissolved in 20 ml of methanol. The methanol solution of nitazoxanide was treated with 10 ml of 5 N hydrochloric acid, and 1 g of zinc granules were added in portions while shaking. After standing for 1 h at room temperature, the solution was filtered through cotton wool. The residue was washed with 10 ml portions of methanol three times, and the total volume of the filtrate was made up to 100 ml with methanol. The final concentration of reduced nitazoxanide was made to 100 μg/ml. If the methanol sample solutions were diluted with distilled water, small particles were found to appear; therefore, directly methanol solutions were used for estimation.
In method I, fresh aliquots of reduced nitazoxanide ranging from 0.5-2.5 ml were transferred into a series of 10 ml volumetric flasks. To each of the above aliquots, 0.5 ml of PDAB solution in methanol (2% w/v) was added and heated at 60-70° for 10 min. After cooling, the volume was brought up to the mark with methanol, and the absorbance of the pink coloured species was measured at 559 nm against reagent blank. The coloured chromogen was stable for more than 3 h. The amount of nitazoxanide present in the sample was computed from calibration curve.
In method II, fresh aliquots of reduced nitazoxanide ranging from 0.5-2.5 ml were transferred into a series of 10 ml volumetric flasks. To each of above aliquots, 3 ml of PDAC solution in methanol (0.4% w/v) was added and heated at 60-70º for 10 min. After cooling, the volume was brought up to the mark with methanol, and the absorbance of the orange red coloured species was measured at 534.5 nm against reagent blank. The coloured species was stable for more than 4 h. The amount of nitazoxanide present in the sample was computed from calibration curve.
In method III, fresh aliquots of reduced nitazoxanide ranging from 1-5 ml (1 ml=100 μg) were transferred into a series of 10 ml volumetric flasks. To each of the above aliquots, 1.5 ml of vanillin solution in methanol (3% w/v) was added and heated at 60-70° for 20 min. After cooling, the volume was brought up to the mark with methanol, and absorbance of orange yellow coloured species was measured at 475 nm against reagent blank. The coloured species was stable for more than 3 h. The amount of nitazoxanide present in the sample was computed from calibration curve.
The developed methods were used for the estimation of nitazoxanide in two batches of Nizonide tablet (Lupin Limited, Mumbai) formulations. Ten tablets of formulation, each containing 500 mg of nitazoxanide, were accurately weighed and powdered. Weight of tablet powder equivalent to 100 mg of drug was taken.
The results of the above methods are compared with the results obtained with visible spectrophotometric method, where nitazoxanide exhibits absorption maxima at 402 nm in methanol and Beer’s law is obeyed in the concentration range of 2-10 μg/ml. Solution of nitazoxanide (100 μg/ml) in methanol, either pure or formulation, was prepared. Aliquots of nitazoxanide ranging from 0.2-1.0 ml were transferred into a series of 10 ml volumetric flasks. The volume was made up to the mark with methanol, and the absorbance of the solutions was measured at 402 nm against the solvent blank. The amount of nitazoxanide present in the sample was computed from calibration curve.
The optical characteristics such as absorption maxima, Beer’s law limits, molar absorptivity, and Sandell’s sensitivity are presented in Table 1. The regression analysis using the method of least squares was made for the slope (b), intercept (a), and correlation (r) obtained from different concentrations, and the results are summarized in Table 1. The percent relative standard deviation and percent range of error (0.05 and 0.01 level of confidence limits) calculated from eight measurements, ¾ of the upper Beer’s law limits of nitazoxanide are given in Table 1. The results showed that the proposed methods have reasonable precision.
| Parameters | PDAB (I) | PDACA (II) Vanillin (III) | |
|---|---|---|---|
| λmax (nm) | 559 | 534.5 | 475 |
| Beer’s law limits (µg/ml) | 5-25 | 5-25 | 10-50 |
| Molar absorptivity | 0.6123×104 | 0.9231×104 | 0.2808×104 |
| (l/mol.cm) | |||
| Sandell’s sensitivity | 0.036 | 0.021 | 0.052 |
| (µg/cm2/0.001 | |||
| absorption units) | |||
| Regression equation (Y*) | |||
| Slope (b) | 0.0201 | 0.0291 | 0.0091 |
| Intercept (a) | 0.0035 | 0.0006 | 0.0006 |
| Correlation coefficient (r) | 0.9999 | 0.9999 | 0.9999 |
| % RSD | 0.3378 | 0.2044 | 0.8904 |
| Range of errors** | |||
| Confidence limits with | |||
| 0.05 level | 0.0008 | 0.0008 | 0.0022 |
| Confidence limits with | |||
| 0.01 level | 0.0012 | 0.0011 | 0.0032 |
*Y=bC+a, where C is the concentration of nitazoxanide in μg/ml and Y is the absorbance at the respective λmax. **For eight measurements
Table 1: Optical Characteristics And Precision
Results obtained with the proposed methods confirm the suitability of these methods for pharmaceutical dosage forms. The optimum conditions for colour development for methods I, II, and III have been established by varying the parameters one at a time and keeping the other parameters fixed and observing the effects of product on the absorbance of the coloured species and incorporated in the procedure. The other active ingredients and excipients usually present in pharmaceutical dosage forms like starch, talc, and magnesium stearate did not interfere at their regularly added levels. The proposed methods (I, II, and III) were applied for assay of nitazoxanide in tablets (T1 and T2, where T1 and T2 were tablets from different batches), and the results were compared with reference method. The amounts obtained were 498.91, 499.51, 498.32, 499.82 mg for sample T1; and 498.94, 499.63, 498.71, 499.87 mg for sample T2 for 500 mg marketed tablets by proposed methods I, II, III, and reference method, respectively. The accuracy of the methods was confirmed by performing recovery studies at two levels by adding 200 mg and 400 mg of pure drug to the formulation already analysed by these methods. The percentage recovery values (average ± SD of eight determinations) are 99.39±0.2, 99.41±4.08, and 99.21±0.3 for the first level; 99.41±0.1, 99.43±0.09, and 99.23±0.4 for the second level for sample T1; and 99.33±0.4, 99.42±0.2, and 99.25±0.03 for the first level and second level for sample T2 for methods I, II, and III, respectively. The methods reported here are found to be simple, sensitive, accurate, precise, and economical and can be used for the determination of nitazoxanide in pharmaceutical dosage forms in a routine manner.
Acknowledgements
The authors are thankful to M/s Lupin Limited, Mumbai, for providing the drug sample for research; and the Head of the Department, Department of Pharmaceutical Sciences; Principal, College of Engineering, Andhra University, Visakhapatnam, for providing the laboratory facilities to carry out the present work. The authors are also thankful to the Principal, H. K. E. Society’s College of Pharmacy, Gulbarga, for providing additional
References
- Sweetman, S.C. Eds., In; Martindale, The Complete Drug Reference, 33rd Edn., Pharmaceutical Press, London, 2002, 598.
- O’Neil, M.J. Eds., In; The Merck Index, An Encyclopedia of Chemicals, Drugs and Biologicals, 13th Edn., Merck & Co. Inc., Whitehouse Station, NJ, 2001, 1177.
- Cavier, R., Eur. J. Med. Chem. Chim. Ther., 1978, 13, 539.
- Dubreuil, L., Antimicrob. Ag. Chemotherm., 1996, 40, 2266.
- Murphy, J.R. and Friedmann, J.C., J. Appl. Toxicol., 1985, 5, 49.