- Corresponding Author:
- B. N. Nalluri
Department of Pharmaceutical Analysis, K. V. S. R. Siddhartha College of Pharmaceutical Sciences, Pinnamaneni Polyclinic Road, Siddhartha Nagar, Benz Circle, Vijayawada-520 010, India
E-mail: buchinalluri@yahoo.com
| Date of Submission | 25 November 2011 |
| Date of Revision | 13 April 2013 |
| Date of Acceptance | 22 April 2013 |
| Indian J Pharm Sci 2013;75(3):372-376 |
Abstract
A stability-indicating reverse-phase high-pressure liquid chromatography method with photodiode array detector was developed and validated for estimation of riluzole in the bulk and tablet dosage forms. Riluzole was subjected to stress conditions (light, heat, humidity, acid/base hydrolysis and oxidation) and the stressed samples were analyzed by developed method. Degradation was observed in acidic, basic, oxidative and thermal conditions. The degradation products were well resolved from riluzole peak. An inertsil-ods column (250×4.6 mm, 5 μ) with a mobile phase comprising 0.02% v/v formic acid:acetonitrile(35:65 v/v) at a flow rate of 1.0 ml/min was used and eluents were monitored at 260 nm. The retention time of riluzole was 5.7 min. Complete validation for the method was carried out according to Internation Conference on Harmonization guidelines. Linearity was achieved in the range 10-50 μg/ml with a correlation coefficient (r) 0.9998. The percent assay was 100.92 and mean percentage recovery was found to be 101.10.
Keywords
Riluzole, reverse phase liquid chromatography, forced degradation, validation
Riluzole (RIL) is a glutamate antagonist used in the treatment of amyotrophic lateral sclerosis, a neurodegenerative disease [1]. RIL chemically is 2-amino-6-trifluoromethoxy-benzothiazole. In vivo, it has neuroprotective, anticonvulsant, and sedative properties [2]. Stability testing plays an important role in the drug development process. The purpose of these studies is to provide evidence on how the quality of drug substance or drug product varies with time under a variety of environmental conditions, for example temperature, humidity and light, which enables storage conditions, retest periods and shelf life to be recommended. Results of stress studies can facilitate stability indicating method (SIM) development, drug formulation design, selection of storage conditions and packaging, better understanding of potential liabilities of drug molecule chemistry and solving of stability-related problems.
A recent literature survey revealed that very few high pressure liquid chromatography (HPLC) [3] methods were reported for the estimation of RIL in pharmaceutical formulations and in biological fluids [4-6]. A few spectrophotometric methods for the determination of RIL in dosage forms [7,8] were also reported. None of the reported procedures enable estimation of RIL in the presence of its degradation products. This manuscript describes the development and validation of stability-indicating reverse-phase high-pressure liquid chromatography method with photodiode arry detector (RP-HPLC-PDA) for the determination of RIL as bulk drug and in pharmaceutical dosage forms. The method enables the separation of drug from the degradation products under the stress conditions (hydrolysis, oxidation, photolysis and thermal). A rapid, robust and economic method was developed which separates the degradation products from the main RIL peak and the method was validated as per ICH guidelines. The developed method is stability indicating and can be used for assessing the stability of RIL in bulk drugs and tablet formulations.
RIL was a gift sample from Veeda CR Las, Ahmedabad, India. Acetonitrile, water and formic acid were purchased from E. Merck, Mumbai, India. All the solvents and reagents are of HPLC grade. RIL tablets of Rilutor® 50 mg were commercially purchased. Chromatographic separation was performed on a Shimadzu LC-20AD dual pump, DGU-20A degasser, SPD-M20A PDA detector, and SIL-20A HT auto sampler. LC solution software was used for analyzing the data.
Mobile phase of 0.02% v/v formic acid: acetonitrile (35:65) at 1 ml/min flow rate was used and the mobile phase was filtered through membrane filter (Millipore Nylon disc filter of 0.45 μm) and sonicated for 5 min in ultrasonic bath before use. For quantitative analytical purpose wavelength was set at 260 nm and the Inertsil-ODS column (250×4.6 mm) was used at ambient temperature.
Stock solution of RIL (1 mg/ml) was prepared using methanol. Appropriate volumes of this stock solution was then further diluted with the diluent, acetonitrile (ACN) to get the required concentrations of standard solutions at a concentration range of 10-50 μg/ml.
The method described above was validated as per the ICH guidelines [9] for the parameters like specificity, accuracy, linearity, precision, detection limit (LOD), quantitation limit (LOQ), and robustness.
For assay, 20 tablets were weighed and finely powdered and a powder quantity equivalent to 25 mg RIL was accurately weighed and transferred to a 25 ml volumetric flask and 10 ml of diluent, was added to the same. The flask was sonicated for 5 min and volume was made up to the mark with diluent. The above solution was filtered using nylon disposable syringe filter (13 mm, 0.45 μm) and the filtrate was diluted with diluent to obtain a concentration of 30 μg/ml and from this solution, 20 μl was injected into HPLC system.
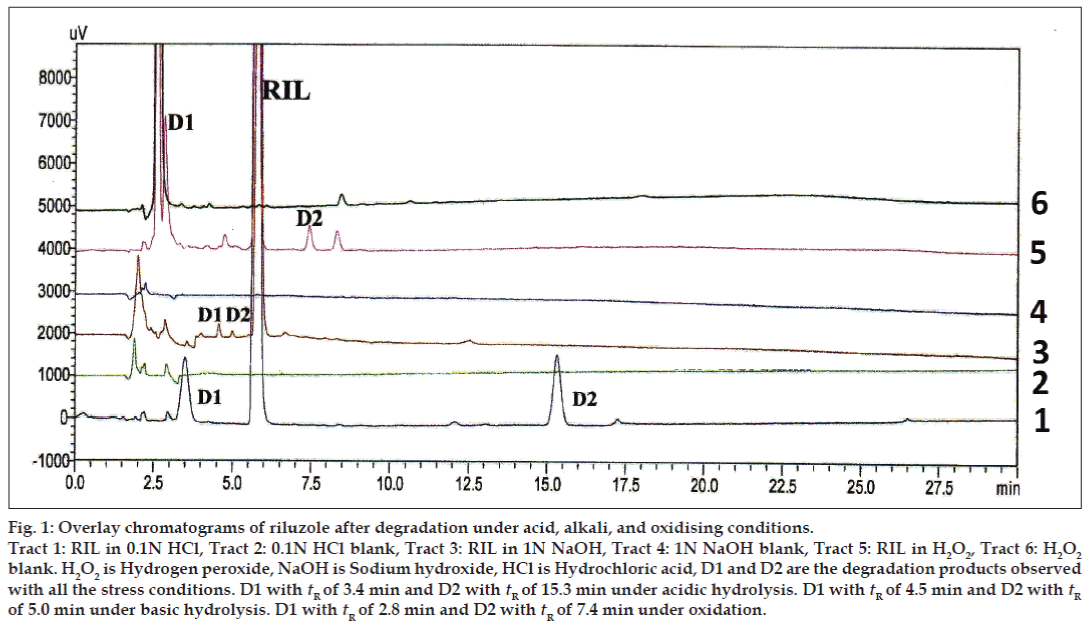
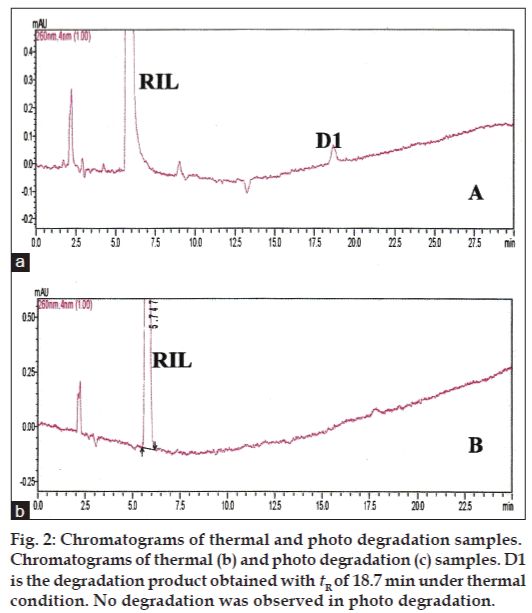
The primary target in developing this stability-indicating LC method was to achieve the resolution between riluzole and its degradation products. Different analytical columns with various stationary phases and mobile phase combinations were tested. Initial trial was done with Phenomenex column using 0.02% v/v formic acid:methanol (50:50 v/v), the RIL peak was eluted at 27.0 min with peak splitting. Whereas with the same mobile phase composition using Inertsil ODS column a broad peak was eluted at 22.0 min. So, further trials were carried out on Inertsil ODS column with 0.02% v/v formic acid:acetonitrile as mobile phase (35:65 v/v). The RIL was eluted at 5.7 min with all the parameters like tailing factor, theoretical plates and symmetry, which are with in the limits. These LC conditions were further used to establish SIM development. Samples of the forced degradation conditions like acidic and basic hydrolysis, oxidation, thermal and photo degradation were run with the above LC conditions. The forced degradation studies showed the method was highly specific where the entire degradation products were well resolved from the main RIL peak. The results from forced degradation studies are given in Table 1. Chromatograms obtained from after degradation under different stress conditions are shown in figs 1 and 2. No peaks co-eluted with the drug peak, suggesting the method enabled specific analysis of RIL in the presence of its degradation products.
| (Stress condition/duration) | tR of RIL and its degradation products (min) | Percent peak area | ||||
|---|---|---|---|---|---|---|
| RILpeak | PeakD1 | PeakD2 | RILpeak | PeakD1 | PeakD2 | |
| Control | 5.7 | – | – | 100 | – | – |
| Acidic/0.1N HCl/40º/2days | 5.72 | 3.493 | 15.31 | 93.804 | 3.986 | 2.210 |
| Alkali/1N NaOH/40º/2days | 5.71 | 4.563 | 5.037 | 95.281 | 2.526 | 2.194 |
| Oxidizing/3%H O /40º/2 days 2 2 |
5.73 | 2.840 | 7.429 | 88.085 | 8.343 | 3.577 |
| Thermal/70º/24h | 5.72 | 18.77 | – | 97.810 | 2.190 | – |
| Photo degradation/2 days | 5.74 | – | – | – | – | – |
Table 1: Forced degradation studies of ril
Figure 1: Overlay chromatograms of riluzole after degradation under acid, alkali, and oxidising conditions.
Tract 1: RIL in 0.1N HCl, Tract 2: 0.1N HCl blank, Tract 3: RIL in 1N NaOH, Tract 4: 1N NaOH blank, Tract 5: RIL in H2O2, Tract 6: H2O2 blank. H2O2 is Hydrogen peroxide, NaOH is Sodium hydroxide, HCl is Hydrochloric acid, D1 and D2 are the degradation products observed
with all the stress conditions. D1 with tR of 3.4 min and D2 with tR of 15.3 min under acidic hydrolysis. D1 with tR of 4.5 min and D2 with tR of 5.0 min under basic hydrolysis. D1 with tR of 2.8 min and D2 with tR of 7.4 min under oxidation.
The peak purity (or peak homogeneity) analysis of the main peak, to assess for the presence of impurities within the main peak, is an essential part of the validation of a SIM. The peak purity test results confirm that analyte peak is homogeneous in all the stress conditions tested. The peak purity of RIL was found to be greater than 0.999. These results confirm the enabling of the developed LC conditions to quantify RIL from bulk and pharmaceutical dosage forms with out any interferences.
A linear relationship was evaluated across the range (10-50 µg/ml) of the analytical procedure in triplicate. The correlation coefficient (R) was found to be 0.9998 and shows good linearity. The data of the calibration curve were given in Table 2. Precision studies were carried out in terms of repeatability. Five determinations of 100% concentration at 30 µg/ml level was evaluated and the data given in Table 2. The % RSD was found to be less than 2 and fulfilled the ICH guidelines criteria.
| Validation data of RIL | |||
|---|---|---|---|
| Linearity (n=3) | Range 10–50 ug/mL | ||
| y=27076x-21990 | - | - | |
| R² = 0.9997 | |||
| R=0.9998 | |||
| Accuracy (n=3) | Level of Addition | Mean percent recovery | %RSD |
| 80% | 99.26 | 0.74 | |
| 100% | 101.43 | 0.79 | |
| 120% | 101.6 | 0.87 | |
| Precision (n=5) | Average peak area of the standard sample (%RSD) 787954.2 (0.062) | - | - |
| System suitability (10–50 μl) | Tailing factor (%RSD) | Theoretical plates (%RSD) | |
| Mean (%RSD) | 1.246 (1.312) | 6479.022 (1.83) | |
| Robustness | Flow rate (ml/min) | Theoretical plates (N) | Tailing factor |
| 0.8 | 6945.4 | 1.5 | |
| 1 | 6882.9 | 1.3 | |
| 1.2 | 6848.9 | 1.4 | |
| Variation in Mobile phase composition |
|||
| 50:50:00 | 6976.4 | 1.5 | |
| 35:65 (actual) | 6882.9 | 1.3 | |
| 40:60 | 6848.9 | 1.5 | |
RIL=Riluzole
Table 2: Validation data of ril.
Accuracy of the method was examined by performing recovery studies by standard addition method and the analyte peak was also evaluated by 3D plots of the chromatogram in order to confirm the existence of one component at 5.7 min elution time of RIL as the impurities are not available. The recovery of the added standard to the drug product sample was calculated and it was found to be 99.26-101.6%, which indicates a good recovery of the method to that of the labeled claim. The obtained recovery results were given in Table 2.
As a part of the robustness, deliberate changes in the flow rate, mobile phase composition, was made to evaluate the impact on the method. Retention times were significantly changed with the flow rate and mobile phase compositions (Table 2). However, the percent assay values of the RIL obtained with these changed conditions are within the limits, indicating the robustness of the LC method.
System suitability parameters like theoretical plate count, tailing factor, and capacity factor were found to be within the limits and the results are given in Table 2. Assay was found to be 100.92±1.2% indicating good compliance with the label claim. None of the tablet ingredients interfered with the analysis of RIL.
It can be concluded that the method developed for the estimation of RIL is rapid, precise, accurate, and selective. The method was completely validated as per ICH guidelines and satisfactory results were obtained for all characteristics tested. The method is stability indicating and can be used to assess the stability of RIL in bulk and pharmaceutical dosage forms and can be conveniently used in quality control laboratory.
References
- O’Neil MJ, editors. In: The Merck Index, An encyclopedia of chemicals, drugs and biologicals, 13th ed. White Hosue Station, NJ: Merck and Co Inc.; 1997. p. 8305.
- Sweetman SC. Martindale: The complete drug reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 1658.
- Maltese A, Maugeri F, Drago F, Bucolo C. Simple determination of riluzole in rat brain by HPLC method. J Chromatogr B 2005;817:331-334.
- Chandu BR, Nama S, Kanala K, Challa BR, Shaik RP, Khagga M. Quantitative estimation of riluzole in human plasma by LC-ESI-MS/ MS and its application to bioequivalence study. Anal Bioanal Chem 2010;398:1367-74.
- Sharma MC, Sharma S, Sharma AD. Validation of riluzole by densitometry application. J Pharm Res 2011;4:1545-7.
- Van Kan HJ, Spieksma M, Groeneveld GJ, Toraño JS, Van den Berg LH, Guchelaar HJ. A validated HPLC assay to monitor riluzole plasma or serum concentrations in patients with amyotrophic lateral sclerosis. Biomed Chromatogr 2004;18:723-6.
- Telekone RS, Shah AN, Khan MJ, Deshpande SV, Mahaparale SP. Spectrophotometric estimation of riluzole in tablet dosage form. Int J Pharma Res Dev 2010;2:12.
- Saminathan J, Vetrichelvan T. Validation of UV spectrophotometric method of riluzole in dosage forms. Int J Chem Tech Res 2011;3:560-4.
- ICH Q2B. Stability Testing of New Drug Substances and Products; International Conference on Harmonization IFPMA Geneva: 2005.