- Corresponding Author:
- K. V. Ramana Murthy
A.U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam - 530 003, India
E-mail: drkvrmurthy@hotmail.com
| Date of Submission | 15 September 2004 |
| Date of Revision | 3 November 2006 |
| Date of Acceptance | 4 April 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 269-273 |
Abstract
Dissolution is the rate-limiting step in the absorption of drugs from solid dosage forms. This is to be focused especially when the drug is poorly soluble. Etoposide is a poorly soluble drug and its absorption is dissolution rate limited. The present study is aimed at improving the dissolution of etoposide. Solid dispersions of drug with surfactants were prepared by using kneading technique. Kneading technique is more applicable to industry. Surfactants such as cetrimide, sodium lauryl sulphate, Tween 80 and Cremophor RH40 were used in different proportions. Etoposide gave faster dissolution rates when Cremophor RH40 was used. The DSC thermograms and IR spectra revealed no interaction of etoposide with these surfactants and no degradation in etoposide molecule was observed.
Dissolution is the rate limiting step in the case of poorly water soluble drugs, in the process of drug absorption. Potential bioavailability problems are prevalent with extremely hydrophobic drugs (aqueous solubility less than 0.1 mg/ml at 37°) due to erratic or incomplete absorption from GIT. The kneading approach has been widely and successfully applied to improve the solubility, dissolution rates and consequently the bioavailability of poorly water soluble drugs [1]. A number of surfactants are used frequently for enhancing the solubility of poorly soluble drugs [2-4]. In the present investigation we studied the effect of cetrimide, sodium lauryl sulphate (SLS), Tween 80 and Cremophor RH40 on the dissolution rate of etoposide.
Etoposide an anticancer drug, is chemically designated as 4’-demethylepipodophyllotoxin 9-[4,6-O-(R)-ethylidene-βD-glucopyranoside] [5]. It is a poorly soluble drug in water as per USP [6]. Because of its poor aqueous solubility, etoposide may pose dissolution related absorption problem [7]. It is marketed as capsules and is official in USP. Hence attempt was made to improve the dissolution of etoposide through preparing solid dispersions of drug with surfactants using kneading method. Surfactants like cetrimide, SLS, Tween 80 and Cremophor RH40 were used in different proportions for enhancing the solubility and thereby the dissolution. All the formulations were subjected to evaluation with special emphasis on dissolution rate studies.
Materials and Methods
Etoposide was obtained from Cadila Pharmaceuticals Limited, Ahmedabad as a gift sample, cetrimide and Cremophor RH40 were obtained from Loba Chemicals, Mumbai, SLS was obtained from S.D. Fine Chemicals, Mumbai, Tween 80 was obtained from Sigma Chemical Co, St. Louis, MO., microcrystalline cellulose (MCC), lactose and PVP K30 were obtained from Knoll Pharmaceuticals Ltd., Pune. Empty hard gelatin capsules were obtained from Dr. Reddy’s Laboratories, Hyderabad. All the chemicals were used of AR grade.
Kneading method
Accurately weighed quantity of etoposide and excipients (MCC and Lactose) were added into a mortar. Under constant trituration, different concentrations of surfactant (cetrimide, SLS, Tween 80 and Cremophor RH40) were added with geometrical dilution method. A wet mass was prepared upon addition of sufficient quantity of PVP solution (3% w/v). This wet mass was kneaded for 15 min. The mass obtained in each case was passed through sieve # 20 to get granules. These granules were dried at 40° until granules having not more than 2.5% moisture content. The dried granules were again passed through sieve # 30 to get uniform granules. The resultant granules equivalent to 100 mg of etoposide were filled in size ‘2’ hard gelatin capsules.
Preparation of physical mixtures
The physical mixtures of etoposide, MCC and lactose with cetrimide, SLS, Tween 80 and Cremophor RH40 were prepared by mixing accurately weighed quantities of drug, surfactants and carrier in stated proportions in a glass mortar and sifted through sieve # 30. The resultant granules equivalent to 100 mg of etoposide were filled in size ‘2’ hard gelatin capsules.
Drug content
An accurately weighed quantity of granules equivalent to 10 mg of etoposide (theoretically) were taken into a 100 ml volumetric flask and dissolved in 1:9 ratio of methanol and chloroform. One millilitre of drug solution was diluted to 100 ml with pH 4.5 acetate buffer and assayed for drug content using double beam spectrophotometer at 254 nm [6].
Reproducibility of method
The reproducibility of the method was checked by preparing four batches of one formulation for each surfactant under similar set of conditions. The percentage yield and drug contents were estimated. The results were subjected to statistical analysis (ANOVA) to assess whether the drug content was uniformly distributed in the kneading preparations and the reproducibility of the technique.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) of etoposide and its kneading preparations were performed in the temperature range of 30° to 300° using Shimadzu DSC-50 Thermal Analyzer. Samples were placed in an aluminum pan and heated at 10°/min with an empty pan as reference.
In vitro dissolution study
Dissolution studies were conducted by using USP XXIV paddle method (apparatus 2) official in USP6. The stirring rate was 50 rpm. An acetate buffer (pH 4.5) was used as dissolution medium (900 ml) and was maintained at 37±1°. Hard gelatin capsules containing prepared mixtures were taken to conduct the dissolution study. Samples of 5 ml were withdrawn at predetermined time intervals with a pipette fitted with a filter. The collected samples were analyzed for etoposide content by UV spectrophotometric method at 254 nm. The volume withdrawn at each time interval was replaced with fresh quantity of 5 ml of dissolution medium. Dissolution studies were performed for three times and mean values were taken.
Results and Discussion
All the formulations were found to be free flowing under dry conditions. Low values of standard deviation in respect of drug content indicated uniform drug distribution in all the formulations. Statistical treatment with ANOVA test on prepared kneading and physical mixtures with pure drug in water shown significant difference in the mean percent of drug dissolved at p=0.05.
DSC thermogram of etoposide in prepared formulations (figure not shown) showed an endothermic peak at 189.3°, which indicates the melting point of etoposide. There is no peak appeared other than the principal peak, indicating that the etoposide is in solid solution form and no interaction between the drug and carrier.
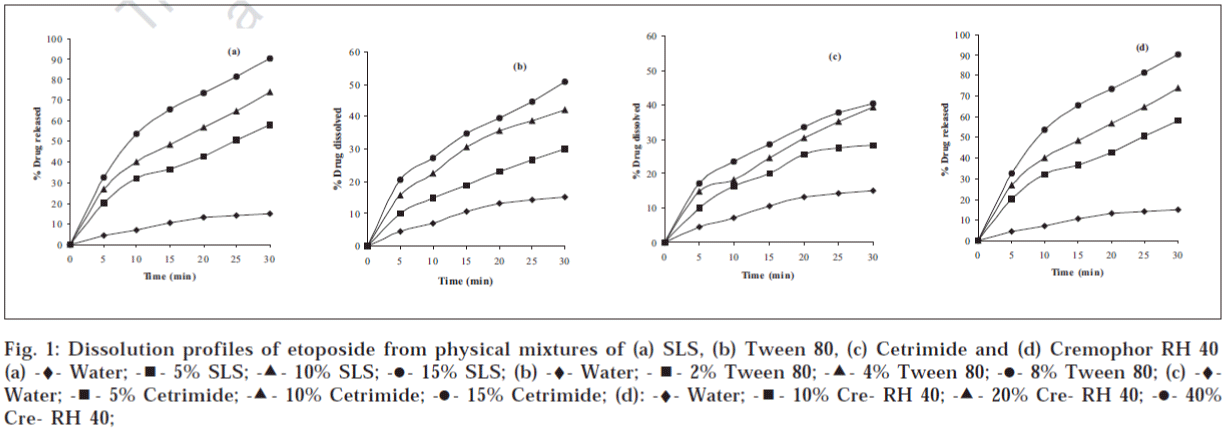
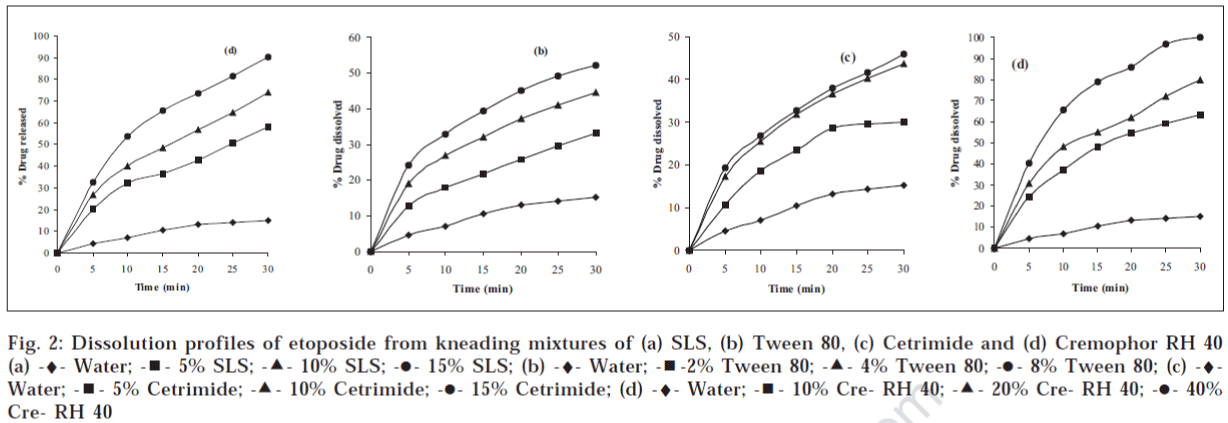
The dissolution profiles of etoposide from kneading mixtures prepared with different surfactants are shown in fig. 1. Though the dissolution rate of the drug increased with increasing concentrations of surfactants, the solid mixtures prepared with SLS, Tween 80 and cetrimide failed to meet the USP dissolution rate requirement which states that not less than 80% (Q) of the labeled amount of DE30etoposide is to be dissolved in 30 min in acetate buffer (pH 4.5). However, the dissolution rate of etoposide was increased significantly in all the kneaded dispersions using Cremophor RH40. Solid mixtures prepared with Cremophor RH40 with concentrations above 20% passed the USP official requirement of dissolution rate testing. The dissolution profiles of etoposide from physical mixtures prepared with different surfactants are shown in fig. 2. The dissolution rate increased with the increased amounts of surfactants, but they did not meet the USP requirement.
Figure 1: Dissolution profiles of etoposide from physical mixtures of (a) SLS, (b) Tween 80, (c) Cetrimide and (d) Cremophor RH 40 (a) -♦- Water; -◾- 5% SLS; -▲- 10% SLS; -⚫- 15% SLS; (b) -♦- Water; -◾- 2% Tween 80; -▲- 4% Tween 80; -⚫- 8% Tween 80; (c) -♦-Water; -◾- 5% Cetrimide; -▲- 10% Cetrimide; -⚫- 15% Cetrimide; (d): -♦- Water; -◾- 10% Cre- RH 40; -▲- 20% Cre- RH 40; -⚫- 40% Cre- RH 40;
Figure 2: Dissolution profiles of etoposide from kneading mixtures of (a) SLS, (b) Tween 80, (c) Cetrimide and (d) Cremophor RH 40 (a) -♦- Water; -◾- 5% SLS; -▲- 10% SLS; -⚫- 15% SLS; (b) -♦- Water; -◾-2% Tween 80; -▲- 4% Tween 80; -⚫- 8% Tween 80; (c) -♦-Water; -◾- 5% Cetrimide; -▲- 10% Cetrimide; -⚫- 15% Cetrimide; (d) -♦- Water; -◾- 10% Cre- RH 40; -▲- 20% Cre- RH 40; -⚫- 40% Cre- RH 40
The mean percent of etoposide dissolved with function of time in minutes from kneading mixtures of various surfactants are shown along with standard deviation in Table 1. The results of in vitro studies of etoposide physical mixtures with various surfactants are given in Table 2. Dissolution efficiency values at 10 minutes (DE10) and 30 minutes (DE30) were calculated for drug release from different formulations prepared by using various surfactants in different ratios. The results indicated that in each case of surfactant the DE10 and DE30 values increased as the surfactant concentration increased. When DE10 and DE30 values were calculated the solid mixture prepared with Cremophor RH40 gave significantly high values compared to pure drug in water and solid mixture prepared with other surfactants. T50 and T90 are the values that the time requires to dissolve the 50% and 90% of the drug from the solid mixtures, respectively. The results are shown in Table 3.
| Time (min) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Medium | 5 | 10 | 15 | 20 | 25 | 30 | ||||
| Water | 4.58±0.25 | 7.05±0.87 | 10.52±1.04 | 13.15±0.47 | 14.25±0.68 | 15.17±0.55 | ||||
| 5% SLS | 5.52±0.98 | 8.24±0.98 | 10.97±0.86 | 14.05±0.99 | 15.10±0.58 | 17.03±0.49 | ||||
| 10% SLS | 6.87±0.75 | 9.07±0.89 | 11.20±0.40 | 16.10±0.58 | 18.32±0.66 | 20.04±0.98 | ||||
| 15% SLS | 10.25±0.99 | 18.30±0.96 | 25.41±0.98 | 32.49±0.88 | 38.60±0.57 | 42.07±1.05 | ||||
| 2% Tween 80 | 12.7±0.87 | 17.96±0.25 | 21.68±0.19 | 25.91±0.56 | 29.64±0.73 | 33.04±1.08 | ||||
| 4% Tween 80 | 19.00±0.40 | 26.80±0.32 | 32.08±0.71 | 37.14±0.56 | 41.09±0.82 | 44.60±0.59 | ||||
| 8% Tween 80 | 24.20±0.91 | 32.97±0.75 | 39.40±0.88 | 45.08±0.76 | 49.15±0.93 | 52.06±0.95 | ||||
| 5% Cetrimide | 10.57±0.24 | 18.61±0.89 | 23.51±0.70 | 28.73±0.81 | 29.52±0.36 | 30.00±0.88 | ||||
| 10% Cetrimide | 17.35±0.96 | 25.47±0.89 | 31.82±0.99 | 36.54±0.92 | 40.17±0.93 | 43.61±0.97 | ||||
| 15% Cetrimide | 19.30±0.91 | 26.85±0.46 | 32.75±0.52 | 38.04±0.81 | 41.70±0.78 | 45.82±0.90 | ||||
| 10% Cre-RH 40 | 24.09±0.98 | 37.24±0.98 | 48.34±0.86 | 54.73±0.99 | 59.27±0.58 | 63.08±0.49 | ||||
| 20% Cre-RH 40 | 30.70±0.75 | 48.29±0.89 | 55.08±0.40 | 61.79±0.58 | 72.03±0.66 | 80.00±0.98 | ||||
| 40% Cre-RH 40 | 40.18±0.99 | 65.37±0.96 | 78.92±0.98 | 85.61±0.88 | 96.85±0.57 | 99.99±1.05 | ||||
| (N=3±sd) | ||||||||||
Table 1: Dissolution profiles of etoposide from kneading mixtures.
| Time (min) | ||||||
|---|---|---|---|---|---|---|
| Medium | 5 | 10 | 15 | 20 | 25 | 30 |
| Water | 4.58 ± 0.25 | 7.05 ± 0.87 | 10.52 ± 1.04 | 13.15 ± 0.47 | 14.25 ± 0.68 | 15.17 ± 0.55 |
| 5% SLS | 5.07 ± 0.23 | 8.56 ± 0.79 | 10.76 ± 0.33 | 13.89 ± 0.82 | 14.77 ± 0.96 | 16.22 ± 0.37 |
| 10% SLS | 6.55 ± 0.88 | 8.89 ± 0.47 | 10.97 ± 0.72 | 15.26 ± 0.37 | 17.89 ± 0.63 | 19.70 ± 0.67 |
| 15% SLS | 9.87 ± 0.89 | 16.22 ± 0.44 | 21.32 ± 0.98 | 29.36 ± 0.86 | 35.44 ± 0.98 | 40.21 ± 0.99 |
| 2% Tween 80 | 10.23 ± 0.66 | 14.82 ± 0.73 | 18.74 ± 0.86 | 23.02 ± 0.82 | 26.77 ± 0.43 | 29.92 ± 0.93 |
| 4% Tween 80 | 15.77 ± 0.76 | 22.37 ± 0.44 | 30.46 ± 0.78 | 35.73 ± 0.98 | 38.66 ± 0.49 | 42.01 ± 0.98 |
| 8% Tween 80 | 20.41 ± 0.88 | 27.33 ± 0.98 | 34.82 ± 0.77 | 39.66 ± 0.78 | 44.57 ± 0.62 | 50.63 ± 0.82 |
| 5% Cetrimide | 9.98 ± 0.66 | 16.43 ± 0.26 | 20.07 ± 0.98 | 25.67 ± 0.47 | 27.42 ± 0.58 | 28.33 ± 0.99 |
| 10% Cetrimide | 14.72 ± 0.44 | 18.2 ± 0.96 | 24.66 ± 0.52 | 30.45 ± 0.68 | 35.28 ± 0.84 | 39.46 ± 0.42 |
| 15% Cetrimide | 17.22 ± 0.48 | 23.46 ± 0.54 | 28.66 ± 0.72 | 33.45 ± 0.63 | 37.82 ± 0.81 | 40.42 ± 0.97 |
| 10% Cre- RH 40 | 20.27 ± 0.99 | 31.96 ± 0.67 | 36.72 ± 0.96 | 42.66 ± 0.98 | 50.84 ± 0.88 | 58.07 ± 0.92 |
| 20% Cre- RH 40 | 27.03 ± 0.32 | 39.92 ± 0.47 | 48.45 ± 0.80 | 56.62 ± 0.73 | 64.88 ± 0.98 | 73.82 ± 0.66 |
| 40% Cre- RH 40 | 32.47 ± 0.96 | 53.56 ± 0.65 | 65.6 ± 0.72 | 73.36 ± 0.48 | 81.67 ± 0.79 | 90.24 ± 1.05 |
Table 2: Dissolution profiles of etoposde from physical mixtures
| Medium | Kneading mixture | Physical mixture | ||||||
|---|---|---|---|---|---|---|---|---|
| DE10 | DE30 | T50 | T90 | DE10 | DE30 | T50 | T90 | |
| Water | 4.05 | 9.52 | - | - | 4.05 | 9.52 | - | - |
| 5% SLS | 4.82 | 10.4 | - | - | 4.68 | 10.19 | - | - |
| 10% SLS | 5.7 | 11.93 | - | - | 5.5 | 11.57 | - | - |
| 15% SLS | 9.7 | 24.35 | - | - | 8.99 | 22.05 | - | - |
| 2% Tween 80 | 10.84 | 20.74 | - | - | 8.82 | 18.09 | - | - |
| 4% Tween 80 | 16.2 | 29.74 | - | - | 27.33 | - | - | 13.48 |
| 8% Tween 80 | 20.34 | 36.14 | 26.46 | - | 17.04 | 32.02 | 29.48 | - |
| 5% Cetrimide | 9.94 | 20.99 | - | 9.1 | 18.96 | - | - | - |
| 10% Cetrimide | 15.04 | 28.86 | - | - | 11.91 | 23.84 | - | - |
| 15% Cetrimide | 16.36 | 30.26 | - | . | 26.8 | - | - | 14.48 |
| 10% Cre- RH 40 | 21.36 | 42.54 | 16.3 | - | 16 | 27.31 | - | - |
| 20% Cre- RH 40 | 27.42 | 51.32 | 11.26 | - | 23.5 | 45.64 | 15.95 | - |
| 40% Cre- RH 40 | 36.43 | 69.49 | 6.95 | 21.95 | 29.63 | 58.64 | 9.16 | 29.86 |
Table 3: Dissolution parameters of etoposide from prepared mixtures.
The increase in the dissolution rate of the solid mixtures might be due to size reduction and increases in the wettability of the drug molecules in presence of the surfactants and Cremophor might be acting as a powerful surfactant compared to other surfactants used in the study. The increase in dissolution rate is proportional to the concentration of surfactant in all cases. The dissolution of etoposide in pure form and from various kneading preparations obeyed Hixson-Crowell’s cube root dissolution rate equation. All the formulations follow the first order dissolution kinetics as shown in Table 4.
| Formula | Kneading Mixture | Physical Mixture | ||||||
|---|---|---|---|---|---|---|---|---|
| First order | Hixson-Crowell | First order | Hixson-Crowell | |||||
| r | K | r | K | r | K | r | K | |
| Water | 0.9962 | 0.0073 | 0.9958 | 0.0112 | 0.9962 | 0.0076 | 0.9958 | 0.0112 |
| 5% SLS | 0.9909 | 0.0086 | 0.9900 | 0.0131 | 0.9957 | 0.009 | 0.9952 | 0.0136 |
| 10% SLS | 0.9866 | 0.0095 | 0.9853 | 0.0145 | 0.9894 | 0.0093 | 0.9881 | 0.0142 |
| 15% SLS | 0.9997 | 0.0202 | 0.9991 | 0.0302 | 0.9983 | 0.0177 | 0.9981 | 0.0266 |
| 2% Tween 80 | 0.9812 | 0.0198 | 0.9771 | 0.0296 | 0.9893 | 0.0161 | 0.9866 | 0.0242 |
| 4% Tween 80 | 0.9801 | 0.0312 | 0.9775 | 0.0458 | 0.9891 | 0.0253 | 0.9858 | 0.0376 |
| 8% Tween 80 | 0.9862 | 0.0400 | 0.9727 | 0.0579 | 0.9848 | 0.0319 | 0.9784 | 0.0469 |
| 5% Cetrimide | 0.9894 | 0.0008 | 0.9983 | 0.0308 | 0.9950 | 0.0179 | 0.9943 | 0.027 |
| 10% Cetrimide | 0.9991 | 0.0014 | 0.9835 | 0.0433 | 0.9903 | 0.0201 | 0.9871 | 0.0301 |
| 15% Cetrimide | 1 | 0.0015 | 0.9751 | 0.0459 | 0.9751 | 0.0267 | 0.9703 | 0.0396 |
| 10% Cre- RH 40 | 0.9969 | 0.0035 | 0.9922 | 0.0668 | 0.9625 | 0.0267 | 0.9548 | 0.0396 |
| 20% Cre- RH 40 | 0.9963 | 0.006 | 0.9954 | 0.0916 | 0.9940 | 0.051 | 0.9914 | 0.0725 |
| 40% Cre- RH 40 | 0.9937 | 0.0147 | 0.9995 | 0.1382 | 0.9999 | 0.0767 | 0.9987 | 0.1047 |
Table 4: Correlation coefficient (r) and release rate constants (k) of etoposide from relprepared mixtures.
In Conclusion, Kneading preparations of etoposide with different categories of surfactants like sodium lauryl sulphate (anionic type), cetrimide (cationic Type), Tween 80 (nonionic type) and Cremophor RH40 (nonionic type), resulted in increased dissolution rate. The increase in dissolution might be due to increase in the wettability of hydrophobic drug like etoposide and also lowering effect of surface tension, hence increasing the solubilizing effect. Etoposide dissolution rate was meeting the USP requirement when Cremophor RH40 was used as surfactant at 40% level with respect to drug. This kind of technique can be extended for improvement dissolution rate of drugs showing poor dissolution profiles and causing erratic bioavailability. This kneading technique was more applicable to industrial practice and production.
References
- Murali Mohan Babu GV, Prasad CH, Ramana Murthy KV. Evaluation of modified gum karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine.Int J Pharm 2002;234:1-17
- Rodriguez NH, Murphy D. Surfactant-facilitated crystallization of dihydrate carbamazepine during dissolution of anhydrous polymorph, J Pharm Sci 2004;93:449-60.
- Perng CY, Kearney AS, Patel K, Palepu NR, Zuber G. Investigation of formulation approaches to improve the dissolution of SB-210661, a poorly water soluble 5-lipoxygenase inhibitor. Int J Pharm 1998;176:31-8.
- Sjokvist E, Nystrom C, Alden M, Carame-Lelham N. Physicochemical aspects of drug release. XIV. The effects of some ionic and non-ionic surfactants on properties of a sparingly soluble drug in solid dispersions. Int J Pharm 1992;79:123-33.
- The Merck Index, 12th ed., Merck and Co Inc: White House, NJ; 1996. p. 3934.
- United States Pharmacopoeia, 24th ed. The United States Pharmacopeial convention Inc., Rockville, MD 20852; 1999.p. 703.
- Shah JC, Chen JR, Chow D. Preformulation study of etoposide: II. Increased solubility and dissolution rate by solid-solid dispersions, Int J Pharm 1995;113:103-11.