- Corresponding Author:
- R. Lakshmi
Department of Stroke Medicine, Amrita Institute of Medical Sciences and Research Centre, Kochi−682 041, India
E−mail: lakshmir87@gmail.com
| Date of Submission | 04 June 2012 |
| Date of Revision | 16 January 2013 |
| Date of Acceptance | 20 January 2013 |
| Indian J Pharm Sci 2013;75(1):53-59 |
Abstract
Anticoagulants are very useful medications but can also lead to haemorrhagic as well as thromboembolic complications when not used correctly or without proper medical attention. Anticoagulant's complex pharmacology and pharmacokinetics contribute to its narrow margin of safety. Pharmacist's unique knowledge of pharmacology, pharmacokinetics and interactions makes them well-suited to assist patients in maintaining safe and effective anticoagulation. Successful anticoagulation therapy implies fewer incidences of therapeutic failures and bleeding complications. The anticoagulation management service staffed by clinical pharmacists is a service established to monitor and manage oral and parenteral anticoagulants. In this research work, 40 patients each were included in the intervention and the control groups. In the intervention group, patient's knowledge score on anticoagulation increased from an average of 5.6±3.2 to 13.8±0.94 (P=0.000) after clinical pharmacist's counselling, whereas in the control group there was no significant improvement in patient's baseline knowledge over the knowledge score at the end of the study (8.0±1.59 vs. 8.3±2.6) (P=0.218). In the intervention group, 73.45% of the international normalised ratio test results were within the therapeutic range, 8.45% supratherapeutic and 18.5% subtherapeutic during the 6 months data collection period. The corresponding data for the control group were 53.2 (P=0.000), 18.4 (P=0.000) and 28.4% (P=0.002), respectively. Forty four adverse drug reactions (ADRs) related to anticoagulants were identified in the intervention group as compared to 56 in the control group. These results revealed that the clinical pharmacist's involvement in the anticoagulation management improved the therapeutic outcome of patients and demonstrate the benefits of clinical pharmacist guided anticoagulation clinics in India.
Keywords
Anticoagulation management in India, clinical pharmacist, international normalised ratio, thromboembolism, warfarin.
Anticoagulants are substances that prevent coagulation; that is, they stop blood from clotting. A clot can create a potentially dangerous medical condition if it causes a blockage of blood flow to a vein or artery. Anticoagulants act by inhibiting the hepatic vitamin K dependent synthesis of coagulation factors II (prothrombin), VII, IX and X and of the anticoagulant protein C and its cofactor protein S[1]. A group of pharmaceuticals called anticoagulants can be used as a medication for thrombotic disorders[2].
Anticoagulants are commonly used for both treatment and prevention of cardiac diseases, cerebral vascular accidents, and thromboembolism in the inpatient and outpatient settings. Their use or misuse carries a significant potential for patient harm. Subtherapeutic levels can increase the risk of thromboembolic complications while supratherapeutic levels can increase the risk of bleeding complications. Anticoagulants have been implicated in adverse drug events due to many factors such as complexity of dosing and monitoring, patient compliance, and numerous drug–drug and drug–food interactions. The demand for anticoagulation services is increasing, particularly in the elderly population[3,4].
The anticoagulation management service (AMS) is a service established to monitor and manage oral and parenteral anticoagulants that decrease formation of blood clots[5]. In a survey of a pharmacist−managed anticoagulation clinic, to find out patients’ perceptions of pharmacist involvement with anticoagulation services, it was indicated that majority of patients were comfortable with pharmacists providing monitoring and dosage adjustments of warfarin[6,7]. When the clinical pharmacists assume responsibility and accountability for achieving therapeutic goals, they are called upon to be more than consultants.
During the course of anticoagulation therapy, a variety of health−care professionals are involved in patient care. The pharmacist’s role is multifactorial and can include monitoring, dosing and provision of drug information, patient education, drug interaction screening and research[8,9]. Pharmacist guided anticoagulation clinics play an important role in managing anticoagulation therapy for both hospitalised patients and outpatients in western countries[10]. Trained in the basic pathophysiology of blood clotting and the essentials of clinical clotting disorders, pharmacists bring their expertise in clinical pharmacology and knowledge of drug interactions to the arena of patient management.
These pharmacists evaluate and manage essentially all hospitalised patients treated with warfarin, as well as most patients treated with full dose heparin or low molecular weight heparin. The pharmacists give the attending physicians and house−staff important information about potential drug interactions, in addition to daily dosing recommendations[11]. Many anticoagulation clinics are staffed only by clinical pharmacists, and there is an indication that this practice has led to nothing except excellent care. The multidisciplinary team effort provides truly optimal care for a population of patients having a high level of comorbidity[12].
In a study to assess the anticoagulation knowledge of patients new to warfarin therapy conducted by Winans et al. demonstrated that inpatient warfarin education programme by AMS may empower patients to achieve a larger degree of initial warfarin knowledge than those educated by usual care[13].
Materials and Methods
It was a prospective randomised controlled study extended over a period of 6 months. Patient data were collected from 1st February 2011 to 31st July 2011. The study was carried out at the Department of Stroke Medicine and at the Department of Cardiology of a tertiary care teaching hospital in Kochi, Kerala, India. The study got ethical clearance from the institutional ethical committee and written informed consent was obtained from all the study participants. The study population consisted of patients admitted to the Departments of Stroke Medicine and Cardiology and who were prescribed warfarin or other oral anticoagulants. Intervention group consisted of all the 41 patients admitted to the department of stroke medicine during the study period and who satisfied the inclusion and exclusion criteria. Forty randomly selected patients from the cardiology ward who were newly started on oral anticoagulants served as the control group. Patient data relevant to the study were obtained by direct interview of the patient and/or caregiver, and from the patient’s medical record and documented in the standardised data collection form.
The data collection form provided information regarding the demographic details of the patient, comorbid conditions, indication for anticoagulation, lab data including international normalised ratio (INR) values, date of INR measurement, and goal INR. Data regarding adverse drug reactions (ADRs) related to anticoagulants, drug and food interactions with anticoagulants, intake of over the counter (OTC) medications and patient’s knowledge score on oral anticoagulants were also included.
The percentage of INRs within the therapeutic range for the intervention group was compared with that of the control group. The time within therapeutic range was calculated using the method of ‘The fraction of INRs in the range’, which was calculated by taking total number of INRs within therapeutic range for all patients divided by the total number of INRs checked during the selected time interval i.e., 6 months period[14]. All the patients, after discharge, were followed−up during the entire period of study through telephonic contacts on a weekly basis. The patients were free to call the clinical pharmacist for clarification of any anticoagulation related issues between 8 am and 8 pm thorough an on call mobile allocated by the hospital administration for the AMS. The frequencies of INR monitoring and dose adjustments were based on the patients INR values. If the values were within therapeutic range, the testing frequency was once in every 2 weeks.
Patient’s knowledge on oral anticoagulation was assessed using a questionnaire (Table 1) comprising of 15 questions. Scores 1 and 0 were given for each right and wrong answer, respectively. During the study period, the baseline knowledge on oral anticoagulation therapy of both intervention and control group patients was assessed. The intervention group patients were counselled on anticoagulation therapy and its importance, common ADRs and management, importance of patient compliance, dose titration, dietary modifications, and the need for INR monitoring by a clinical pharmacist. Patient information booklets were also provided to all the patients in the intervention group. The knowledge of the intervention group was reassessed using the same questionnaire when they came for review after 1 month. A patient satisfaction survey on anticoagulation service provided by the AMS was also assessed by a third person.
Inclusion criteria
The patients inclusion criteria were as follows, patients prescribed warfarin or other oral anticoagulants; patients of age ≥18 years; patients and/or their caregivers (with informed; consent) willing to participate in the study; patients who are able to read and speak English or the local language Malayalam.
Exclusion criteria
The excliusion criteria for the patients were as follows, patients on parenteral anticoagulants; patients with severe renal insufficiency who are on dialysis; patients with active liver disease; patients having visual or hearing impairment.
| Questions |
|---|
| What is warfarin? Why you have been prescribed warfarin? What is your current dose of warfarin? |
| Who is responsible for adjusting your warfarin dose? What is the importance of INR testing? |
| What is your target INR? |
| How frequently should you check your INR? When should warfarin be taken and why? What will you do in case of a missed dose? |
| What will happen when you take a double dose of warfarin? |
| What will you do in case of surgery, dental work, or some type of invasive procedures while on warfarin? |
| Which types of foods affect warfarin therapy? |
| Do you know that some of the drugs, alcohol, herbal medicines can affect warfarin’s action? |
| What will you do in case of bleeds from nose/gum? What should you do if you plan to go on holidays? What are the possible side effects of warfarin? |
Table 1: Questionnaire To Assess The Patient’s Knowledge On Oral Anticoagulation Therapy.Inr=International Normalised Ratio
Statistical analysis
Frequency distributions and descriptive statistics were used to calculate baseline characteristics of patients. Calculation of the mean and standard deviation were carried out by using statistical calculators. Data was shown as mean±SD and number (%) of patients unless otherwise stated. The significance of the study results were assessed using independent sample t−test and paired sample t−test. A P value of <0.05 was considered statistically significant.
Results and Discussion
Table 2 summarises the baseline characteristics of patients in the intervention and control groups. 60% (26) of patients in both the groups were males and 40% (14) were females. Hence, the gender distribution of patients in both the groups was similar. The male−female ratio in both groups was 1.5:1. The mean age of the patients in intervention group was 55.98±13.47 years with minimum of 30 and maximum of 87 years and that of control group patients was 55.95±12.23 years with minimum of 26 and maximum of 80 years.
In the intervention group, 25% of patients had no comorbidities whereas 20% of them had one comorbidity, 32.5% of them two comorbidities, 20% of them three comorbidities and 2.5% of them had four comorbidities. The largest number of patients (50%) in the intervention group had hypertension as the comorbidity followed by diabetes mellitus (32.5%) and dyslipidaemia (15%). However, in the control group 20% of the patients had no comorbidities whereas 20% of them had one comorbidity, 50% of them had two comorbidities, 7.5% of them had three comorbidities and 2.5% of them had four comorbidities. The largest number of patients (47.5%) had hypertension as the comorbidity followed by diabetes mellitus (37.5%) and dyslipidaemia (27.5%) similar to the intervention group (Table 3).
| Characteristics | Intervention | Control |
|---|---|---|
| group (n=40) | group (n=40) | |
| (P=0.01) | (P=0.01) | |
| Total no. of patients | 40 | 40 |
| Males (%, no.) | 60 (26) | 60 (26) |
| Mean age of male patients | 58.35±11.64 | 56.28±12.84 |
| (years)±SD | ||
| Mean age of female patients | 51.5±11.61 | 55.47±14.02 |
| (years)±SD | ||
| Patients with age >60 years | 18 | 15 |
| Patients with no comorbidity | 10 | 8 |
| Patients with ≥1 comorbidity | 30 | 32 |
| Baseline knowledge score of | 5.0±1.59 | 8.0±2.19 |
| patients on oral anticoagulation | ||
| % of patients with goal INR of | 77.5 | 82.5 |
| 2.0-3.0 | ||
| % of patients with goal INR of | 22.5 | 17.5 |
| 2.5-3.5 | ||
| Ethnicity | Asian | Asian |
Table 2: Summary Of Baseline Characteristics Of The Study Patients.
INR=International normalised ratio, SD=Standard deviation
During the study period the most common indication for anticoagulation was found to be the atrial fibrillation followed by the mitral valve replacement in both the groups. Anticoagulants were also prescribed for secondary prevention in stroke patients who had cardiac risk factors. Majority of patients (70%) in the intervention group the target INR was 2.0−3.0 followed by a target INR of 2.5−3.5 in 22.5% of patients and a target INR of 2.0−2.5 in 7.5% of the patients whereas in the control group, 82.5% of the patients had 2.0−3.0 and 17.5% had 2.5−3.5 as their target INR range (Table 4). Thus, the base line characteristics of patients in the both the control and intervention groups were comparable.
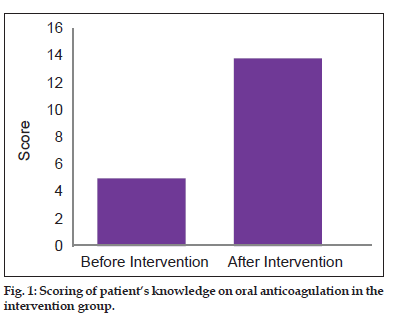
Patient’s baseline knowledge was assessed by means of an internally validated questionnaire and the mean base line knowledge score of the patients in the intervention group was found to be 5.0±2.19. One month postcounselling, the knowledge of the intervention group patients was reassessed and the mean score increased to 13.8±0.9. Here, it is evident that patient’s knowledge on oral anticoagulation improved after counselling by a clinical pharmacist in the intervention group. Baseline knowledge of the patients in the control group regarding oral anticoagulation was also assessed using the same questionnaire and the score was estimated to be 8.0±1.59 (fig. 1) and the score after 1 month was 8.3±2.6. The control group patients received only the ‘usual care’ by the physicians.
| Comorbidities | Intervention group (no. (%) of patients) | Control group (no. (%) of patients) |
|---|---|---|
| n=40, P=0.01% | n=40, P=0.01% | |
| None | 25 (10) | 20 (8) |
| Asthma | 20 (2) | 12.5 (5) |
| BPH | 2.5 (1) | 2.5 (1) |
| COPD | 2.5 (1) | 2.5 (1) |
| Coronary heart disease | 17.5 (7) | 10 (4) |
| Diabetes mellitus | 32.5 (13) | 37.5 (15) |
| Dyslipidemia | 15 (6) | 27.5 (11) |
| Hypertension | 50 (20) | 47.5 (19) |
| Hypothyroidism | 10 (4) | 2.5 (1) |
| Rheumatic heart disease | 12.5 (5) | 10 (4) |
Table 3: Pattern of comorbidities in the intervention and control groups. Bph=benign prostrate ypertrophy, COPD=Chronic obstructive pulmonary disease.
The baseline patient knowledge score for the intervention and control groups was 5.0±2.19 and 8.0±1.59, respectively. The P value by independent sample t−test was found to be 0.078 and hence, the baseline patient knowledge scores are not statistically significant. Paired sample t−test was used to compare the patient knowledge score before and after the intervention in the intervention group. A P value of 0.000 indicates that there is statistically significant difference between the knowledge scores of intervention group patients after counselling. However, in the control group there is no significant difference between the two scores (8.0±1.59 and 8.3±2.6) i.e., it is evident that clinical pharmacist’s intervention improved patient’s knowledge on oral anticoagulation. In a study to assess the anticoagulation knowledge in patients new to warfarin therapy conducted by Winans et al. found that the intervention group (n=20) scored significantly higher on the oral anticoagulation knowledge test than the usual care group (n=20): 74% versus 55%, respectively (P=0.004). They demonstrated a large amount of variability regarding patient knowledge of warfarin on discharge from an inpatient facility. They concluded that inpatient warfarin education program may empower patients to achieve a larger degree of initial warfarin knowledge than those educated by usual care[13].
| Indications for | Intervention group | Control group (n=40) |
|---|---|---|
| anticoagulation and | (n=40) (no. (%) of | (no. (%) of patients) |
| (target INR) | patients) P=0.01 (%) | P=0.01 |
| Mitral valve | 9 (22.5) | 7 (17.5) |
| replacement (2.5-3.5) | ||
| Atrial fibrillation | 13 (32.5) | 32 (80) |
| (2.0-3.0) | ||
| Deep vein thrombosis | 2*(5) | 0 |
| (2.0-3.0) | ||
| Pulmonary embolism | 2*(5) | 0 |
| (2.0-3.0) | ||
| Valvotomy (2.0-3.0) | 1 (2.5) | 0 |
| Bioprosthetic valve | 1 (2.5) | 1 (2.5) |
| (2.0-3.0) | ||
| Other cardiac risk | 12 (30) | 0 |
| factors (2.0-3.0) |
Table 4: Indications For Anticoagulation In The Study Patients.
Tang et al. studied the relationship between patients’ warfarin knowledge and anticoagulation control and they concluded that patient’s warfarin knowledge is an important determinant of anticoagulation control[15].
The study on ‘Comparison of an anticoagulation clinic with usual medical care’ carried out by Chiquette et al.[16] confirmed that a clinical pharmacist managed ‘Anticoagulation Clinic’ improved anticoagulation control, reduced bleeding and thromboembolic event rates, and saved $162,058/100 patients annually due to reduced hospitalisations and emergency department visits.
Table 5 depicts the anticoagulation control achieved in both intervention and control group patients. In the control group patients, 46.5% of INRs measured were within the target range, 23.56% were above the target range and 29.3% were below the target range, whereas in the intervention group % of INRs within, above and below the target range were 77.4, 8.31 and 14.29, respectively. In the intervention group, 1.56% of the INRs were >5 and none of them were >8, whereas in the control group 6.05% of INRs were >5 and 0.96% of the INRs were >8. The risk of bleeding is increased when the INR is >5 and an INR >8 is a medical urgency.
| Intervention group (n=40) (P=0.01) (%) | Control group (n=40) (P=0.01) (%) | |
|---|---|---|
| Total no. of INR checks | 385 | 314 |
| No. (%) of INRs within the target range | 298 (77.4) | 146 (46.5) |
| No. (%) of INRs above the target range | 32 (8.31) | 74 (23.56) |
| No. (%) of INRs below the target range | 55 (14.29) | 92 (29.30) |
| No. (%) of INRs>5 | 6 (1.56) | 19 (6.05) |
| No. (%) of INRs>8 | 0 | 3 (0.96) |
Table 5: Evaluation of international normalised ratio results in the intervention and control group patients. Inr=international normalised ratio.
Independent sample t−test was used to compare the INR levels of the intervention and control groups. Total number of INRs monitored by the patients in the intervention and control groups were 385 and 314, respectively (P value 0.097). The P value is nonsignificant and it indicates that the clinical pharmacist’s involvement did not increase the number of INRs monitored. The INRs within target range (P value 0.000), above target range (P value 0.000), below target range (P value 0.002) and INR values >5 (P value 0.001) of the intervention and control groups were compared. All the above P values were significant indicating that there was a significant difference between the two groups. It reveals that the INR levels in normal, above and below range and >5 are significantly different in the intervention and control groups at 5% level of significance.
In the intervention group, 44 ADRs occurred and three of which were major bleeding events whereas in the control group 56 ADRs were identified and out of which seven were major bleeding events[17]. The number of ADRs especially, the no of major bleeding events were less in the intervention group compared to the control group (Table 6). However, the number of patients experiencing major bleeding events was the same in both groups, i.e., 3 (7.5%). In the intervention group, each of the three patients had only one event of bleeding but in the control group three of the patients experienced a total of seven episodes. The control group patients monitored their INR values less frequently and at irregular intervals. The reasons for major bleeding events in the intervention group patients were due to drug interaction between warfarin and the fluconazole capsule the patient was taking for oral candidiasis in one case and due to injury resulting from falls in two of the other cases.
| ADRs | Intervention group (n=40) (P=0.01) | Control group (n=40) (P=0.01) |
|---|---|---|
| Major bleeding | ||
| Bleed requiring blood/FFP tansfusion | 2 | 5 |
| Intracranial bleed | 1 | 2 |
| Minor bleeding | ||
| Bleeding from blood draw site | 9 | 12 |
| Haematoma | 1 | 1 |
| Blood in stool | 1 | 2 |
| Gum bleed | 10 | 12 |
| Gastrointestinal discomfort | 12 | 13 |
| Rash | 8 | 9 |
| Total | 44 | 56 |
Table 6: adverse drug reactions observed during the study period for the patients in the intervention and control groups. Ffp=fresh frozen plasma.
During the study period, 18 drug interactions were observed and resolved in the intervention group. Out of 18, 10 were due to warfarin−food interactions. Two interactions with OTC medications (ibuprofen) were also observed whereas eight interactions were identified in the control group (Table 7). More drug interactions were identified and resolved in the intervention group due to the clinical pharmacist’s involvement.
Assessment of patient satisfaction with the anticoagulation service was carried out using a Questionnaire. The results showed that all the patients were satisfied with the service (Table 8).
| Interaction | Intervention group (n=40) (P=0.01) | Control group (n=40) (P=0.01) |
|---|---|---|
| Food interactions | ||
| Green leafy vegetables | 6 | 2 |
| Protein powder* | 3 | 0 |
| Glucerna* | 1 | 0 |
| Drug interactions | ||
| Fluconazole | 3 | 2 |
| Atorvastatin | 1 | 4 |
| OTC medications | 2 | 0 |
| Ayurvedic medications | 2 | 0 |
| Total | 18 | 8 |
Table 7: drug and food interactions observed during the study period in the intervention and control groups. Otc=over the counter, *these nutritional supplements contain small amounts of vitamin k.
| Question | Response (n=40) P=0.01 | |
|---|---|---|
| Yes | No | |
| Are you satisfied with the follow−up arrangements by the anticoagulation management service? | 40 | 0 |
| Did you find any problems associated with the follow−up arrangements? | 2 | 38 |
| Are you satisfied with the counselling provided by the clinical pharmacist? | 40 | 0 |
| Were the written information booklet provided easy to read and understand? | 40 | 0 |
| Overall, are you satisfied with the clinical pharmacist’s involvement in the management of your anticoagulation therapy? | 40 | 0 |
Table 8: Assessment Of Patient Satisfaction With The Pharmacist Managed Anticoagulation Service.
Patient knowledge is the key to safe and effective use of warfarin and other oral anticoagulants. Patients should be aware of the indications, monitoring requirements, drug–drug and drug–food interactions and the adverse reactions to watch for. There is a positive relationship between their knowledge and the outcomes of therapy. The present study shows that the patient’s knowledge on oral anticoagulation was improved in intervention group patients and they achieved better therapeutic outcome compared with the control group patients. Thus, this study highlights the role of a clinical pharmacist in improving the overall therapeutic outcome of patients on oral anticoagulants. Hence, a clinical pharmacist driven anticoagulation service can empower patients to achieve better therapeutic outcome with added safety. More such studies are required in India to elucidate and clarify the above results. It is therefore, worthwhile to address these improvements in anticoagulation management and to make an attempt to unify the concept of clinical pharmacist driven anticoagulation clinics in India.
References
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:204S-233.
- Fagan SC, Hessjoseph DC. Stroke. In: Dipiro JT, editor. Pharmacotherapy a Pathophysiologic Approach. 7th ed. New York: The McGraw-Hill Companies; 2008. p. 373-85.
- Fitzmaurice DA, Hobbs FD, Murray JA. Monitoring oral anticoagulation in primary care. BMJ 1996;312:1431-2.
- Ansell JE, Hughes R. Evolving models of warfarin management: Anticoagulation clinics, patient self -monitoring, and patient self-management. Am Heart J 1996;132:1095-100.
- Testa S, Alatri A, Paoletti O, Morstabilini G, Medagliani MA, Denti N, et al. Reorganisation of an anticoagulation clinic using a telemedicine system: Description of the model and preliminary results. Intern Emerg Med 2006;1:24-9.
- Lewis SM, Kroner BA. Patient survey of a pharmacist-managed anticoagulation clinic.Manag Care Interface 1997;10:66-70.
- Bond CA, Raehl CL. Pharmacist-provided anticoagulation management in United States hospitals: Death rates, length of stay, Medicare charges, bleeding complications, and transfusions. Pharmacotherapy 2004;24:953-63.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998;105:91-9.
- Crowther MA, Ginsberg JB, Kearon C, Harrison L, Johnson J, Massicotte MP, et al. A randomized trial comparing 5-mg and 10-mg warfarin loading doses. Arch Intern Med 1999;159:46-8.
- Tait RC, Sefcick A. A warfarin induction regimen for out-patient anticoagulation in patients with atrial fibrillation. Br J Haematol 1998;101:450-4.
- Freedman MD. Oral anticoagulants: Pharmacodynamics, clinical indications and adverse effects. J ClinPharmacol 1992;32:196-209.
- Baglin TP, Keeling DM, Watson HG, British Committee for Standards in Haematology. Guidelines on oral anticoagulation (warfarin): Third edition – 2005 update. Br J Haematol 2006;132:277-85.
- Winans AR, Rudd KM, Triller D. Assessing anticoagulation knowledge in patients new to warfarin therapy. Ann Pharmacother 2010;44:1152-7.
- 14.Meier DJ, Sonnad SS, Merz JC, Fay WP. Comparison of narrow versus standard target INR ranges. J Am Coll Cardiol 2002;39Suppl 2:S272.
- Tang EO, Lai CS, Lee KK, Wong RS, Cheng G, Chan TY. Relationship between patients’ warfarin knowledge and anticoagulation control. Ann Pharmacother 2003;37:34-9.
- Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: Anticoagulation control, patient outcomes, and health care costs. Arch Intern Med 1998;158:1641-7.
- Ansell JE. Anticoagulation management as a risk factor for adverse events: Grounds for improvement. J Thromb Thrombolysis 1998;5:13-8