- *Corresponding Author:
- Yan Xue
Department of Obstetrics and Gynecology, First Ward, Yan'an People's Hospital, Yan’an, Shaanxi Province 716000, China
E-mail: 280608215@qq.com
| Date of Received | 19 December 2022 |
| Date of Revision | 14 August 2023 |
| Date of Acceptance | 16 November 2023 |
| Indian J Pharm Sci 2023;85(6):1719-1724 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore beta-sitosterol involved in regulating the mechanism of postmenopausal osteoporosis in the elderly through the phosphoinositide-3-kinase–protein kinase B. 90 Sprague Dawley grade female rats were selected as the research subjects and randomly divided into a control, beta-sitosterol treatment, and the model group. Establishing an elderly postmenopausal osteoporosis model using oophorectomy, beta-sitosterol treatment group was given 20 μmol/l, the model group and control group were given equal volume of 0.9 % physiological saline by gavage of sitosterol drugs. In terms of bone mineral density, the bone mineral density in the beta-sitosterol group was raised than that in the model group. The results of hematoxylin and eosin staining showed that the degree of osteoporosis in the beta-sitosterol group was less than that in the model group. Enzyme-linked immunosorbent assay results showed that the concentrations of serum estradiol, bone alkaline phosphatase, osteocalcin and procollagen N-terminal propeptide in the beta-sitosterol group were raised than those in the model group. Quantitative polymerase chain reaction results showed that the expression of phosphoinositide- 3-kinase–protein kinase B messenger ribonucleic acid in bone tissue of beta-sitosterol treated group was raised than that of model group. Western blot analysis showed that the expression of phosphoinositide-3- kinase–protein kinase B was increased in beta-sitosterol treated group. Further experiments confirmed that the protective effect of beta-sitosterol on senile postmenopausal osteoporosis could be reversed by inhibiting phosphoinositide-3-kinase–protein kinase B. Beta-sitosterol can regulate senile postmenopausal osteoporosis and regulate the expression of bone mineral density and osteogenesis-related factors through phosphoinositide- 3-kinase–protein kinase B signal pathway.
Keywords
Elderly postmenopausal osteoporosis, beta-sitosterol, phosphoinositide-3-kinase, protein kinase B, bone density
Senile Postmenopausal Osteoporosis (PMOP) refers to the decrease of Bone Mineral Density (BMD) and the destruction of bone tissue microstructure in postmenopausal women. Its pathogenesis is mainly related to endocrine, bone metabolism, bone remodeling and bone destruction[1]. After menopause, the level of estrogen in women decreases sharply, and estrogen has a protective effect on bones[2]. Estrogen cannot only inhibit the production of regulatory factors of bone resorption, but also promote the production of regulatory factors of bone formation. Therefore, the lack of estrogen in postmenopausal women leads to the imbalance of bone remodeling and the increase of bone destruction, leading to the occurrence of osteoporosis[3]. Beta (β)-sitosterol is a kind of phytosterol, which is similar to estrogen. Phosphoinositide-3-Kinase–Protein Kinase B (PI3K-Akt) signal pathway is an important cellular signal transduction pathway, which is involved in regulating a variety of biological functions such as cell growth, survival and metabolism[4]. PI3K-Akt signaling pathway is considered to be an important regulatory pathway in elderly PMOP. β-sitosterol can promote the proliferation and differentiation of osteocytes, inhibit the activity of bone resorption cells and promote bone formation[5]. It is not clear whether β-sitosterol participates in the regulation of senile PMOP through PI3K-Akt signal pathway. This study aimed to investigate the mechanism of β-sitosterol regulating senile PMOP through PI3K-Akt.
Materials and Methods
Experimental animals:
Ninety 3 mo-old Specific-Pathogen-Free (SPF) rats weighing 230-257 g were purchased from Changsha Biological Co., Ltd. All experimental mice meet the requirements of animal ethics examination and approval [SCXK (Xiang) 2019- 0014]. All the experimental rats were reared in a single cage with a laboratory temperature of 20°-24° and a relative humidity of 52 %-62 %. The rats were fed adaptively for 7 d.
Grouping and administration:
90 Sprague Dawley (SD) female rats were selected as the subjects, 30 rats were randomly selected as the control group, and the other 60 rats were ovariectomized to establish the model of PMOP, and then randomly divided into model group (n=30) and β-sitosterol group (n=30). The model group and the control group were given 0.9 % normal saline every day, and the β-sitosterol group was treated with 20 μ mol/l β-sitosterol.
Hematoxylin-Eosin (HE) staining:
The bone specimens of femur of experimental animals were collected, and the bone samples were treated with dehydration, degreasing and cleaning. The processed bone specimens were embedded in paraffin, fixed and cut into thin slices with a thickness of 5-10 μm. After heating, the slices were fixed on glass slides and stained with HE dye. Hematoxylin staining was used first, and then eosin was used for staining. In the dyeing process, the slices are first soaked in dye solution for a period of time. After dyeing, the slices are rinsed in a solvent. The sections were covered with a transparent medium and observed and analyzed under a microscope. Observe the section with a microscope and photograph the tissue structure with appropriate magnification by observing the density and structure of bone trabeculae, as well as the number and arrangement of osteocytes.
BMD measurement:
BMD of rats was measured by Dual energy X-ray Absorptiometry (DXA). The rats were placed in an appropriate position to measure the BMD. The BMD of rats in each group was measured and the results were recorded.
Enzyme-Linked Immunosorbent Assay (ELISA):
The serum samples of experimental rats were collected by centrifuging venous blood, transferred to the centrifuge tube marked with sample number, and stored in cold storage. Prepare the standard, which is a sample of known concentrations of Estradiol (E2), Bone Alkaline Phosphatase (BALP), Osteocalcin (OC) and Procollagen N-Terminal Propeptide (PINP), used to create a standard curve. Take a refrigerated serum sample. Add an appropriate amount of reagent to each sample to dilute the sample, mix well, and follow the instructions of the ELISA kit. The standard and sample were added to the corresponding pore of the ELISA plate, and the target molecules (E2, BALP, OC and PINP) were captured on the plate with specific antibodies. The secondary antibody labeled with enzyme is added and binds to the captured molecule. Wash the plate again and add a substrate to allow the enzyme-catalyzed reaction to occur to produce a measurable signal. The absorbance of each hole was measured by enzyme labeling instrument, and the absorbance was proportional to the molecular concentration. The standard curve is used to determine the concentration of the target molecule in the sample. According to the experimental conditions and the concentration of the standard, the concentrations of E2, BALP, OC and PINP in the sample were calculated.
Detection of PI3K-Akt messenger Ribonucleic Acid (mRNA) expression in bone tissue by quantitative Polymerase Chain Reaction (qPCR):
Bone tissue samples were collected from the right femur of experimental rats to avoid RNA degradation and bone tissue samples were completely broken up with appropriate tissue decomposition reagents. The total RNA was extracted from the sample using RNA extraction reagent. The concentration and purity of extracted RNA were determined by spectrophotometer, and the A260/A280 ratio of RNA (1.8 to 2.0). The extracted RNA was transcribed into complementary Deoxyribonucleic Acid (cDNA) by reverse transcriptase. Reverse transcription reactions usually include RNA templates, random primers or special primers, reverse transcriptase and reverse transcription buffers. Prepare qPCR reaction mixture, including cDNA template, primers (specific PI3K-Akt primers and reference gene primers, Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)), nuclease water, primers and SYBR Green, etc. PCR reaction was carried out with qPCR instrument, including cyclic denaturation, annealing and extension steps. The expression level of reference gene was measured, and the PCR results were analyzed by qPCR software or other data analysis tools. The expression level of target gene was calculated by relative expression method (2–ΔΔCt method). The primers seen are shown in Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| PI3K | CACCTTAAATGGTGAGCACGG | ACACCCCAGCCAATCAAGTC |
| AKT | CCGCCTGATCAAGTTCTCCT | TTCAGATGATCCATGCGGGG |
Table 1: qPCR Primer Sequence
Western blot:
The bone tissue samples of experimental rats were put into protein extraction buffer to extract protein, and medical instruments (ultrasonic crusher or liquid nitrogen) were used to break the tissue and release protein. Use a centrifuge to separate cell fragments and proteins, and collect the supernatant. The concentration of extracted protein was determined by protein quantitative Bradford. The extracted protein was loaded into Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel according to concentration, and the protein was separated by electrophoresis. The separated proteins were transferred to PVDF or membranes using semi-wet or completely wet methods. Bovine Serum Albumin (BSA) was used to block non-specific binding sites and reduce false positive signals. The first antibody against proteins related to PI3K-Akt signal pathway was added to the membrane and incubated overnight at 4°. When the second antibody combined with the first antibody is added, the second antibody will bind to the first antibody and form a complex. Use chemiluminescence, fluorescence staining, or other visualization methods to detect proteins. Use an imaging device (X-ray film or fluorescence imaging system) to capture images of protein bands.
Statistical processing:
Statistical Package for the Social Sciences (SPSS) 20.0 software was used for statistical analysis, the measurement data were expressed by x±s, and the differences among groups were compared by analysis of variance. Least Significant Difference (LSD)-t test was used for pairwise comparison. The difference was statistically significant p<0.05. Compared with the control group, ap<0.05; compared with the model group, bp<0.05.
Results and Discussion
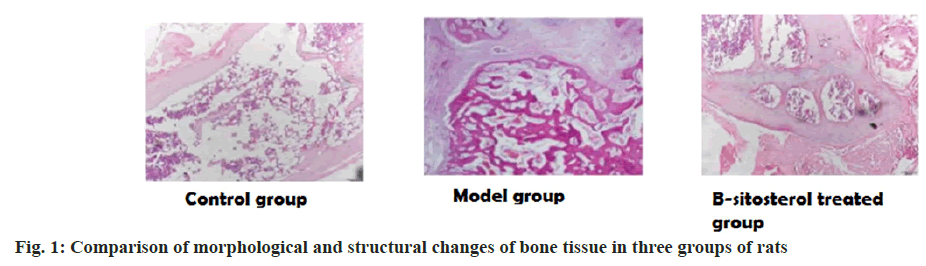
In the control group, the index of osteoporosis increased significantly, the trabecular density decreased and the bone structure was loose in the elderly postmenopausal women. In the model group, the index of osteoporosis increased after ovariectomy, and the decrease of bone trabecula and osteoporosis appeared. The osteoporosis index of β-sitosterol group showed an improvement trend, the trabecular density increased and the bone structure became tighter as shown in fig. 1.
While BMD decreased in the model group compared to the control group, it increased in the sitosterol group, but it was still lower reduced than in the control group as shown in Table 2.
| Group | n | BMD |
|---|---|---|
| Control | 30 | 0.28±0.05 |
| Model | 30 | 0.15±0.03a |
| β-sitosterol | 30 | 0.25±0.04ab |
| F | 83.4 | |
| p | <0.001 |
Note: Compared with the control group, ap<0.05 and compared with the model group, bp<0.05
Table 2: Comparison of BMD Among the Three Group (x͞±s, g/cm2)
While BMD decreased in the model group compared to the control group, it increased in the sitosterol group, but it was still lower than in the control group, while these in the β-sitosterol treated group were raised than those in the control group, but lower than those in the control group as shown in Table 3.
| Group | n | E2 (ng/l) | BALP (ng/l) | OC (μg/l) | PINP (U/l) |
|---|---|---|---|---|---|
| Control | 30 | 46.11±3.84 | 114.57±13.26 | 1655±128 | 24.19±1.88 |
| Model | 30 | 15.45±1.52a | 65.38±7.52a | 975±84a | 13.23±1.34a |
| β-sitosterol | 30 | 27.63±3.34ab | 102.44±10.34ab | 1143±107ab | 18.87±1.76ab |
| F | 760.27 | 174.2 | 323.64 | 320.79 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Compared with the control group, ap<0.05 and compared with the model group, bp<0.05
Table 3: Comparison of Three Groups of Osteogenic Factors (x͞ ±s)
While the levels of PI3K and AKT mRNA decreased in the model group compared to the control group, these increased in the β-sitosterol group, but these were still lower than in the control group as shown in Table 4.
| Group | n | PI3K mRNA | AKT mRNA |
|---|---|---|---|
| Control | 30 | 1.00±0.03 | 1.14±0.04 |
| Model | 30 | 0.23±0.01a | 0.85±0.08a |
| β-sitosterol | 30 | 0.32±0.04ab | 1.01±0.07ab |
| F | 6135 | 147.21 | |
| p | <0.001 | <0.001 |
Note: Compared with the control group, ap<0.05 and compared with the model group, bp<0.05
Table 4: PI3K and AKT Protein Expression in Bone Tissue
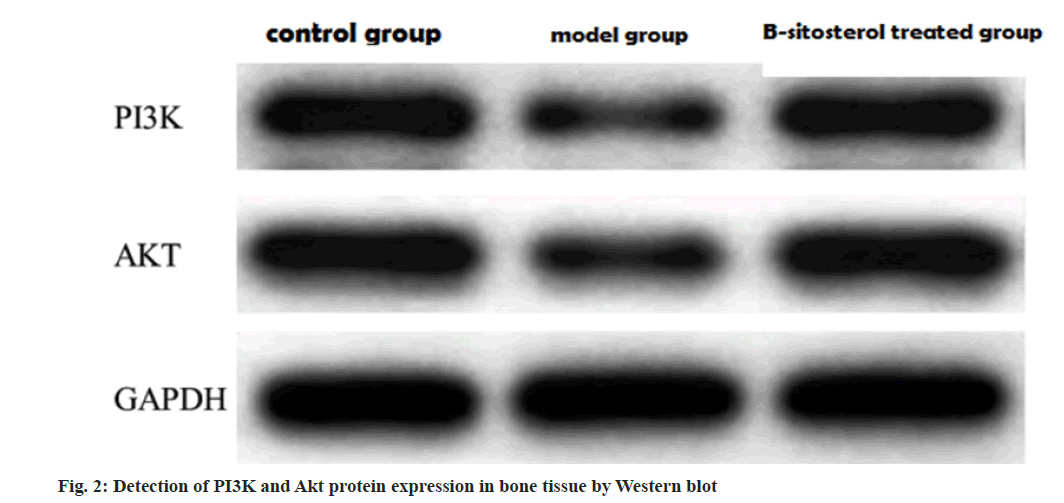
Compared with the control group, the protein expression levels of PI3K and Akt in the model group were decreased, and the protein expression levels of PI3K and Akt in the β-sitosterol group were higher than those in the model group as shown in Table 5 and fig. 2.
| Group | n | PI3K | AKT |
|---|---|---|---|
| Control | 30 | 1.14±0.08 | 0.89±0.11 |
| Model | 30 | 0.51±0.05a | 0.42±0.06a |
| β-sitosterol | 30 | 0.87±0.06ab | 0.75±0.08ab |
| F | 719.28 | 237.15 | |
| p | <0.001 | <0.001 |
Note: Compared with the control group, ap<0.05 and compared with the model group, bp<0.05
Table 5: Comparison of PI3K-AKT Signal Pathway Activity among Three Groups of Rats
Senile PMOP is a common bone metabolic disease caused by decreased estrogen levels[6]. Anti-absorptive drugs (such as bisphosphate drugs), drugs that promote bone formation (such as bone resorption inhibitors), calcium and vitamin D supplements and hormone replacement therapy, etc.,[7] as a common drug to reduce osteoporosis, but there are many deficiencies. Pathological studies show that[8], there is a reduction in bone mass and destruction of bone microstructure, resulting in decreased bone strength and easy fractures in senile PMOP. In the development of osteoporosis, the decrease of estrogen leads to the imbalance of bone remodeling and resorption, the increase of bone resorption and the decrease of bone formation. In addition, the imbalance of cytokines, hormones and factors will also play critical role in osteoporosis. The main complications of PMOP in the elderly are fractures, especially spinal fractures, hip fractures and wrist fractures. The elderly can suffer serious complications from these fractures, including long-term bed rest, pneumonia, and thrombus[9]. β-sitosterol is a kind of phytosterol, which has been shown to have potential bone protective effect. It is thought to be involved in the regulation of senile PMOP, but it is not clear until now.
In this study, the model of senile PMOP was established by ovariectomy. It was observed that after treatment with β-sitosterol, the BMD was increased, the related indexes of osteogenic factors were significantly improved, and the degree of osteoporosis was reduced. Further experiments confirmed that compared with the model group, the expression of PI3K and Akt protein in β-sitosterol group increased[10]. This study proved that β-sitosterol can regulate and control senile PMOP by activating PI3K-Akt signal pathway. The results showed that β-sitosterol had a certain protective effect. As a plant sterol, β-sitosterol can mimic the effect of estrogen and thus has a protective effect on bones[11,12]. PI3K-Akt signal pathway is an important cellular signal transduction pathway, which is involved in regulating a variety of biological functions such as cell growth, survival and metabolism. PI3K-Akt signaling pathway is considered to be an important regulatory pathway in elderly PMOP[13]. β-sitosterol can promote the proliferation and differentiation of osteocytes, inhibit the activity of bone resorption cells and promote bone formation by activating PI3K-Akt signal pathway. β-sitosterol binds to the receptor on the cell surface and activates PI3K. Activated PI3K further activates Akt protein kinase, which regulates multiple downstream signal pathways. Activated Akt can inhibit the synthesis and release of bone resorption factor and reduce the damage of bone resorption cells to bone tissue. Activated Akt can also promote the synthesis and release of bone morphogenetic factors and increase the activity of bone morphogenetic cells. PI3K-Akt signaling pathway promotes the proliferation and differentiation of osteocytes, thus increasing bone formation. PI3K-Akt pathway plays an important role in regulating cell proliferation and survival[14].
It can be seen that β-sitosterol can inhibit the activity of bone resorption cells and promote bone formation by activating PI3K-Akt signal pathway. To sum up, β-sitosterol can inhibit PMOP and regulate the expression of BMD and osteogenesis- related factors through PI3K-Akt signal pathway.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wanby P, Nobin R, Von SP, Brudin L, Carlsson M. Serum levels of the bone turnover markers dickkopf-1, sclerostin, osteoprotegerin, osteopontin, osteocalcin and 25-hydroxyvitamin D in Swedish geriatric patients aged 75 years or older with a fresh hip fracture and in healthy controls. J Endocrinol Invest 2016;39(8):855-63.
[Crossref] [Google Scholar] [PubMed]
- Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014;59(4):1406-14.
[Crossref] [Google Scholar] [PubMed]
- Chen CH, Elsalmawy AH, Ish-Shalom S, Lim SJ, AlAli NS, Cunha-Borges JL, et al. The effect of teriparatide treatment on the risk of fragility fractures in postmenopausal women with osteoporosis: Results from the Asian and Latin America Fracture Observational Study (ALAFOS). Calcif Tissue Int 2022;110(1):74-86.
[Crossref] [Google Scholar] [PubMed]
- Shi X, Wang J, Lei Y, Cong C, Tan D, Zhou X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer. Mol Med Rep 2019;19(6):4529-35.
[Crossref] [Google Scholar] [PubMed]
- Moon DO, Kim MO, Choi YH, Kim GY. β-Sitosterol induces G2/M arrest, endoreduplication, and apoptosis through the Bcl-2 and PI3K/Akt signaling pathways. Cancer Lett 2008;264(2):181-91.
[Crossref] [Google Scholar] [PubMed]
- Hereth JE, Durand B. Incorporating transgender-affirmative practice models into substance use treatment and prevention. J Soc Work Pract Addict 2023;23(2):152-60.
- Xiong Z, Zheng C, Chang Y, Liu K, Shu L, Zhang C. Exploring the pharmacological mechanism of Duhuo Jisheng decoction in treating osteoporosis based on network pharmacology. Evid Based Complement Altern Med 2021;2021:5510290.
[Crossref] [Google Scholar] [PubMed]
- Lee JS, Shin JK, An SJ, Suh KT, Kang SS. Correlation between clinical outcomes and spinopelvic parameters in osteoporosis. Acta Orthop Belg 2014;80(4):522-8.
[Google Scholar] [PubMed]
- Huang J, Ge HA, Zhu X, Xue C, Su Q, Chen X, et al. Risk factors analysis and nomogram construction for postoperative pulmonary infection in elderly patients with hip fractures. Aging Clin Exp Res 2023;35(9):1891-9.
[Crossref] [Google Scholar] [PubMed]
- Moon DO, Kim MO, Kang SH, Lee KJ, Heo MS, Choi KS, et al. Induction of G2/M arrest, endoreduplication, and apoptosis by actin depolymerization agent pextenotoxin-2 in human leukemia cells, involving activation of ERK and JNK. Biochem Pharmacol 2008;76(3):312-21.
[Crossref] [Google Scholar] [PubMed]
- Wei QS, Huang L, Tan X, Chen ZQ, Chen SM, Deng WM. Serum osteopontin levels in relation to bone mineral density and bone turnover markers in postmenopausal women. Scand J Clin Lab Invest 2016;76(1):33-9.
[Crossref] [Google Scholar] [PubMed]
- Yang ZQ, Wei MF, Chen L, Xi JN. Research progress in the application of motor-cognitive dual task training in rehabilitation of walking function in stroke patients. J Neurorestoratol 2022;11(1):100028.
- Wu X, Li S, Xue P, Li Y. Liraglutide inhibits the apoptosis of MC3T3-E1 cells induced by serum deprivation through cAMP/PKA/β-catenin and PI3K/AKT/GSK3β signaling pathways. Mol Cells 2018;41(3):234-43.
[Crossref] [Google Scholar] [PubMed]
- Tian-Jiao Li, Yong-Rui B, Shuai W, Xin-Xin Y, Xian-Sheng M. Study on proliferation and mechanism of hepatocarcinoma cells induced by ophicalcitum based on PTEN/PI3K/AKT signaling pathway. Lishizhen Med Mater Med Res 2017;45:2-9.