- *Corresponding Author:
- T. K. Giri
Rungta College of Pharmaceutical Sciences and Research, Kohka Road, Kurud, Bhilai-491 024, Malaysia
E-mail: tapan_ju01@rediffmail.com
| Date of Submission | 21 October 2012 |
| Date of Revision | 01 September 2013 |
| Date of Acceptance | 10 September 2013 |
| Indian J Pharm Sci 2013;75(6):619-627 |
Abstract
Interpenetrating polymer network hydrogel beads of pectin and sodium carboxymethyl xanthan were prepared by ionotropic gelation with Al(+3) ions and covalent cross-linking with glutaraldehyde for sustained delivery of diltiazem hydrochloride. Fourier transform infrared spectroscopy, X-ray diffraction, differential scanning colorimetry and scanning electron microscopy were used to characterise the hydrogel beads. The swelling of the hydrogel and the release of drug were relatively low in pH 1.2 buffer solutions. However, higher swelling and drug release were observed in pH 6.8 buffer solutions. The carboxyl functional groups of hydrogels undergo ionisation and the osmotic pressure inside the beads increases resulting in higher swelling and drug release in higher pH. The release of drug depends on concentration of polymer, amount and exposure time of cross-linker and drug content in the hydrogel matrices. The present study indicated that the hydrogel beads minimised the drug release in pH 1.2 buffer solutions and to prolong the drug release in pH 6.8 buffer solutions.
Keywords
Entrapment efficiency, swelling ratio, release kinetics, biodegradation, modification
Over the past decade the use of biodegradable polymers for development of sustained drug delivery systems has increased dramatically [1-3]. Synthetic polymers offer greater advantages than natural ones, wherein they can be tailored to develop a wide range of properties. However, there is a trend to restrict the use of synthetic polymers in pharmaceutical formulations since processing techniques of these polymers are based on organic solvents. Toxicity in long term dosing due to the residual organic solvents and the environmental pollution associated with the use of large volume of organic solvents have raised question for long term use of synthetic polymer and its viability [4]. Therefore, a safer carrier has been demanded. Natural polymers may generally be considered safer than synthetic polymers. Recently, extensive research efforts have been concentrated on natural polymers in sustained drug delivery systems as they are derived from natural sources and do not require organic solvents for processing [5]. Among the various natural polymers, pectin appeared to be highly promising due to its nontoxic and biocompatible nature. It consists of D-galacturonic acid residues joined together by α-(1→4) linkages. It is widely used as a gelling and stabilising agent in food, pharmaceuticals and cosmetics [6]. However, high swelling in physiological environments is a major limitation of pectin gel for use in sustained drug delivery [7]. Drugs with high solubility in physiological conditions may exhibit premature release due to the expanded pore size of pectin gels. To overcome this problem, divalent cations such as Ca++ have been used to cross-link the polymer. The carboxylic acid groups present in the pectin can react with calcium ions to form an insoluble compound with “egg box” structure [8]. This unique property of calcium pectinate gel prepared by ionotropic gelation of pectin with Ca++ ions has been utilised to encapsulate therapeutic agents like indomethacin [9], theophylline [10], sulphamethoxazole [11], methotrexate [12], metronidazole [13] and atenolol [14]. However, the major drawback of calcium pectinate gel beads is rapid release of the encapsulated drug in the simulated gastric fluid [8]. Therefore, improved pectin-based drug delivery systems are still desired.
Xanthan gum (XG) is a carbohydrate polymer produced by bacteria Xanthomonas campestris and is extensively used in food, pharmaceutical and cosmetic [15]. Its backbone consists of β-glucose rings and side chains consist of substituted α-mannose, β-glucose and β-mannose rings. It contains up to two carboxyl groups per repeat unit [16] and cannot form gel beads in the presence of multivalent cations. However, XG is modified to sodium carboxymethyl xanthan (SCMX), which is able to form gel beads through ionotropic gelation with Al3+ ions [17]. SCMX beads prepared by ionotropic gelation with Al3+ ions have been found capable of encapsulating ibuprofen [18], diltiazem hydrochloride (DTZ) [19], bovine serum albumin [20] for sustained delivery of drug. However, in physiological environment Al3+ ion cross-linked SCMX beads showed poor mechanical strength and the drug was released rapidly in acidic environment of the stomach.
DTZ is widely used for the treatment of angina pectoris, arrhythmias and hypertension [21]. Its short biological half-life and frequency of administration (usually three to four times a day) make it a potential candidate for sustained release preparation [22]. The objective of the present work was to prepare interpenetrating polymer network (IPN) hydrogel beads using SCMX and pectin for minimising the release of drug in acidic environment of the stomach and prolonged the release of drug in intestinal environment. The development of IPN hydrogel beads of SCMX and pectin is beneficial because it contains two cross-linked polymers in a network form to give a three dimensional network structure, which produces more free volume for the easy entrapment of drugs and improves mechanical strength. As the hydrogel beads carry –COOH functional groups, they remain unionised at gastric pH leading to low swelling and drug release, but they undergo ionisation at higher pH leading to maximum swelling and drug release in the intestine.
Materials and Methods
DTZ was obtained as a gift sample from ZIM Laboratories, Nagpur, India. Pectin, XG, aluminium chloride hexahydrate (AlCl3, 6H2O) and monochloroacetic acid were obtained from Loba Chemie, Mumbai, India. The glutaraldehyde (GA) (25% v/v) was purchased from S. D. Fine-Chem. Ltd., Mumbai, India. All other chemicals were used as received.
Preparation of SCMX
The SCMX was synthesised from XG having degree of substitution of 1.26 following a reported method [17]. Briefly, 2 g of XG was dispersed in ice cold solution of 45% w/v sodium hydroxide. The dispersion was kept at 5-8° with continuous stirring for 1 h. Monochloroacetic acid solution (75% w/v) was added with stirring in the reaction mixture and the temperature was raised slowly to 15-18°. After 30 min, the temperature was raised to 75° and maintained for another 30 min. The reaction mixture was then cooled to room temperature. The precipitate obtained was cut into small pieces and dried at 50°. The dried product was milled, washed with 80% v/v methanol and again dried to constant weight.
Preparation of DTZ-loaded IPN beads
An accurately weighed quantity of DTZ corresponding to 20-40% of dry mass of the polymer was dispersed in an aqueous solution of pectin and SCMX (8% w/v, 1:1 ratio). The dispersion was added drop-wise through 18-gauze flat-tipped needle into 100 ml of AlCl3 solutions of different concentration. The beads formed were removed after the gelation period of 30 min and washed with distilled water repeatedly to remove unreacted reagents and dried at room temperature for 24 h and then at 40° till constant weight.
In another experiment the wet beads prepared by the method described above were transferred to 20 ml solution of acetone and water (3:1) containing varying concentration of GA solution for different periods of time to introduce covalent cross-link. The beads were removed and washed repeatedly with distilled water. The complete removal of GA was confirmed by the negative test of washing with Brady’s qualitative reagent (2,4-dinitrophenyl hydrazine). The same procedure was followed for preparation of blank beads (without drug). The composition of beads was given in Table 1.
| Codes | Drug (% of dry polymer) |
Concentration of AlCl3 (% w/v) |
Concentration of GA (% v/v) |
Mean bead size (µm) ± SD |
DEE (%) ± SD |
|---|---|---|---|---|---|
| F1 | 20 | 1 | - | 982.08 ± 8.66 | 45.27 ± 1.22 |
| F2 | 20 | 2 | - | 936.67 ± 7.33 | 39.08 ± 3.12 |
| F3 | 20 | 3 | - | 853.33 ± 4.38 | 37.25 ± 4.46 |
| F4 | 20 | 4 | - | 814.58 ± 2.68 | 36.23 ± 1.32 |
| F5 | 20 | 6 | - | 808.33 ± 3.68 | 27.08 ± 1.68 |
| F6 | 20 | 8 | - | 795.00 ± 5.31 | 21.41 ± 2.34 |
| F7 | 30 | 1 | - | 996.50 ± 4.45 | 46.15 ± 0.16 |
| F8 | 40 | 1 | - | 1012.67 ± 7.33 | 47.62 ± 0.18 |
| F9 | 40 | 1 | 0.5 | 845.83 ± 6.42 | 44.46 ± 3.76 |
| F10 | 40 | 1 | 1 | 737.50 ± 7.16 | 41.31 ± 2.54 |
| F11 | 40 | 1 | 2 | 686.67 ± 5.78 | 36.91 ± 1.86 |
| F12 | 40 | 1 | 3 | 669.17 ± 3.06 | 34.17 ± 2.24 |
SD=Standard deviation, AlCl3=aluminium chloride, GA=glutaraldehyde, DEE= drug entrapment efficiency
Table 1: Composition, Mean Bead Size And Drug Entrapment Efficiency Of Different Formulations Of Ipn Beads
Bead size analysis
Bead size analysis was carried out by sieving method. Several British standard sieves ranging from 10 to 80 meshes were arranged in a nest with the coarsest at the top. A weighed amount of samples were placed on the top and the sieve set was shaken with a mechanical sieve shaker (Labtech Instrument, India) for 10 min. The samples retained on each sieve were collected and weighed. The arithmetic mean diameter was calculated following the method reported earlier [23].
Estimation of drug entrapment efficiency
Nearly 20 mg of dried beads were crushed with a mortar-pestle and were transferred into 250 ml of phosphate buffer solution (pH 6.8). After 2 h, the suspension was filtered and the samples were analysed with a spectrophotometer (UV1800, Shimadzu, Japan) at 236 nm. The drug entrapment efficiency (DEE) (%) was calculated using the equation: Drug entrapment efficiency (%)=(actual drug content/theoretical drug content)×100.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) spectra of pure drug, polymers and beads containing drug and without drug were measured in a FTIR spectrophotometer (Varian 640-IR, USA). The spectra were recorded within 4000-400 cm-1 wave numbers.
Differential scanning colorimetry study
Differential scanning colorimetry (DSC) thermograms of pure drug, drug-loaded beads and drug free beads were obtained in an atmosphere of nitrogen in the following way: 2-5 mg of samples were kept in hermetically sealed aluminium pan and heated at a scan speed of 20 °/min over a temperature range of 30-300° in a differential scanning calorimeter (DSC-8500 with Hyper DSC, Perkin Elmer, USA).
X-ray diffraction study
X-ray diffraction (XRD) analyses were performed using an X-ray diffractometer (Regaku ultima IV, Japan). Pure drug, drug free bead and drug-loaded bead were scanned from 0° to 60° (2θ) under the measurement conditions of voltage, 40 kV, current 20 mA, scan speed 20 °/min.
Scanning electron microscopy
The beads were mounted onto stubs using double sided adhesive tape and sputter coated with a palladium layer in a JFC-1600 autofine coater, in order to make them conductive. The coated beads were observed under scanning electron microscopy (SEM, JSM-6701F, Jeol, Japan) at room temperature. Pictures were taken at an excitation voltage of 20 kV.
Swelling study
The swelling of the beads was studied in 25 ml of pH 1.2 buffer solutions and pH 6.8 USP phosphate buffer solutions. The beads were removed at different time intervals by filtration and blotted carefully to remove excess surface water. The swollen beads were weighed. The swelling ratios were calculated using equation: Swelling ratio=(final weight-initial weight)/ initial weight.
In vitro biodegradation study
The in vitro biodegradation of the beads was carried out in presence of 0.2% lysozyme in pH 7.4 phosphate buffer solutions [24]. Predetermined amounts of beads were transferred to test tubes and incubated in 5 ml of lysozyme solution with shaking (120 rpm) in an incubator (CIS-24BL, Remi, Mumbai, India) at 37° for 3 h for equilibrium swelling. The weights of the swollen beads were measured after removing the solvent. Then 5 ml of fresh lysozyme solution was added to the swollen beads. The percent weight remaining of the samples due to enzymatic degradation were calculated as follows:
Percent weight remaining of beads=100- [(weight of sample after 3 h exposure in lysozyme solution-weight of the sample after incubating with fresh lysozyme for a given time)/weight of sample after 3 h exposure in lysozyme solution].
In vitro drug release study
In vitro drug release study was carried out in pH 1.2 and pH 6.8 phosphate buffer using USP-II dissolution rate test apparatus (TDT-08L, Electro lab, Mumbai, India). Nearly 20 mg of dried beads was placed in 500 ml buffer solutions and maintained at 37±0.5°. The paddle was rotated at 50 rpm. Aliquots were withdrawn at different time intervals. The volume of dissolution medium was replenished immediately with the same volume of fresh solution. After suitable dilution the samples were analysed spectrophotometrically at 236 nm. Each sample was tested and analysed in triplicate.
Statistical analysis
The statistical analyses were carried out using Graph Pad Instant package. The one way analysis of variance (ANOVA) was used to determine the statistically significant differences between the results. Differences were considered significant when P<0.05.
Results and Discussion
The beads were formed instantly when a dispersion of drug and SCMX was extruded through the needle into a solution containing Al3+ ions. However, the beads exhibited very poor mechanical strength. The IPNs were prepared with SCMX and pectin by ionotropic gelation method using AlCl3 as a common crosslinking agent for both the polymers to improve the mechanical strength. As soon as the Al3+ was brought in contact with pectin and SCMX solution, they formed ionic cross-links between two polymers and also with different parts of the same polymer chain. The prepared IPN beads were spherical in shape and were in size range of 669.17-1012.67 μm (Table 1). Size of the beads was decreased with increasing the concentration of AlCl3 (Table 1). This may be due to rapid shrinking of hydrogel beads at higher concentration of AlCl3. Similar results were reported by other workers [25]. Increase in size of the beads was observed with an increase in drug-loading (Table 1).
As the amount of drug increases, the interfacial viscosity of the polymeric dispersion increases which inhibits the formation of smaller droplets during extrusion through needle. Hence, bigger droplets are formed resulting increased size of beads. Soppimath and Aminabhavi had the similar experience [26]. When these ionically cross-linked beads were treated with GA, an acetal structures was formed between the –CHO group of GA and –OH groups of two polymer strands. Further with an increase of concentration of GA, decrease in size of the beads was observed (Table 1). This may be due to rapid shrinking of hydrogel, as reported by other authors [27].
The DEE of the beads decreased from 45.27 to 21.41% with the increase in concentration of AlCl3 from 1 to 8% w/v (Table 1). Such difference in DEE was statistically significant (P<0.05). Increase in the concentration of AlCl3 increases the influx of Al3+ ions that moves towards the gelled boundaries inwardly and formed a thicker gelled structure and accordingly displaces more drugs with consequent decrease in DEE. Reddy and Tammishetti [28] reported that increase in AlCl3 concentration decreased the bovine serum albumin entrapment efficiency of carboxymethyl guar gum beads. DEE was found to vary within 45.27- 47.62% with changing the drug-load in hydrogel beads (Table 1). The effect of GA on the DEE of beads was also studied. It was observed that by increasing amount of GA in the matrix, DEE decreased significantly (P<0.05, Table 1). This could be because increase of rigidity depends on cross-link density. A similar finding was reported by Kulkarni and his coworkers [29].
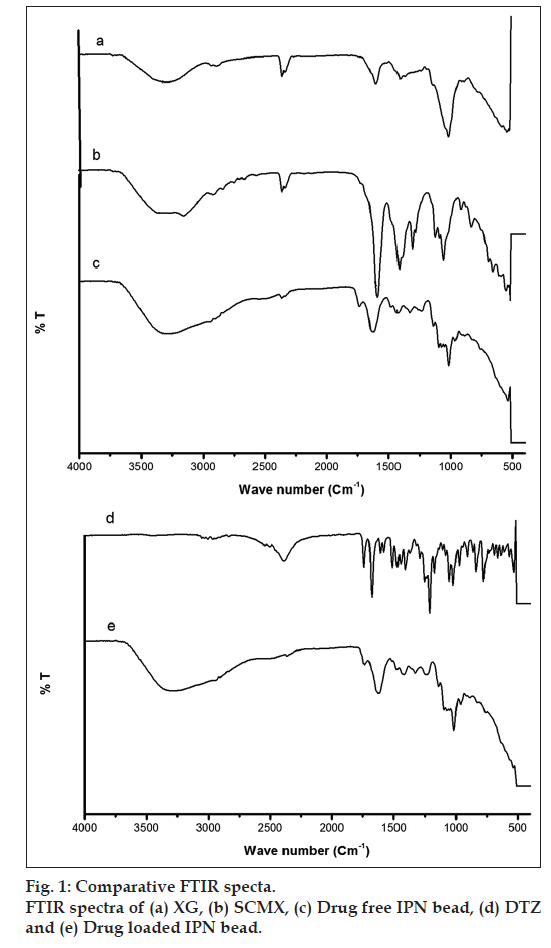
FTIR spectra of XG, SCMX, drug free bead, DTZ and drug-loaded beads are presented in fig. 1. The spectrum of XG (fig. 1a) shows the presence of peaks of two important functional groups, one at 3305.42cm-1 for -OH stretching vibration and the others at 1604.49 and 1403.93cm-1 for asymmetric and symmetric carboxylate anions. The spectrum of SCMX (fig. 1b) exhibits a peak at 3320.31cm-1 corresponding to stretching vibration of hydroxyl group, the peaks at 1592.99 and 1415.50cm-1 are due to asymmetric and symmetric carboxylate anions. Peak appeared at 1311.36cm-1 is due to -CO stretching of the O-carboxymethyl group. This indicates that acetyl group of XG was hydrolysed by sodium hydroxide and subsequently converted into carboxymethyl group. In case of drug free IPN beads, the carboxylate peaks (1592.92 and 1415.50cm-1) shifted to a higher absorption frequency 1627.64 and 1427.07cm-1, and this indicates ionic interaction between COO- and Al3+ ions (fig. 1c). The spectrum of DTZ (fig. 1d) exhibited the characteristic peaks of carboxyl lactum, carboxyl acetate and amine group, respectively, at 1677.77, 1741.48 and 2388.37cm-1. The peaks of the drug were also present almost at the same wave numbers in the spectrum (fig. 1e) of drug-loaded beads. This confirms the chemical stability of DTZ in the hydrogel beads.
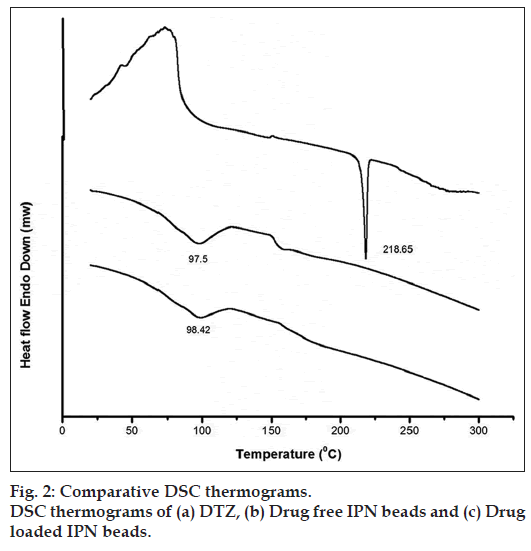
DSC thermograms of DTZ, drug free bead and drug-loaded beads are shown in fig. 2. A sharp endothermic peak corresponding to the melting point of DTZ is found at 218.65° (fig. 2a). The DSC thermogram of drug free bead (fig. 2b) exhibits a broad endothermic peak at 97.5°. However, drug-loaded bead (fig. 2c) does not show sharp endothermic peak; instead a broad endothermic peak at 98.42°. The disappearance of the melting endothermic peak of the drug indicates that the drug might have been molecularly dispersed or converted into amorphous form during the preparation of IPN bead. [29,30].
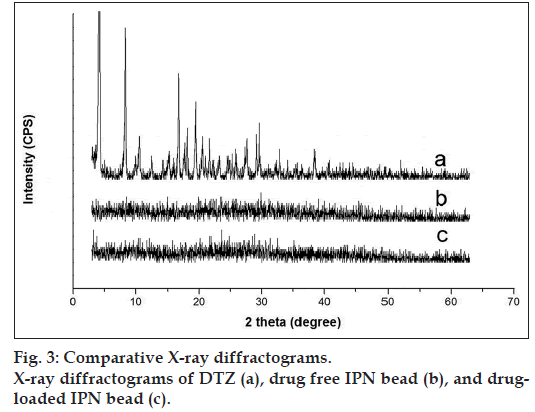
X-ray diffractograms of DTZ, drug free bead and drug-loaded bead are presented in fig. 3. DTZ has shown characteristic intense peaks between 0° and 60° (2θ) due to its crystalline nature. No intense peaks were observed between 0° and 60° (2θ) in both drug free and drug-loaded hydrogel beads. The diffractograms of both drug free and drug-loaded beads are almost identical, indicating the amorphous dispersion of the drug after entrapment into the hydrogel beads.
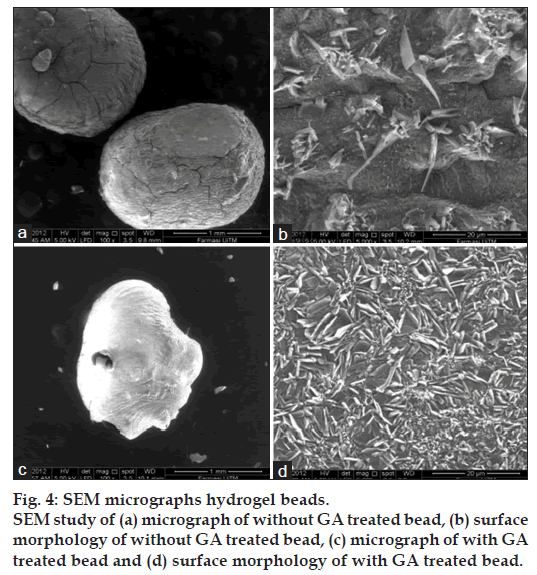
Scanning electron microscopic studies revealed that the beads were more or less spherical having rough surface (fig. 4). GA was not present in the surface of the beads since complete removal of the GA was confirmed by the negative test of washing with Brady’s qualitative reagent. There may be some drug particles deposited on the surface of the beads.
To explain the drug release behaviours the swelling study of beads was conducted in pH 6.8 phosphate buffer and pH 1.2 buffer solutions for 1.5 h (Table 2). Change of pH of the medium from 1.2 to 6.8 resulted in considerable increase in swelling of all the beads. In acidic solution the carboxyl functional group of hydrogel remains protonated and exerts insignificant electrostatic repulsive force consequently the beads swell to very low extent. However, at higher pH values the carboxyl groups of hydrogel can undergo ionisation and the osmotic pressure inside the beads increases. It was observed that increase in AlCl3 concentration, decreased the swelling of beads in both the media. Gradual increase of AlCl3 concentration to 8% reduced the swelling ratio by 65.29 and 77.46% in acidic and alkaline medium, respectively. The increase in concentration of Al3+ ions in the gelation medium increases the availability of Al3+ ions, which increase the number of interactions with COO- groups present in SCMX and pectin. This results in increase in cross-linking density, which hinders inward diffusion of swelling medium. Also by increasing the drug-loading in the beads, a decrease in swelling of the beads was observed. The increase in drug-loading from 20 to 40% reduced the swelling ratio by 67.52 and 77.78% in acidic and alkaline medium, respectively. Low drug-load in beads forms larger pore fraction resulting in higher swelling. In contrast, at higher drug-load, smaller pore fraction was affected. It was further observed that swelling of the beads decreased with an increasing amount of GA. Increase in GA concentration to 3% reduced the swelling ratio by 43.40 and 35.67% in acidic and alkaline medium, respectively. At low cross-link density, the hydrogel network is loose with a greater hydrodynamic free volume and can absorb more of the solvent resulting in higher swelling.
| Codes | Swelling ratio in 1.5 h mean ± SD (medium) | Drug release in acidic medium mean ± SD | Drug release in alkaline medium mean ± SD | Korsemeyer–Peppas model (solution) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acidic | Alkaline | % drug release in 2 h | % drug release in 2 h | t50% (h) | Acidic | Alkaline | ||||
| n | r2 | n | r2 | |||||||
| F1 | 6.28 ± 0.06 | 9.36 ± 0.28 | 20.44 ± 0.45 | 32.76 ± 0.30 | 5.38 ± 08 | 0.363 | 0.915 | 0.439 | 0.937 | |
| F2 | 5.45 ± 0.13 | 5.49 ± 0.08 | 18.67 ± 0.32 | 30.09 ± 0.22 | 5.59 ± 04 | 0.306 | 0.890 | 0.506 | 0.975 | |
| F3 | 2.75 ± 0.05 | 5.35 ± 0.16 | 16.26 ± 0.55 | 28.23 ± 0.32 | 5.83 ± 03 | 0.145 | 0.910 | 0.449 | 0.907 | |
| F4 | 2.41 ± 0.02 | 4.88 ± 0.11 | 14.14 ± 0.50 | 26.37 ± 0.45 | 6.42 ± 07 | 0.241 | 0.830 | 0.465 | 0.771 | |
| F5 | 2.20 ± 0.04 | 2.48 ± 0.08 | 13.10 ± 0.56 | 24.11 ± 0.61 | 6.91 ± 06 | 0.223 | 0.939 | 0.398 | 0.778 | |
| F6 | 2.18 ± 0.02 | 2.11 ± 0.06 | 11.39 ± 0.31 | 22.09 ± 0.55 | 7.35 ± 01 | 0.202 | 0.781 | 0.398 | 0.778 | |
| F7 | 4.68 ± 0.01 | 5.30 ± 0.09 | 19.70 ± 0.38 | 30.11 ± 0.58 | 6.27 ± 03 | 0.188 | 0.878 | 0.414 | 0.921 | |
| F8 | 2.04 ± 0.08 | 2.08 ± 0.05 | 18.21 ± 0.33 | 27.97 ± 0.07 | 6.89 ± 08 | 0.133 | 0.885 | 0.437 | 0.924 | |
| F9 | 2.35 ± 0.05 | 6.14 ± 0.12 | 14.07 ± 0.43 | 24.52 ± 0.39 | 7.42 ± 04 | 0.158 | 0.795 | 0.557 | 0.885 | |
| F10 | 1.83 ± 0.04 | 5.15 ± 0.05 | 12.48 ± 0.39 | 22.48 ± 0.50 | 7.78 ± 05 | 0.195 | 0.782 | 0.642 | 0.865 | |
| F11 | 1.75 ± 0.08 | 4.67 ± 0.01 | 10.52 ± 0.39 | 20.53 ± 0.48 | 8.57 ± 02 | 0.340 | 0.964 | 0.700 | 0.871 | |
| F12 | 1.33 ± 0.04 | 3.95 ± 0.08 | 8.49 ± 0.44 | 18.60 ± 0.35 | 9.57 ± 07 | 0.407 | 0.961 | 0.709 | 0.797 | |
SD=Standard deviation
Table 2: Swelling Ratio And Drug Release Behaviours Of Dtz-Loaded Ipn Beads In Ph 6.8 Phosphate Buffer And Ph Buffer Solutions
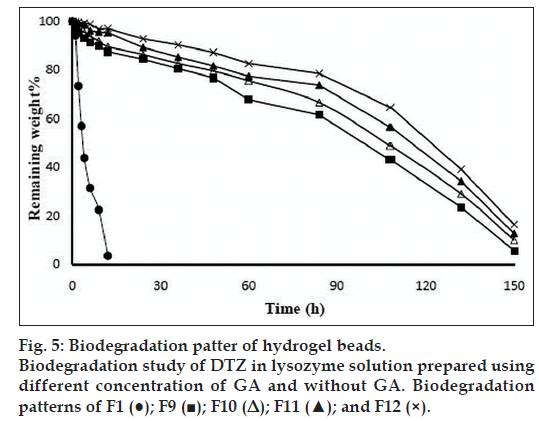
An in vitro biodegradation study of the prepared beads was carried out in presence of lysozyme. The percent of remaining weight of bead was determined as a function of time and considered as a measure of degradation. Fig. 5 shows the degradation patterns of the beads prepared using GA as a cross-linking agent in addition to the control formulation prepared without GA. The IPN beads prepared without GA showed higher extent of degradation (lower percent remaining weights) than the beads prepared with GA (fig. 5). The beads prepared using GA showed less swelling than the beads prepared using without GA (Table 2). This decrease in swelling hinders the diffusion of enzyme through the hydrogel matrix and consequently decreases the rate of degradation. Further, it was observed that increasing the GA content in the IPN beads decreased their degradation rate. This is due to the increase of cross-linking density upon increasing the GA content, which tends to impede the diffusion of enzyme through the beads and consequently retards their degradation.
The drug release behaviour was studied in pH 1.2 buffer solution for 2 h and in pH 6.8 buffer solution for 8 h. The percentage drug release after 2 h was determined to compare the drug release pattern of different formulations in pH 1.2 buffer solution and pH 6.8 buffer solutions. Time required for the release of 50% drug-load (t50%) was determined to compare the release pattern of different formulations in pH 6.8 buffer solution. In acidic medium, the drug release was 20.44±0.45, 18.67±0.32, 16.26±0.55, 14.14±0.50, 13.10±0.56 and 11.39±0.31% in 2 h, respectively, with increasing AlCl3 concentration. In alkaline medium, the beads released 32.76±0.30, 30.09±0.22, 28.23±0.32, 26.37±0.45, 24.11±0.61 and 22.09±0.55% of the drug in 2 h, respectively, with increasing concentration of AlCl3 (Table 2). As the concentration of cross-linking agent (AlCl3) was increased the drug release was decreased. It may be due to the fact that at higher cross-linking, free volume of the polymer matrix decreases, thereby blocking the movement of solutes through the polymer matrix. In order to compare the drug release rate, a time point approach was adopted. The values of t50% were 5.38±0.08, 5.59±0.04, 5.83±0.03, 6.42±0.07, 6.91±0.06 and 7.35±0.11, respectively, in the order of the increasing concentration of AlCl3 (Table 2). A similar result has been explained by other workers [30]. Moreover, the release of drug in pH 1.2 buffer solution was slower compared with that in pH 6.8 buffer solution at all preparative conditions. The slower drug release in pH 1.2 buffer solution from the beads could be due to the limited solubility of beads in the acidic solution as well as due to the weakly acidic nature of the drug. At higher pH values, the carboxyl groups of the beads undergo ionisation and consequently, the osmotic pressure inside the beads increases resulting in higher swelling and faster drug release. The carboxyl groups in unionised form in the acidic environment result in loss of the electrostatic repelling force and hence, lower the swelling of the beads and slower drug release [31].
In acidic medium, the drug release were 20.44±0.45, 19.70±0.38 and 18.21±0.33% in 2 h, respectively, with increasing drug-loading (Table 2) while in alkaline medium the drug release were 32.76±0.30, 30.11±0.58 and 27.97±0.07% in 2 h, respectively, with increasing drug-loading (Table 2). The increase in drug-loading resulted in the decreased drug release from hydrogel beads. The values of t50% were 5.38±0.08, 6.27±0.03 and 6.89±0.08 in 2 h, respectively, with increasing drug-loading (Table 2). Low drug-load in beads forms larger pore fraction resulting in higher swelling and faster drug release. At higher drug-load, larger crystalline domain of drug is formed in the beads. This causes reduction as well as shrinkage of pores of the matrix and results in fall in drug release. A similar finding was reported by other authors [32].
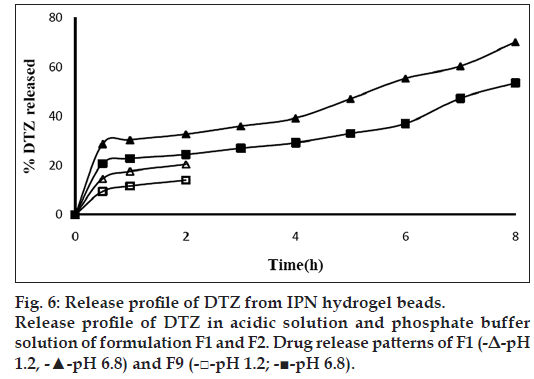
The treatment of GA during preparation of beads suppressed the drug release in weakly acidic as well as weakly basic dissolution medium. In acidic medium the drug release were 14.07±0.43, 12.48±0.39, 10.52±0.39 and 8.49±0.44% in 2 h, respectively, with increasing concentration of GA (Table 2) and resulted in a statistically significant difference in their drug release rate (P<0.05). In alkaline medium, the drug release were 24.52±0.39, 22.48±0.50, 20.53±0.48 and 18.60±0.35 in 2 h, respectively, with increasing concentration of GA (Table 2) and resulted in a statistically significant difference in their drug release rate (P<0.05). In addition to ionic cross-links, GA produced covalent acetal linkages and showed the retarded drug release in both dissolution media than those having ionic linkages only. This could be due to the fact that at higher cross-linking free volume of the matrix will decreases; thereby hinders the transport of drug molecules through the matrix. This could also reduce the swelling along with release rate from the matrix [31]. Optimised formulations were determined by considering DEE and/or t50%. The concentration of AlCl3 was varied in the formulations F1-F6. Formulation F1 showed the lowest t50% and highest DEE among six formulations. Formulation F6 showed the highest t50% and lowest DEE among six formulations. F1 was considered as optimised formulation among six formulations by considering DEE since other formulations showed very low DEE (Table 1). Further, the drug-load was varied in the formulations F1, F7 and F8. Formulation F8 showed the highest t50% and highest DEE among three formulations. Hence, the formulation F8 was considered as the optimised formulation among three formulations. In formulations F9-F12, concentration of GA was varied. Formulation F9 showed the highest DEE and lowest t50%. So the final optimised formulation was F9 by considering DEE since other formulation showed very low DEE. Release of drug from optimised formulation (F9) and basic formulation (F1) are shown in fig. 6.
To understand the mode of drug transport through the IPN beads the release data was fitted to the following empirical equation [33]. Mt/Mα=ktn, where, Mt/Mα was the fraction of drug released at time t, k was a constant, which indicated the structural and geometric characteristics of the device, and n was diffusion exponent. Mt was the amount of drug released at time t and determined from the release study. Mα was the amount of drug released at infinite time and that was the total amount of drug present in the formulation.
The mechanism of drug release from spherical polymeric devices may be Fickian diffusion when the value of n is 0.43 or less, anomalous (non-Fickian) transport when the value of n lies between 0.43 and 0.85, and case II transport when n=0.85. The values above 0.85 indicate super case II transport that relates to polymer relaxation during swelling [34]. The n values have been calculated and given in Table 2 along with correlation coefficients. The values of n were confined within 0.133-0.407 in acidic medium. This indicates that the release of drug followed Fickian transport. The values of n were found to vary within 0.398- 0.709 in alkaline medium indicating that the release deviated from Fickian to anomalous transport.
In conclusion all the formulations showed considerably low release of drug in pH 1.2 buffer solutions. The drug release was significantly increased when the pH of the medium was changed from acidic to alkaline. The drug release depends upon the extent of cross-linking and amount of drug used in the IPN hydrogel beads. The findings of the study indicate that a blend of SCMX and pectin could be a suitable matrix material for the preparation of IPN beads for sustained release of DTZ.
References
- Lu Y, Chen SC. Micro and nano fabrication of biodegradable polymers for drug delivery. Adv Drug Deliv Rev 2004;56:1621-33.
- Alexander A, Dwivedi S, Ajazuddin, Giri TK, Saraf S, Saraf S, et al. Approaches for breaking the barriers of drug permeation throughtransdermal drug delivery. J Control Release 2012;164:26-40.
- Behra A, Giri TK, Tripathi DK, Ajazuddin, Alexander A. An exhaustive review on recent advancement in pharmaceutical bioadhesive used for systemic drug delivery through oral mucosa for achieving maximum pharmacological response and effect.int J Pharmacol 2012;8:283-305.
- Halder A, Maity S, Sa B. Entrapment efficiency and release characteristics of polyethyleneimine treated or untreated calcium alginate beads loaded with propranolol-resin complex. Int J Pharm 2005;302:84-94.
- Ajazuddin, Alexander A, Khan J, Giri TK, Tripathi DK, Saraf S, et al. Advancement in stimuli triggered in situ gelling delivery for local and systemic route. Expert Opin Drug Deliv 2012;9:1573-92.
- Sipahigil O, Cevik HN, Dortunc B. Effect of different salts on the ionotropic gelation of pectin and formulation of oral drug delivery systems based on pectinate gels. Acta Pharma Sci 2011;53:211-23.
- Mura P, Maestrelli F, Cirri M, Gonzalez-Rodriguez ML, Rabasco AM. Development of enteric coated pectin based matrix tablets for colonic delivery of theophylline. J Drug Target 2003;11:365-71.
- Dupuis G, Chambin O, Genelot C. Colonic drug delivery: Influence of cross linking agent on pectin beads properties and role of shell capsule type. Drug Dev Ind Pharm 2006;32:847-55.
- Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in vitro release studies. Int J Pharm 1998;160:207-12.
- Sriamornsak P, Prakongpan S, Puttipipakhachorn S, Kennedy RA. Development of sustained release theophylline pellets coated with calcium pectinate. J Control Release 1997;47:221-32.
- Munjeri O, Collett JH, Fell JT. Hydrogel beads based on amidated pectins for colon-specific drug delivery: The rate of chitosan in modifying drug release. J Control Release 1997;46:273-8.
- Talukder R, Fassihi R. Gastroretentive delivery systems: Hollow beads. Drug Dev Ind Pharm 2004;30:405-12.
- Sriamornsak P, Thirawong N, Puttipipakhachorn S. Emulsion gel beads of calcium pectinate capable on floating on the gastric fluid: Effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur J Pharm Sci 2005;24:363-73.
- Aydin Z, Akbuga J. Preparation and evaluation of pectin beads. Int J Pharm 1996;137:133-6.
- Garcia-Ochoa F, Santos VE, Casas JA, Gomez E. Xanthan gum: Production, recovery, and properties. Biotechnol Adv 2000;18:549-79.
- Kocherbitova V, Ulvenlundb S, Briggnerb LE, Koberb M, Arnebranta T. Hydration of natural polyelectrolyte xanthan gum: Comparison with non-ionic carbohydrates. Carbohydr Polym 2010;82:284-90.
- Maiti S, Ray S, Mandal B, Sarkar S, Sa B. Carboxymethyl xanthan microparticles as a carrier for protein delivery. J Microencapsul 2007;24:743-56.
- Ray R, Pal R, Karan S, Sa B, Chatterjee TK. Evaluation of pharmacological activities of ibuprofen loaded interpenetrating polymer network (IPN) beads from sodium carboxymethyl xanthan and sodium alginate on rats. Pharmacol Online 2010;2:719-36.
- Ray S, Maiti S, Sa B. Polyethyleneimine-treated xanthan beads for prolonged release of diltiazem: In vitro and In vivo evaluation. Arch Pharm Res 2010;33:575-83.
- Maiti S, Ray S, Sa B. Controlled delivery of bovine serum albumin from carboxymethyl xanthan microparticles. Pharm Dev Technol 2009;14:165-72.
- Mandal S, Ray R, Basu SK, Sa B. Evaluation of a matrix tablet prepared with polyacrylamide-g-sodium alginate co-polymers and their partially hydrolyzed co-polymers for sustained release of diltiazem hydrochloride. J Biomater Sci Polym Ed 2010;21:1799-814.
- Mandal S, Basu SK, Sa B. Ca 2+ ion cross-linked interpenetrating network matrix tablets of polyacrylamide-grafted-sodium alginate and sodium alginate for sustained release of diltiazem hydrochloride. Carbohydr Polym 2010;82:867-73.
- Giri TK, Verma S, Alexander A, Ajazuddin, Badwaik H, Tripathy M, et al. Cross-linked biodegradable hydrogel floating beads forstomach site specific controlled delivery of metronidazole. Farmacia 2013;61:533-50.
- EI-Sherbiny IM, Abdel-Moqib M, Dawidar AM, Elsayed A, Smyth HD. Biodegradable pH-responsive alginate-poly (lactic-co-glycolic acid) nano/micro hydrogel matrices for oral delivery of silymarin. Carbohydr Polym 2011;83:1345-54.
- Kulkarni RV, Sa B. Evaluation of pH-Sensitivity and drug release characteristics of (Polyacrylamide-Grafted-Xanthan)–Carboxymethyl cellulose-based ph-sensitive interpenetrating network hydrogel beads. Drug Dev Ind Pharm 2008;34:1406-14.
- Soppimath KS, Aminabhavi TM. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release applications. Eur J Pharm Biopharm 2002;53:87-98.
- Kulkarni RV, Boppana R, Mohan GK, Mutalik S, Kalyane NV. pH-responsive interpenetrating network hydrogel beads of poly(acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: Synthesis, in vitro and in vivo evaluation. J Colloid Interface Sci 2012;367:509-17.
- Reddy T, Tammishetti S. Gastric resistant microbeads of metal ion cross-linked carboxymethyl guar gum for oral drug delivery. J Microencapsul 2002;19:311-8.
- Kulkarni AR, Soppimath KS, Aminabhavi TM. Controlled release of diclofenac sodium from sodium alginate beads crosslinked with glutaraldehyde. Pharm Acta Helv 1999;74:29-36.
- Kulkarni RV, Sreedhar V, Mutalik S, Setty CM, Sa B. Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int J Biol Macromol 2010;47:520-7.
- Maiti S, Ranjit S, Mondol R, Ray S, Sa B. Al+3 ion cross-linked and acetalated gellan hydrogel network beads for prolonged release of glipizide. Carbohydr Polym 2011;85:164-72.
- Berita S, Barkai A, Pathak YU. Effect of drug-loading extent on the in vitro release kinetic behavior of nifedipine from polyacrylate microspheres. J Control Release 1990;12:213-22.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppar NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.
- Ritger PI, Peppar NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 1987;5:37-42.