- *Corresponding Author:
- S. K. Sahu
University Department of Pharmaceutical Sciences, Utkal University, Vani Vihar, Bhubaneswar - 751 004, India
E-mail: tutu_kh@yahoo.com
| Date of Submission | 9 January 2006 |
| Date of Revision | 11 April 2007 |
| Date of Acceptance | 12 October 2007 |
| Indian J Pharm Sci 2007, 69 (5): 689-692 |
Abstract
A series of Schiff's bases have been prepared by condensation of substituted benzaldehydes with primary arylamines and the corresponding 4-thiazolidinones have been prepared by the reaction of Schiff's bases with thioglycolic acid in benzene. The resulting 4-thiazolidinones on reaction with substituted benzaldehydes in anhydrous sodium acetate by Knoevenagel's condensation have afforded 2-phenyl(substituted)-3-aryl-5-benzilidine(substituted) thiazolidine-4-ones, which on cyclization with phenyl hydrazine in anhydrous sodium acetate have furnished the title compounds. The structures have been established on the basis of spectral data. All the compounds have been screened in vitro for their antibacterial activity. The results of antibacterial activity study revealed promising inhibitory activity for 3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d] thiazole derivatives with 4-chloro and 4-nitro phenyl substitutions at 5-position against all the tested strains.
Selected substituted thiazoles [1,2] as well as different pyrazole ring containing heterocycles [3,4] possess marked antibacterial activity. The present investigation deals with the development of a series of nitrogen heterocyclic system from easily available starting materials. We report herein the synthesis of 2-phenyl (substituted)-3-aryl-5-benzilidine (substituted) thiazolidine-4-ones (3), their conversion to the title compounds (4) and evaluation of latter for their antibacterial activity.
Melting points were determined in open capillaries and were uncorrected. Purity of the compounds was checked by TLC on silica gel G plates. IR spectra (KBr) were recorded on a Jasco FTIR 410 spectrophotometer (νmax). 1H NMR spectra (CDCl3) were taken on a Bruker DRX 300-MHz spectrometer using TMS as an internal standard (chemical shifts in δ ppm). Elemental analysis (C, H, N) was carried out on a Euro EA (Italy) analyser. Schiff’s bases (I) and the corresponding 4-thiazolidinones (2) were prepared according to literature method [5].
2-Phenyl (substituted)-3-aryl-5-benzilidine (substituted)- thiazolidine-4-ones (3) [6] were synthesized by reß uxing an equimolar mixture (0.001 mol) of compound (2) and substituted benzaldehydes with anhydrous sodium acetate (0.082 g) in glacial acetic acid (20 ml) for 3 h. The reaction mixture was concentrated, cooled and poured into ice cold water. The solid thus separated was filtered, washed with water and crystallized from glacial acetic acid. The physical and elemental analysis data are given in Table 1 and 2, respectively.
| Compound | Substituents | mp(°) | Yield | ||
|---|---|---|---|---|---|
| Ar | R | RI | (%) | ||
| 3a1 | 4-NO2 Phenyl |
2-OH | 2-OH | 151 | 46.34 |
| b1 | 4-NO2 Phenyl |
4-N(CH3)2 | 2-OH | 88 | 44.05 |
| c1 | 4-NO2 Phenyl |
4-NO2 | 2-OH | 132 | 25.97 |
| d1 | 4-NO2 Phenyl |
4-Cl | 2-OH | 202 | 20.06 |
| e1 | 4-NO2-Phenyl | 4-OCH3 | 2-OH | 190 | 36.74 |
| a2 | 4-Cl Phenyl | 2-OH | 4-N(CH3)2 | 108 | 48 |
| d2 | 4-Cl Phenyl | 4-Cl | 4-N(CH3)2 | 118 | 48 |
| a3 | 4-Br Phenyl | 2-OH | 4-Cl | 98 | 48 |
| c3 | 4-Br Phenyl | 4-NO2 | 4-Cl | 88 | 37 |
| d3 | 4-Br Phenyl | 4-Cl | 4-Cl | 130 | 49 |
| b4 | Naphthyl | 4-N(CH3)2 | 4-Cl | 206 | 64 |
| c4 | Naphthyl | 4-NO2 | 4-Cl | 99 | 64 |
| d4 | Naphthyl | 4-Cl | 4-Cl | 83 | 49 |
| e4 | Naphthyl | 4-OCH3 | 4-Cl | 95 | 81 |
Table 1: Physical data of 2-phenyl-3-aryl-5- Benzilidine (substituted) thiazolidine-4-ones
| Compound | Molecular formula | C % | H % | N % | |||
|---|---|---|---|---|---|---|---|
| Calculated | Found | Calculated | Found | Calculated | Found | ||
| 3a1 | C22H16N2O5S | 62.84 | 62.80 | 3.83 | 3.80 | 6.66 | 6.64 |
| b1 | C24H21N3O4S | 64.41 | 64.37 | 4.73 | 4.69 | 9.39 | 9.37 |
| c1 | C22H15N3O6S | 58.79 | 58.74 | 3.36 | 3.32 | 9.34 | 9.32 |
| d1 | C22H15ClN2O4S | 60.20 | 59.80 | 3.44 | 3.40 | 6.38 | 6.37 |
| e1 | C23H18N2O5S | 63.58 | 63.55 | 4.17 | 4.14 | 6.44 | 6.42 |
| a2 | C24H21Cl N2O2S | 65.96 | 65.92 | 4.84 | 4.81 | 6.41 | 6.30 |
| d2 | C24H20Cl2N2OS | 63.28 | 36.24 | 4.42 | 4.38 | 6.14 | 6.12 |
| a3 | C22H14BrClNO2S | 57.84 | 57.80 | 3.30 | 2.9 | 3.06 | 3.03 |
| c3 | C22H14BrClNO3S | 55.88 | 55.84 | 3.19 | 3.16 | 2.96 | 2.95 |
| d3 | C22H14BrCl2NOS | 53.71 | 53.68 | 2.87 | 2.84 | 2.85 | 2.83 |
| b4 | C28H23ClN2OS | 71.39 | 71.35 | 4.92 | 4.88 | 5.94 | 5.93 |
| c4 | C26H17ClN2O3S | 66.02 | 65.09 | 3.62 | 6.58 | 5.92 | 5.90 |
| d4 | C26H17Cl2NOS | 67.52 | 67.48 | 3.70 | 3.30 | 3.02 | 3.00 |
| e4 | C27H20ClNO2S | 70.01 | 69.97 | 4.52 | 4.48 | 3.14 | 3.12 |
Table 2: Elemental analysis of 2-phenyl-3-aryl-5- benzilidine (substituted) thiazolidine-4-ones.
3a1 : IR(KBr, cm-1): 3285(Ar-OH), 3052 (Ar-CH),1739 (C=O),1542 (Ar-NO2). b1: IR (KBr, cm-1): 3294 (Ar-OH), 3045 (Ar-CH), 1736(C=O), 1538 (Ar-NO2), d1: IR (KBr, cm-1): 3292 (Ar-OH), 3038 (Ar-CH), 1733 (C=O), 747 (C-Cl). a2: IR (KBr, cm-1): 3289 (Ar-OH), 3026 (Ar-CH), 1722 (C=O), 754 (C-Cl). d2: IR (KBr, cm-1): 3049 (Ar-CH ), 1746 (C=O),751 (C-Cl). a3: IR (KBr, cm-1): 3295 (Ar-OH), 3031 (Ar-CH), 1742 (C=O), 742 (C-Cl). C3: IR (KBr, cm-1): 3064 (Ar- CH),1752 (C=O), 1546 (Ar-NO2), 759 (C-Cl). b4: IR (KBr, cm-1): 3068 (Ar-CH), 1756 (C=O), 746 (C-Cl). c4: IR (KBr, cm-1): 3059 (Ar-CH), 1731 (C=O), 1560 (Ar-NO2), 745 (C-Cl). d4: IR (KBr, cm-1): 3071 (Ar- CH ),1729 (C=O), 749 (C-Cl). The NMR Spectra of the synthesized compounds (3) of the series revealed peaks around 5.1-5.8 δ (1H, s, C=CH) and 6.5-8.0 δ due to bulk aromatic protons.
2-Phenyl-3,5-diphenyl(substituted)-6-aryl-3,3a,5,6- tetrahydro-2H-pyrazolo-[3,4-d]thiazoles (4) [6] were synthesized by heating under reflux an equimolar (0.001 mol) of compound (3) and phenylhydrazine with anhydrous sodium acetate (0.082 g) in glacial acetic acid (20 ml) for 6 h and cooled to room temperature. The solid thus separated was filtered, washed thoroughly with water and crystallised from glacial acetic acid. The physical and elemental analysis data are given in Tables 3 and 4, respectively.
| Compound | Substituents | mp(°) | Yield | ||
|---|---|---|---|---|---|
| Ar | R | RI | (%) | ||
| 4a1 | 4-NO2Phenyl | 2-OH | 2-OH | 98 | 99.9 |
| b1 | 4-NO2Phenyl | 4-N (CH3)2 | 2-OH | 138 | 94.96 |
| c1 | 4-NO2Phenyl | 4-NO2 | 2-OH | 108 | 95 |
| d1 | 4-NO2Phenyl | 4-Cl | 2-OH | 140 | 97.1 |
| e1 | 4-NO2Phenyl | 4-OCH3 | 2-OH | 150 | 99 |
| a2 | 4-Cl Phenyl | 2-OH | 4-N (CH3)2 | 242 | 99.75 |
| d2 | 4-Cl Phenyl | 4-Cl | 4-N (CH3)2 | 92 | 99.7 |
| a3 | 4-Br Phenyl | 2-OH | 4-Cl | 121 | 96.8 |
| c3 | 4-Br Phenyl | 4-NO2 | 4-Cl | 105 | 81.7 |
| d3 | 4-Br Phenyl | 4-Cl | 4-Cl | 88 | 90.74 |
| b4 | Naphthyl | 4-N (CH3)2 | 4-Cl | 87 | 99.4 |
| c4 | Naphthyl | 4-NO2 | 4-Cl | 160 | 98.89 |
| d4 | Naphthyl | 4-Cl | 4-Cl | 236 | 95.5 |
| e4 | Naphthyl | 4-OCH3 | 4-Cl | 120 | 97.98 |
Table 3: Physical data of 2-phenyl-3,5-diphenyl (substituted)-6-aryl-3,3a, 5,6-tetrahydro-2hpyrazolo [3,4-d] thiazoles.
| Compound | Molecular formula | C % | H % | N % | |||
|---|---|---|---|---|---|---|---|
| Calculated | Found | Calculated | Found | Calculated | Found | ||
| 4a1 | C28H22N4O4S | 65.86 | 65.82 | 4.34 | 4.30 | 10.97 | 10.95 |
| b1 | C30H27N5O3S | 67.02 | 66.98 | 5.06 | 4.96 | 13.02 | 13.01 |
| c1 | C28H21N5O5S | 62.32 | 62.28 | 3.92 | 3.89 | 12.97 | 12.96 |
| d1 | C28H22ClN4O3S | 68.13 | 68.10 | 4.28 | 4.25 | 11.35 | 11.33 |
| e1 | C29H24N4O4S | 66.39 | 66.35 | 4.61 | 4.57 | 10.67 | 10.66 |
| a2 | C30H27ClN4S | 68.35 | 68.33 | 5.16 | 5.14 | 10.62 | 10.60 |
| d2 | C30H26Cl2N4S | 66.03 | 65.99 | 4.80 | 4.50 | 10.27 | 10.26 |
| a3 | C28H21BrClN3OS | 59.73 | 59.70 | 3.76 | 3.74 | 7.46 | 7.44 |
| c3 | C28H20BrClN4O2S | 65.75 | 65.71 | 3.01 | 2.93 | 3.06 | 3.05 |
| d3 | C28H20BrCl2N3S | 57.83 | 57.80 | 3.46 | 3.44 | 7.22 | 7.20 |
| b4 | C34H29ClN4S | 72.76 | 72.72 | 5.02 | 4.17 | 9.98 | 9.96 |
| c4 | C32H23ClN4O2S | 68.25 | 68.21 | 4.11 | 4.08 | 9.94 | 9.93 |
| d4 | C32H23Cl2N3S | 69.55 | 69.51 | 4.19 | 4.15 | 7.60 | 7.59 |
| e4 | C33H26ClN3OS | 72.3 | 72.00 | 4.78 | 4.75 | 7.66 | 7.64 |
Table 4: Elemental analysis of 2-phenyl-3,5-diphenyl (substituted)-6-aryl-3,3a,5,6-tetrahydro-2hpyrazolo[ 3,4-d] thiazoles.
4a1: IR (KBr, cm-1): 3289 (Ar-OH), 3064(Ar-CH), 1539 (Ar-NO2), 1671 (C=N), 1266 (C-N); 1HNMR δ: 3.15 (s,1H,CH), 5.84 (s,1H,CH), 6.58-8.74 (m,17H,Ar- H), 11.14(s,1H,OH). b1: IR (KBr, cm-1): 3290(Ar-OH), 3031(Ar-CH), 1663(C=N), 1539 (Ar-NO2), 1260(C-N); 1HNMR δ: 1.13 (s.6H,2xCH3), 2.95(s,1H,CH),5.64(s, 1H,CH), 6.5-9.24(m,17H,Ar-H). d1: IR (KBr, cm-1): 3284(Ar-OH), 3045 (Ar-CH), 1548 (ArNO2), 1656 (C=N), 1264 (C-N), 748 (C-Cl); 1HNMR δ: 3.04 (s,1H,CH), 5.78 (s,1H,CH), 6.64-8.78 (m,17H,Ar-H), 10.96 (s,1H,OH). a2: IR(KBr, cm-1): 3286 (Ar-OH), 3063 (Ar-CH), 1683 (C=N), 1276 (C-N), 751 (C-Cl); 1HNMR δ: 1.40 (s,1H, 2×CH3), 3.22 (s,1H, CH), 5.75 (s,1H, CH), 5.90-7.97 (m,17H, Ar-CH), 10.99 (s,1H, OH). d2: IR (KBr, cm-1): 3062 (Ar-CH), 1674 (C=N), 1272 (C-N), 756 (C-Cl); 1HNMR δ: 2.02 (s, 6H, 2×CH3), 3.19 (s,1H,CH), 5.79 (s,1H,CH), 6.52-8.68 (m,17H,Ar-H). a3: IR (KBr, cm-1): 3294 (Ar-OH), 3030 (Ar-CH), 1668 (C=N), 1279 (C-N), 755 (C-Cl); 1HNMR δ: 2.17 (s,1H,CH), 5.85 (s,1H,CH), 7.02-9.18 (m,17H,Ar-H), 11.20 (s,1H,OH). c3: IR (KBr, cm-1): 3076 (Ar-CH), 1672 (C=N), 1552 (Ar-NO2), 1274 (C-N); 1HNMR δ: 3.06 (s,1H,CH), 5.72 (s,1H,CH), 5.90-7.97 (m,17H,Ar-H). b4: IR (KBr, cm-1): 3049 (Ar-CH), 1677 (C=N), 1267 (C-N), 744 (C-Cl); 1HNMR δ: 1.98 (s,6H, 2×CH3), 3.22 (s,1H,CH), 5.85 (s,1H,CH), 6.39-8.88 (m,20H,Ar-H). c4: IR (KBr, cm- 1): 3078 (Ar-OH), 1661 (C=N), 1564 (Ar-NO2), 1270 (C-N), 743 (C-Cl); 1H NMR δ: 2.22 (s,1H,CH), 5.08 (s,1H,CH), 5.83-8.88 (m,20H,ArH). d4: IR (KBr, cm-1): 3067 (Ar-CH), 1673 (C=N), 1273 (C-N), 753 (C-Cl); 1HNMR δ: 3.18 (s,1H,CH), 5.82 (s,1H,CH), 6.60-8.81 (m,20H,Ar-H).
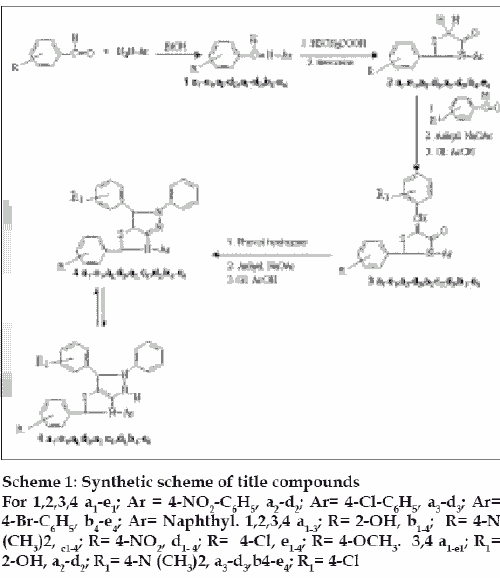
No doublet was seen in the NMR spectrum of any of the title compounds, thus indicating that the initial structure got rapid transformation through tautomeric shift of H-atom to the more stable structure as indicated in Scheme 1.
Scheme 1: Synthetic scheme of title compounds
For 1,2,3,4 a1-e1; Ar = 4-NO2-C6H5, a2-d2; Ar= 4-Cl-C6H5, a3-d3; Ar=
4-Br-C6H5, b4-e4; Ar= Naphthyl. 1,2,3,4 a1-3; R= 2-OH, b1-4; R= 4-N
(CH3)2, c1-4; R= 4-NO2, d1- 4; R= 4-Cl, e1-4; R= 4-OCH3. 3,4 a1-e1; R1=
2-OH, a2-d2; R1= 4-N (CH3)2, a3-d3,b4-e4; R1= 4-Cl
Substituted benzaldehydes on condensation with primary arylamines gave Schiff’s bases (1a1-e1, a2-d2, a3-d3, b4-e4), which on reaction with thioglycolic acid in benzene gave the corresponding 4-thiazolidinone (2a1-e1, a2-d2, a3-d3, b4-c4). The latter on reacting with substituted benzaldehydes in anhydrous sodium acetate afforded 2-phenyl(substituted)-3-aryl-5-benzilidine(sub stituted)thiazolidine-4-ones (3a1-e1, a2 , d2, a3 , c3, d3, b4-e4), which in turn reacted with phenylhydrazine in presence of anhydrous sodium acetate to furnish 2-phenyl-3,5-diphenyl(substituted)-6-aryl-3,3a,5,6- tetrahydro-2H-pyrazolo[3,4-d] thiazoles (4a1-e1, a2, d2, a3, c3, d3, b4-e4).
All compounds were screened for their in vitro antibacterial activity by agar cup plate method [7] at 100 μg concentration. Solutions of the test compounds were kept in dimethylsulphoxide. Ampicillin trihydrate (100 μg/ml) was used as a standard drug for comparison and solvent control was kept. The antibacterial activity of various compounds against pathogenic strains in nutrient agar is shown in Table 5. Compounds 4d1, d2, d3, c4, and d4 were found to be the most active against all the microbes. However, all the compounds were comparatively less active than the standard drug.
| Compound | Inhibition zone diameter (mm)* | |||
|---|---|---|---|---|
| S. a | A. p | E. c | K. a | |
| 4a1 | 18 | 20 | 17 | 19 |
| b1 | 19 | 18 | 21 | 20 |
| c1 | 17 | 20 | 19 | 18 |
| d1 | 19 | 21 | 21 | 22 |
| e1 | 17 | 18 | 20 | 19 |
| a2 | 19 | 18 | 21 | 20 |
| d2 | 21 | 20 | 23 | 22 |
| a3 | 19 | 20 | 18 | 21 |
| c3 | 20 | 18 | 21 | 19 |
| d3 | 21 | 20 | 22 | 24 |
| b4 | 17 | 19 | 18 | 20 |
| c4 | 19 | 20 | 22 | 21 |
| d4 | 20 | 22 | 21 | 23 |
| e4 | 18 | 17 | 20 | 21 |
| Ampicillin trihydrate | 31 | 29 | 30 | 31 |
Table 5: Antibacterial activities of 2-phenyl- 3,5-diphenyl(substituted)-6-aryl-3,3a,5,6- Tetrahydro-2h-pyrazolo [3,4-d] thiazoles
Acknowledgements
The authors wish to thank Dr. G. C. Pradhan, Department of Chemistry, Utkal University, Bhubaneswar for facilities and Prof. C. S. Panda, Department of Chemistry, Berhampur University, Berhampur for his valuable suggestions.
References
- Pattan, S.R., Maste, M. and Angadi, J. , Indian Drugs , 2002, 39(8), 429.
- Javed, S.A. and Sidiqui, N., Indian J. Heterocycl. Chem ., 2004, 13, 287.
- Solanki, P.R. and Wadodkar K.N., Indian J. Heterocycl. Chem ., 2003, 13, 135.
- Havaldar, F.H., Kumar, S. and Mishra, J., Indian J. Heterocycl. Chem ., 2004, 13, 197.
- Sharma, R.C. and Kumar, D., J. Indian Chem .Soc ., 2000, 77, 492.
- Mohan, J. and Khatter, D., Indian J. Heterocycl. Chem . 2004, 13, 327.
- Anonymous, Biritish Pharmacopoeia, Vol II, H.M. S.O. Publication Centre, London, 1998, A205.