- *Corresponding Author:
- M. Singh
Research Scholar,
IKG Punjab Technical University,
Kapurthala,
Jalandhar-144 603,
E-mail: mandeepchadha7@gmail.com
| Date of Submission | 28 January 2020 |
| Date of Revision | 11 May 2020 |
| Date of Acceptance | 17 July 2020 |
| Indian J Pharm Sci 2020;82(4):586-592 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Novel triazolyl-acridine compounds were synthesized in 4 series of 9-(2-(substituted phenyl-1H-1,2,3-triazol-1-yl)ethoxy)acridine, 9-(3-(4-(2-substituted phenyl)-1H-1,2,3-triazol-1-yl)propoxy) acridine, N-(2-(4 substituted phenyl-1H-1,2,3-triazol-1-yl)ethyl)acridin-9-amine and N-(3-(4-(substituted phenyl)-1H-1,2,3-triazol-1-yl)propyl)acridin-9-amine using appropriate synthetic procedures and screened for cytotoxic activity. The structures of all synthesized compounds were confirmed by Fourier-transform infrared spectroscopy, proton nuclear magnetic resonance and mass spectroscopy and these compounds were assayed in vitro for cytotoxic activity against MCF-7 (human breast adenocarcinoma cell line) and HT-29 (human colon adenocarcinoma cell line) cells. Tested compounds showed better cytotoxic activities in terms of IC50 value against MCF-7 and HT-29 cells. Methyl substituted compound MPP-9 exhibited excellent sensitivity with IC50 value 1 and 2 µM, against MCF-7 and HT-29, respectively. Unsubstituted MPP-1 and chloro-substituted MPP-2 and MPP-5 also exhibited good IC50 value ranges from 2-4 µM against both cell lines. These compounds were active at micro molar concentrations. Data study revealed that synthesized compounds are promising leads for future as cytotoxic agents.
Keywords
Acridine, Triazol, Cytotoxic, Cell line
Without a doubt, no one deny the fact that cancer is an epidemic greater than one of the biggest medical challenges of the century[1]. There are many types of cancer e.g. breast cancer, colon cancer, lung cancer, prostate cancer, but all types of cancer have similarity of uncontrolled proliferation or uncontrolled symmetric cell division[2]. Technologies for diagnosis and treatment are highly advanced now a day, but the treatment still remains poor.
Literature survey revealed many heterocyclic organic compounds like acridines, triazoles, which are effective against cancer. Acridine is a tricycle organic compound with a nitrogen heterocycle (fig. 1) having molecular formulae C13H9N, which can also be named as 10-azaanthracene and dibenzo[b,e]pyridine[3]. Inhibitory activity on tumor cells and binding to DNA is the popular molecular strategy. DNA targeting is a recent trend in innovation of new anticancer agents. In 1998, a quantitative structure-activity relationship (QSAR) analysis of 9-anilinoacridines with respect to antitumor activity and binding to DNA was reported by Gao et al.[4]. Intercalative binding of proflavine to bacterial nucleic acids is the site of action[5], which led to the development of acridine derivatives for modern anticancer chemotherapy e.g. m-AMSA, although the actual site of action of such derivatives is now established at the level of DNA coiling enzymes (topoisomerase) rather than DNA itself, damage being caused by the stabilization of the enzyme-DNA cleavage complex[6,7]. Acridine derivatives are not only effective antitumor[8] but also effective antiinflammatory[9], antibacterial[10], antimalarial[11] and antiviral agents[12].
Triazoles are heterocyclic organic compounds containing ?ve-membered ring with 3 nitrogen and 2 carbon atoms. Two isomeric forms of triazoles exist namely 1,2,3-triazole and 1,2,4-triazole[13], with molecular formula C2H3N3, 1,2,3-triazole is a basic aromatic heterocycle. The medicinal chemists have considered the synthesis of 1,2,3-triazole based heterocycles as the corner stone of medicinal chemistry due to their important biological activities like anticancer[14], antitubercular[15], antibacterial[16], antiinflammatory[17], antimicrobial[18] and antiviral[19]. The features possessed by the 1,2,3-triazoles make them pharmaceutically important molecules.
Thus, both acridines and 1,2,3-triazoles are important pharmacophores with anticancer activity. Both compounds are heterocyclic, small in size, with ease of synthesis and reported to possess extensively anticancer activity. Therefore in this investigation both structures were combined to synthesize novel triazolyl-acridine derivatives for evaluating anticancer activity against different cell lines.
Materials and Methods
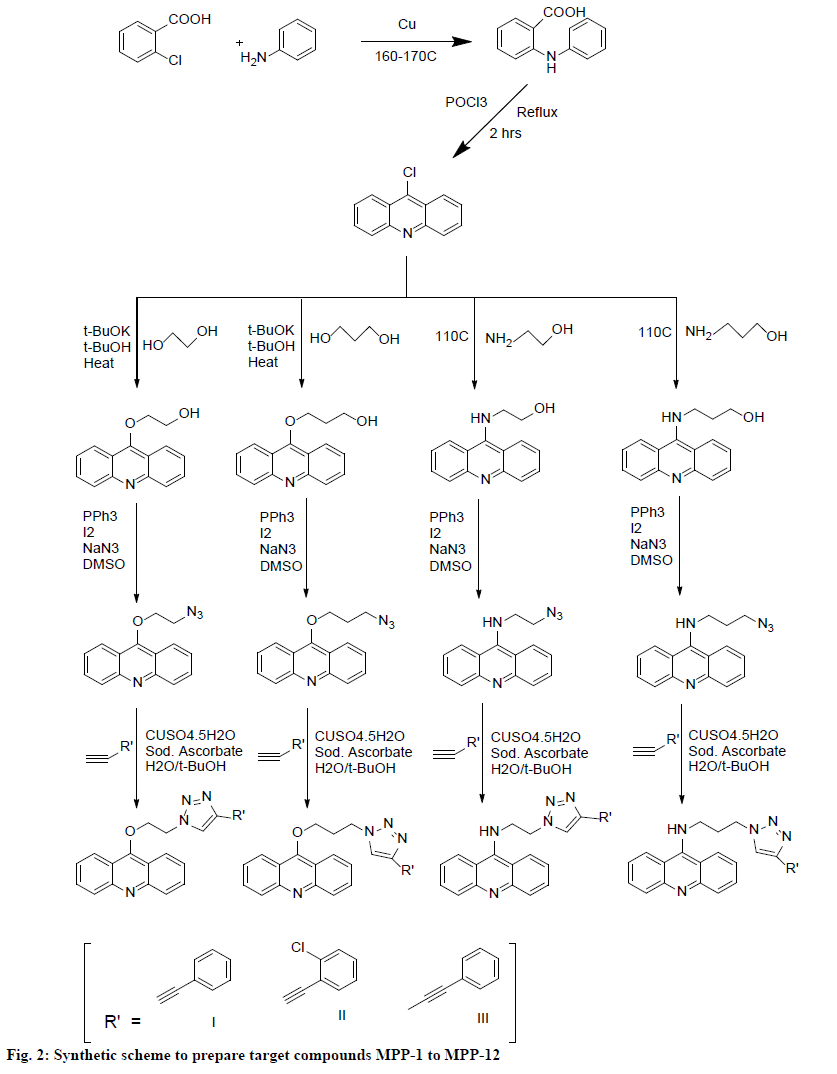
Synthetic grade chemicals were used. Thin layer chromatography (TLC) on precoated TLC plates was used to monitor the synthesis and finally prepared compounds. Chloroform:methanol (9:1) was used as the mobile phase for TLC and iodine chamber to visualized spots. Open glass capillary method was used for melting point determination. Infrared (IR) spectra were recorded on Bruker’s FT-IR spectrophotometer as KBr pellet and values are expressed in cm-1. 1H nuclear magnetic resonance spectroscopy (NMR) spectral analysis of the synthesized compounds were recorded on a Bruker Avance II 400 MHz in deuterated chloroform (CDCl3) using tetramethylsilane as internal standard. Chemical shift values were recorded on δ-scale. Syntheses of the triazolyl-acridine derivatives were performed according to the scheme presented in fig. 2.
Synthesis of 9-chloroacridine and N-phenylanthranilic acid:
9-Chloroacridine was synthesized using modified Ullman-Goldberg reaction[20]. N-phenylanthranilic acid was synthesized by mixing equal quantities of (0.038 mol) of o-chlorobenzoic acid and aniline with 0.12 g of copper powder in 40 ml isoamyl alcohol to which 6 g of dry potassium carbonate was added slowly and the final mixture was refluxed for 6 to 8 h. After reflux, isoamyl alcohol was removed by distillation, the mixture poured into 500 ml of hot water and filtered. The filtrate was acidified with concentrated hydrochloric acid. Yellow precipitate formed, filtered, washed with hot water and collected.
Synthesis of 9-chloroacridineby cyclization of n-phenylanthranilic acid:
Five grams (0.023 mol) of N-phenylanthranilic acid was mixed with 16 ml (27 g, 0.176 moles) of phosphorus oxychloride taken in a 500 ml round bottom flask fitted with a water-cooled condenser and heated about 15 min at 85-90°, then temperature is raised to 135-140°, where it was maintained for 2 h. Excess phosphorus oxychloride was removed by simple distillation. After cooling, the residue was poured into a well-stirred mixture of 20 ml of concentrated ammonia solution, 50 g of ice and 20 ml of chloroform in a separating funnel and allowed to stand for 30-40 min. The chloroform layer was separated and evaporated, greenish gray powder obtained was 9-chloroacridine.
Addition of alkanediol to 9-chloroacridine[21]:
Dissolved 0.2 g (1.0 mmol) of 9-chloroacridine in 2.0 ml of ethylene glycol, under inert atmosphere, a 1.0 M solution of potassium t-butoxide in t-butyl alcohol (1.5 ml, 1.5 mmol, 1.5 equiv) was added, stirred at 80° for 18 h, then quenched with saturated NaHCO3. The mixture was extracted with CH2Cl2, dried over anhydrous MgSO4, concentrated in vacuum, and purified by recrystallization from CHCl3 to yield 9-acridinyl alkanediol as the product.
Conversion of 9-chloroacridine to amino alcohols:
9-chloroacridine (1.0 g, 5.1 mmol) and alkanolamine (5 equiv) was heated at 110° for 6 h, under inert atmosphere and then cooled. NaOH 1 N was added and extracted with CHCl3. The organic layers were washed with brine, dried over anhydrous Na2SO4 and evaporated under reduced pressure to yield 9-acridinyl alkanolamine as the product.
Conversion of alcohols into azides[22]:
Firstly, 3 mmol of 9-acridinyl alkanediol or 9-acridinyl alkanolamine, 3.6 mmol of triphenylphosphine and 3.6 mmol of iodine was triturated in a mortar and pestle for 10 min, when exothermic reaction took place, paste like consistency appeared. Separately, 12 mmol of sodium azide was dissolved in DMSO, the solution was mixed with acridine paste and stirred for 30 min on a magnetic stirrer. Upon completion of the reaction, icecooled solution of 50 ml of sodium thiosulphate was added and extracted with 30 ml of diethyl ether 3 times. The combined organic layer was washed with brine (50 ml). On evaporation a crude product obtained, which was purified by column chromatography using 5 % ethyl acetate in hexane.
Conversion of azides in to triazoles[23]:
Three mmol of alkyne and, 3 mmol of azide were suspended in 12 ml of a 1:1 water/tert-butanol mixture. Freshly prepared 1 M sodium ascorbate solution was added, followed by 0.03 mmol of copper (II) sulfate pentahydrate in 100 ml of water. The heterogeneous mixture was stirred vigorously overnight. When TLC analysis indicated complete consumption of the reactants, the reaction mixture was diluted with 50 ml of water and cooled in ice, and the white precipitate was collected as filtrate. After being washed with cold water (225 ml), the precipitate was dried under vacuum to obtain pure triazole compounds, MPP-1 to MPP-12.
MPP-1, 9-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethoxy) acridine, yield: 85 %, melting point (MP): 223o, Rf: 0.45;FT-IR (KBr): cm-1 3051 C-H str. (triazole), 1463 C-H str. (methylene), 1651 C=N str. (Ar), 1592 N=N str., 1524 C=C str. (Ar), 1308 C-N str., 1254 C-O str. (ether), C-Cl str. (Ar); 1H NMR (CDCl3, 400 MHz): δ; 8.14 (d, 2H, J =9.44, CH), 7.99 (d, 2H, J =8.2, CH), 7.82 (t, 2H, J =4.96,4.2, CH), 7.79 (d, 2H, J =6.92, CH), 7.63 (t, 2H, J =4.08, 3.68, CH), 7.59 (s, 1H, CHtriazole), 7.53 (t, 2H, J =2.8, 2.96, CH-Ar), 7.45 (t, 1H, J =2.48, 4.72, CH-Ar), 4.50 (t, 2H, J =6.56, 7.32, CH), 4.16 (t, 2H, J =5.24, 5.08, CH) ppm; MS (m/z): 367 (M+1).
MPP-2, 9-(2-(4-(2-chlorophenyl)-1H-1,2,3-triazol-1- yl)ethoxy)acridine, yield: 83 %, MP: 242o, Rf: 0.68;FTIR (KBr): cm-1 3048 C-H str. (triazole), 1466 C-H str. (methylene), 1656 C=N str. (Ar), 1541 N=N str., 1510 C=C str. (Ar), 1313C-N str., 1278 C-O str. (ether), 836 C-Cl str. (Ar); 1H NMR (CDCl3, 400 MHz): δ 8.12 (d, 2H, J =10.28, CH), 7.94 (d, 2H, J =6.8, CH), 7.79 (t, 2H, J =5.08,6.84, CH-Ar), 7.74 (d, 1H, J =8.76, CH), 7.64 (t, 2H, J =5.24, 3.6, CH), 7.59 (s, 1H, CH-triazole), 7.57 (d, 1H, J =7.84, 2.96, CH-Ar), 7.39 (t, 1H, J =4.0, 4.2, CH-Ar),7.35 (t, 1H, J =2.88, 6.44, CH-Ar), 4.49 (t, 2H, J =2.08, 7.52, CH), 4.16 (t, 2H, J =7.24, 2.56, CH) ppm. MS (m/z); 401 (M+1).
MPP-3, 9-(2-(4-(o-tolyl)-1H-1,2,3-triazol-1-yl)ethoxy) acridine, yield: 82 %, MP: 212o, Rf: 0.58; FT-IR (KBr): cm-1 3052 C-H str. (triazole), 1455 C-H str. (methylene), 1436 C-H str. (methyl), 1651 C=N str., 1524 N=N str., 1308 C-N str., 1254 C-O str. (ether). 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, 2H, J =6.8, CH), 7.98 (d, 2H, J =6.84, CH), 7.83 (t, 2H, J =6.44, 1.16, CH-Ar), 7.63 (t, 2H, J =3.6, 5.32, CH), 7.60 (s, 1H, Ar-CH), 7.56 (d, 1H, J =9.64, CH-Ar), 7.33-7.27 (m, 3H, CH-Ar), 4.51 (t, 2H, J =4.88, 4.64,CH), 4.15 (t, 2H, J =3.6, 5.2, CH), 2.58 (s, 3H,CH3) ppm. MS (m/z): 381 (M+1).
MPP-4, 9-(3-(4-phenyl-1H-1,2,3-triazol-1-yl)propoxy) acridine, yield: 90 %, MP: 242o, Rf: 0.46; FT-IR (KBr): cm-1 3054C-H str. (triazole), 1452 C-H str. (methylene), 1654 C=N str., 1541 N=N str., 1511 C=C str. (Ar), 1307 C-N str., 1259 C-O str. (ether); 1H NMR (CDCl3, 400 MHz): δ 8.13 (d, 2H, J =9.28, CH), 7.97 (d, 2H, J =8.84, CH), 7.82 (t, 2H, J =1.84, 6.08, CH-Ar), 7.79 (d, 2H, J =8.44, CH), 7.63 (t, 2H, J =4.64, 6.0, CH), 7.59 (s, 1H, Ar-CH), 7.53 (t, 2H, J =4.6, 5.32, CH-Ar), 7.42 (t, 1H, J =4.12, 4.6, CH-Ar), 4.46 (t, 2H, J =1.6, 5.44, CH), 4.06 (t, 2H, J =5.08, 2.0, CH), 2.21-2.17 (p, 2H, CH) ppm. MS (m/z): 381 (M+1)
MPP-5, 9-(3-(4-(2-chlorophenyl)-1H-1,2,3-triazol-1- yl)propoxy)acridine, yield: 81 %, MP: 221o, Rf: 0.43; FT-IR (KBr): cm-1 3072 C-H str. (triazole), 1436 C-H str. (methylene), 1658 C=N str., 1545 N=N str., 1504 C=C str.(Ar), 1309 C-N str., 1256 C-O str. (ether), 836 C-Cl str.(Ar); 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, 2H, J =6.12, CH), 7.99 (d, 2H, J =5.12, CH), 7.82 (t, 2H, J =3.4, 2.88, CH-Ar), 7.76 (d, 1H, J =8.0 CH), 7.63 (t, 2H, J =2.8, 3.6, CH), 7.59 (s, 1H, Ar-CH), 7.55 (d, 1H, J =7.36, CH-Ar), 7.39 (t, 1H, J =8.28, 3.36, CHAr), 7.35 (t, 1H, J =5.08, 3.08, CH-Ar), 4.48 (t, 2H, J =7.32, 3.44, CH), 4.07 (t, 2H, 4.68, J =2.84, 4, CH) 2.21-2.17 (p, 2H, CH) ppm. MS (m/z): 415 (M+1).
MPP-6, 9-(3-(4-(o-tolyl)-1H-1,2,3-triazol-1-yl) propoxy)acridine: yield, 79 %, MP: 215oC, Rf: 0.61; FT-IR (KBr): cm-1 3073C-H str. (triazole), 1468 C-H str. (methylene), 1438 C-H str. (methyl), 1652 C=N str., 1546 N=N str., 1507 C=C str. (Ar), 1313 C-N str., 1255C-O str. (ether); 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, 2H, J =6.8, CH), 7.98 (d, 2H, J =9.84, CH), 7.81 (t, 2H, J =2.36, 3.6, CH-Ar), 7.62 (t, 2H, J =7.08, 2.48, CH), 7.59 (s, 1H, Ar-CH), 7.56 (d, 1H, J =10.04, CH), 7.35 (t, 1H, J =2.88, 6.44, CH-Ar), 7.30-7.26 (m, 2H, CH-Ar), 4.48 (t, 2H, J =4.2, 5.16, CH), 4.09 (t, 2H, J =6.4, 3.96, CH), 2.59 (s, 3H, CH3), 2.21-2.17 (p, 2H, CH) ppm. MS (m/z): 395 (M+1).
MPP-7, N-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethyl) acridin-9-amine: yield, 82 %, MP: 232o, Rf: 0.49; FTIR (KBr): cm-1 3339 N-H str. (secondary amine), 3075 C-H str. (triazole), 1468 C-H str. (methylene), 1655C=N str., 1545 N=N str., 1510C=C str. (Ar), 1311C-N str.; 1H NMR (CDCl3, 400 MHz): δ 8.18 (d, 2H, J =7.44, CH), 7.98 (d, 2H, J =6.8, CH), 7.81 (d, 2H, J =8.64, CH), 7.78 (t, 2H, J =2.56, 4.44, CH-Ar), 7.62 (t, 2H, J =5.24, 5.08, CH), 7.59 (s, 1H, Ar-CH), 7.52 (t, 2H, J =3.24, 7.2, CH-Ar), 7.42 (t, 1H, J =5.36, 3.36, CH-Ar), 5.61 (t, 2H, J =4.2, 4.8, CH), 4.01 (s, 1H, Ar-NH), 3.56 (t, 2H, J =4.56, 6.48, CH) ppm. MS (m/z): 366 (M+1).
MPP-8, N-(2-(4-(2-chlorophenyl)-1H-1,2,3-triazol- 1-yl) ethyl)acridin-9-amine, yield: 85 %, MP: 236o, Rf: 0.54; FT-IR (KBr): cm-1 3334-N-H str. (secondary amine), 3061-C-H str. (triazole), 1452-C-H str. (methylene), 1650-C=N str., 1545-N=N str., 1505-C=C str. (Ar), 1310 C-N str., 832C-Cl str.; 1H NMR (CDCl3, 400 MHz): δ 8.19 (d, 2H, J =8.24, CH), 7.95 (d, 2H, J =7.52, CH), 7.78 (t, 2H, J =1.32, 5.32, CH-Ar), 7.74 (d, 1H, J =8.76, CH), 7.61 (t, 2H, J =1.64, 3.92, CH), 7.59 (s, 1H, Ar-CH), 7.55 (d, 1H, J =9.32, CH-Ar), 7.40- 7.35 (m, 2H, CH-Ar), 5.60 (t, 2H, J =3.88, 3.36, CH), 4.00 (s, 1H, NH), 3.56 (t, 2H, J =2.12, 4.92, CH) ppm. MS (m/z): 400 (M+1).
MPP-9, N-(2-(4-(o-tolyl)-1H-1,2,3-triazol-1-yl)ethyl) acridin-9-amine: yield, 86 %, MP: 227o, Rf: 0.62; FT-IR (KBr): cm-1 3379-N-H str. (secondary amine), 3069-CH str. (triazole), 1475-C-H str. (methylene), 1435-C-H str. (methyl), 1655-C=N str., 1590-N=N str., 1538-C=C str. (Ar), 1308-C-N str.; 1H NMR (CDCl3, 400 MHz): δ 8.20 (d, 2H, J =11.12, CH), 7.96 (d, 2H, J =7.32, CH), 7.78 (t, 2H, J =1.36, 4.72, CH-Ar), 7.62 (t, 2H, J =3.04, 5.24, CH), 7.59 (s, 1H, Ar-CH), 7.57 (d, 1H, J =10.28, CH-Ar), 7.33-7.28 (m, 3H, CH-Ar), 5.59 (t, 2H, J =3.36, 4.96, CH), 4.01 (s, 1H, NH), 3.56 (t, 2H, J =3.24, 3.2, CH), 2.59 (s, 3H, CH3) ppm. MS (m/z): 380 (M+1).
MPP-10, N-(3-(4-phenyl-1H-1,2,3-triazol-1-yl)propyl) acridin-9-amine, yield: 89 %, MP: 217o, Rf: 0.67; FTIR (KBr): cm-1 3365-N-H str. (secondary amine), 3078- C-H str. (triazole), 1457-C-H str. (methylene), 1656- C=N str., 1597-N=N str., 1535-C=C str. (Ar), 1302- C-N str.; 1H NMR (CDCl3, 400 MHz): δ 8.20 (d, 2H, J =10.48, CH), 7.98 (d, 2H, J =9.36, CH), 7.82-7.77 (m, 4H, CH-Ar), 7.62 (t, 2H, J =1.52, 4.64, CH), 7.59 (s, 1H, Ar-CH), 7.52 (d, 2H, J =3.68, CH-Ar), 7.43 (t, 1H, J =5.24, 5.08, CH-Ar), 4.01 (s, 1H, NH), 3.37 (t, 2H, J =0.52, 6.96, CH), 2.58-2.54 (p, 2H, CH) ppm. MS (m/z): 480 (M+1).
MPP-11, N-(3-(4-(2-chlorophenyl)-1H-1,2,3-triazol- 1-yl)propyl)acridin-9-amine, yield: 83%, MP: 242o, Rf: 0.62; FT-IR (KBr): cm-1 3374-N-H str. (secondary amine), 3031-C-H str. (triazole), 1449-C-H str. (methylene), 1647-C=N str., 1513-C=C str. (Ar), 1338- C-N str., 794-C-Cl str.; 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, 2H, J =7.48, CH), 7.97 (d, 2H, J =7.24, CH), 7.79 (t, 2H, J = 1.92, 3.96, CH), 7.73 (d, 1H, J =6.52, CH), 7.62 (t, 2H, J =2.88, 5.64, CH), 7.59 (s, 1H, Ar- CH), 7.57 (d, 1H, J =7.0, CH-Ar), 7.41 (t, 1H, J =6.64, 2.92, CH-Ar), 7.36 (t, 1H, J =4.56, 4.32, CH-Ar), 4.48 (t, 2H, J =4.24, 4.72, CH-Ar), 4.01 (s, 1H, NH), 3.36 (t, 2H, J =3.36, 3.96, CH), 2.57-2.53 (p, 2H, CH) ppm. MS (m/z): 414 (M+1).
MPP-12, N-(3-(4-(o-tolyl)-1H-1,2,3-triazol-1-yl) propyl)acridin-9-amine, yield: 88 %, MP: 252o, Rf: 0.57; FT-IR (KBr): cm-1 3332-N-H str. (secondary amine), 3082-C-H str. (triazole), 1463-C-H str. (methylene), 1401-C-H str. (methyl), 1683-C=N str., 1579-N=N str., 1544-C=C str. (Ar), 1266-C-N str.; 1H NMR (CDCl3, 400 MHz): δ 8.20 (d, 2H, J =5.68, CH), 7.98 (d, 2H, J =4.84, CH), 7.79 (t, 2H, J = 4.32, 3.92, CH), 7.64 (t, 2H, J =3.48, 6.2, CH), 7.59 (s, 1H, Ar-CH), 7.58 (d, 1H, J =8.32, CH-Ar), 7.33-7.28 (m, 3H, CH-Ar), 4.48 (t, 2H, J =5.0, 3.32, CH), 4.01 (s, 1H, NH), 3.36 (t, 2H, J =5.36, 1.4, CH), 2.59 (s, 3H, CH3), 2.57-2.53 (p, 2H, CH) ppm. MS (m/z): 394 (M+1).
Biological evaluation:
All newly synthesized triazolyl-acridines were assayed in vitro for cytotoxic activity against MCF-7 (human breast adenocarcinoma cell line) and HT-29 (human colon adenocarcinoma cell line) cells, which were maintained at 37° in a humidified atmosphere (90 %) containing 5 % CO2[24].
MTT Assay:
All the synthesized triazolyl-acridine compounds were dissolved in DMSO and serially diluted with complete medium to get a range of test concentrations. DMSO concentration was kept <0.1 % in all the samples. HT-29 colon carcinoma and MCF7 cells breast adenocarcinoma maintained in appropriate conditions were seeded in 96 well plates and treated with different concentrations of the test samples and incubated at 37°, 5 % CO2 for 96 h. MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazoliumbromide) reagent was added to the wells and incubated for 4 h; the dark blue formazan product formed by the cells was dissolved in DMSO under a safety cabinet and read at 550 nm in triplicate. Percent inhibitions were calculated and plotted with the concentrations used to calculate the inhibitory concentration (IC50) values[25]. The results of MTT assay summarized in Tables 1 and 2.
| Compound code | Concentration (µM) | IC50 value (µM) | ||||
|---|---|---|---|---|---|---|
| 10 | 1 | 0.1 | 0.01 | 0.001 | ||
| MPP-1 | 74.3±0.23 | 42.55±0.00 | 23.66±0.04 | 14.29±0.08 | 10.31±0.01 | 2 |
| MPP-2 | 55.48±0.71 | 43.26±0.82 | 29.21±1.07 | 7.54±0.23 | 1.22±0.09 | 2 |
| MPP-3 | 68.36±1.58 | 50.1±2.55 | 13.24±0.45 | 1.26±0.14 | 2.3±0.00 | >10 |
| MPP-4 | 56.64±1.59 | 33.64±0.22 | 18.54±1.49 | 12.35±0.92 | 3.69±0.27 | >10 |
| MPP-5 | 56.64±0.86 | 33.21±0.59 | 21.54±0.38 | 2.38±0.91 | 1.25±0.53 | 4 |
| MPP-6 | 58.95±0.17 | 45.56±0.24 | 12.54±0.21 | 3.36±0.09 | 2.05±0.09 | 10 |
| MPP-7 | 41.26±0.47 | 32.35±1.16 | 23.64±0.39 | 10.11±0.73 | 2.37±0.38 | >10 |
| MPP-8 | 38.65±0.80 | 28.66±0.33 | 12.64±0.13 | 10.56±0.22 | 2.3±0.21 | >10 |
| MPP-9 | 68.1±0.27 | 50.2±0.69 | 32.15±0.98 | 13.65±0.44 | 10.1±0.46 | 1 |
| MPP-10 | 32.15±0.09 | 16.54±0.24 | 10.2±0.09 | 2.5±0.12 | 1.1±0.25 | >10 |
| MPP-11 | 31.21±0.09 | 23.5±0.30 | 12.3±0.39 | 6.27±0.59 | 2.13±0.10 | >10 |
| MPP-12 | 39.21±0.19 | 25.32±0.59 | 13.5±0.20 | 8.24±0.00 | 1.34±0.08 | >10 |
Results expressed as mean±SD of % inhibition, IC50 values calculated from MTT assay results
Table 1: Cytotoxic Activity Of Synthesized Compounds Against Mcf-7 Cell Line
| Compound code | Concentrations (µM) | IC50 value (µM) | ||||
|---|---|---|---|---|---|---|
| 10 | 1 | 0.1 | 0.01 | 0.001 | ||
| MPP-1 | 72.3±0.13 | 44.22±0.74 | 32.7±0.06 | 15.74±0.63 | 11.2±0.12 | 2 |
| MPP-2 | 58.17±0.18 | 44.6±0.12 | 32.91±1.26 | 10.8±0.01 | 2.47±0.29 | 2 |
| MPP-3 | 32.87±0.55 | 15.66±0.21 | 12.74±0.81 | 2.47±0.21 | 1.45±0.28 | >10 |
| MPP-4 | 52.7±0.47 | 32.84±0.46 | 22.85±0.33 | 18.19±0.72 | 2.47±0.32 | >10 |
| MPP-5 | 53.44±0.23 | 37.6±0.35 | 22.5±0.17 | 7.24±0.18 | 3.54±0.45 | 4 |
| MPP-6 | 54.25±0.42 | 22.61±0.2 | 18.4±0.18 | 1.2±0.07 | 1.1±0.12 | 10 |
| MPP-7 | 45.26±0.01 | 25.63±0.78 | 18.54±0.37 | 12.31±0.35 | 1.25±0.16 | >10 |
| MPP-8 | 38.65±0.32 | 28.66±0.28 | 12.64±0.10 | 10.56±0.33 | 2.3±0.16 | >10 |
| MPP-9 | 55.3±0.63 | 44.21±0.24 | 32.26±0.52 | 15.6±0.36 | 2.36±0.21 | 2 |
| MPP-10 | 45.26±0.44 | 32.15±0.41 | 21.26±0.62 | 6.35±0.42 | 1.25±0.32 | >10 |
| MPP-11 | 42.3±0.12 | 22.21±0.36 | 12.42±0.36 | 6.12±0.41 | 2.31±0.01 | >10 |
| MPP-12 | 32.3±0.37 | 20.1±0.56 | 11.21±0.89 | 4.91±0.71 | 1.22±0.56 | >10 |
Results expressed as mean±SD of % inhibition, IC50 values calculated from MTT assay results
Results and Discussion
Structures of novel synthesized triazolyl-acridine compounds, MPP-1 to MPP-12 were confirmed by FT-IR, 1HNMR and mass spectroscopy data as well as their distinct Rf values in TLC analysis. FT-IR spectroscopic data clearly confirmed the formation of target compounds, by showing stretching of triazole’s C-H bond at 3000 cm-1, methylene’s C-H bond about 1465 cm-1, aryl alkyl ether’s C-O between 1200-1275 cm-1 (MPP-1 to MPP-6 compounds), secondary amine’s N-H bond between 3310-3350 cm-1 (MPP-7 to MPP-12 compounds) and disappearance of azide’s peaks found between 2120-2160 cm-1. Similarly proton NMR signal characterized the target compounds by δ-value of, CH at triazole ring at 7.59 ppm, CH2 ranges 2.1-5.5 ppm and NH at 4.01 ppm (compound MPP-7 to compounds MPP-12).
All synthesized triazolyl-acridine compounds (MPP- 1 to MPP-12) were evaluated in vitro for cytotoxicity against MCF-7 and HT-29. IC50 values were calculated by MTT assay, using five different concentrations of all triazolyl-acridine compounds. MCF-7 cell line was found highly sensitive against methyl substituted compound (MPP-9) with an IC50 of 1 μM, unsubstituted compound (MPP-1) and chlorine substituted compounds (MPP-2, MPP-5) exhibited IC50 of 2, 4 μM, respectively. HT-29 cell line was found sensitive with IC50 of 2 μM against MPP-1, MPP-2, MPP-9 and MPP- 5 with IC50 of 4 μM. The data clearly showed inhibition of growth of MCF-7 and HT-29 cells, which decreased with increasing doses administered. At the final dose, there was only minimal amount of cells survived.
Present study concluded that novel synthesized triazolyl-acridine compounds inhibited the growth of cells tested in a dose-dependent manner. MPP-1, MPP- 2 and MPP-9 demonstrated greater activity while MPP- 5 was moderately active in inhibiting the growth of both cell line tested. These can still be considered good candidates for the development of anticancer drugs since these demonstrated activities against cancer cells. These compounds also can be used as drug leads for the development of better candidate drugs or these can be used in combination with other antineoplastic drugs to complement their therapeutic effects. However, more in vitro and in vivo mechanistic studies are required to understand the full potential of these compounds.
Acknowledgements
The authors thank the IKG Punjab Technical University, Kapurthala, Jalandhar where the author is registered as a research scholar. The author also thanks Deshpande Laboratories Pvt. Ltd. Bhopal for evaluating the synthesized compounds for cytotoxic studies and sophisticated analytical instruments facility, Panjab University, Chandigarh for 1H-NMR, IR and Mass spectroscopic data.
Conflict of interest
Nil.
Financial support/scholarship
Nil.
References
- Mohammad AA. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. BioMed Res Int 2020;7:8602879
- Hahn W.C. Cancer: Surviving on the Edge. Cancer Cell 2004;6:215-22.
- Rupar SJ, Dobricic V, Aleksic MM, Brboric SJ, Cudina O. A review of published data on acridine derivatives with different biological activities. Kragujevac J Sci 2018;40:83-101.
- Gao H, Denny WA, Garg R, Hansch C. Quantitative structure-activity relationships (QSAR) for 9-anilino- acridines: a comparative analysis. Chem Biol Interaction 1998;116:157-80.
- Lerman LS. The structure of the deoxyribonucleic acid (DNA)–acridine complex. Proc Nat Acad Sci USA 1963;49: 94-102.
- Demeunynck M. Antitumour Acridines. Expert Opin 2004;14:55-70.
- Belmont P, Dorange I. Acridine: A Simple Scaffold with a Wide Range of Application in Oncology. Expert Opin 2008;18:1211-24.
- Su TL, Lin YW, Chou TC, Zhang X, Bacherikov VA, Chen CH, et al. Potent Antitumor 9-Anilinoacridines and Acridines Bearing an Alkylating N-Mustard Residue on the AcridineChromophore. Synthesis and Biological Activity. J Med Chem 2006;49:3710-18.
- Chandra T, Garg N, Lata S, Saxena KK, Kumar A. Synthesis of substituted acridinylpyrazoline derivatives and their evaluation for anti-inflammatory activity. Eur J Med Chem 2010;45:1772-76.
- Rahimizadeh M, Pordel M, Bakavoli M, Bakhtiarpoor Z, Orafaie A. Synthesis of imidazo [4,5-a]acridones and imidazo [4,5-a] acridines as potential antibacterial agents. Monatsh Chem 2009;140:633-38.
- Valdes AFC. Acridine and Acridinones: Old and New Structures with Antimalarial Activity. Open Med Chem J 2011;5:11-20.
- Goodell JR, Madhok AA, Hiasa H, Ferguson DM. Synthesis and evaluation of acridine- and acridone-based anti-herpes agents with topoisomerase activity. Bioorg Med Chem 2006;14:5467-80.
- Praveena KSS, Murthy NYS, Pal S. Synthesis and biological activities of 1,4-disubstituted-1,2,3-triazoles. J Chem Pharm Res 2015;7:506-22;
- Reddy PB, Agrawal SK, Singh S, Bhat BA, Saxena AK, Kumar HMS, et al. Synthesis and Biological Evaluation of 4b-[(4-Substituted)-1,2,3-triazol-1-yl] podophyllotoxins as Potential Anticancer Agents. Chem Biodivers 2008;5:1792-1802.
- Jordao AK, Sathler PC, Ferreira VF, Campos VR, de Souza M, Castro HC, et al. Synthesis, antitubercular activity, and SAR study of N-substituted-phenylamino-5-methyl-1H-1,2,3-triazole-4-carbohydrazides. Bioorg Med Chem 2011;19:5605-11.
- Bengtsson C, EG Lindgren A, Uvell H, Almqvist F. Design, synthesis and evaluation of triazole functionalized ring-fused 2-pyridones as antibacterial agents. Eur J Med Chem 2012;54:637-46.
- Haider S, Alam MS, Hamid H, Shafi S, Nargotra A, Mahajan P, et al. Synthesis of novel 1,2,3-triazole based benzoxazolinones: Their TNF-a based molecular docking with in-vivo anti-inflammatory, antinociceptive activities and ulcerogenic risk evaluation. Eur J Med Chem 2013;70:579-88.
- Garudachari B, Isloor AM, Satyanarayana MN, Fun HK, Hegde G. Click chemistry approach: Regioselective one-pot synthesis of some new 8-trifluoromethylquinoline based 1,2,3-triazoles as potent antimicrobial agents. Eur J Med Chem 2014;74:324-32.
- Piotrowska DG, Balzarini J, Glowacka IE. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur J Med Chem 2012;47:501-09.
- Ullmann, F. On a new formation of diphenylamine derivatives. [machine translation]. Ber Dtsch Chem Ges 1903;36:2382-84.
- Jayakumar KN, John NA, Kimberly Y, Kekeli A. Ekoue-Kovi, Leah B, et al. 4-N-, 4-S-, and 4-O-Chloroquine Analogues: Influence of Side Chain Length and Quinolyl Nitrogen pKaon Activity vsChloroquine Resistant Malaria. J Med Chem 2008;51:3466-79.
- Rokhum L, Bez G. A practical one-pot synthesis of azides directly from alcohols. J Chem Sci 2012;124(3):687-91.
- Fahmi H, Timothy L, Robert H, Vsevolod V. Rostovtsev, Louis N, et al. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J Am Chem Soc 2005;127:210-16
- Van de LAA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 1994;174:311-20.
- Slater TF, Sawyer B, Straeuli U. Studies on succinate-tetrazoliumreductase systems.&Iii Points of Coupling of Four Different Tetrazolium Salts. Biochim Biophys Acta&1963;77:383-93.