- *Corresponding Author:
- Nalini v. Purohit
Department of Chemistry, Faculty of Science, The Maharaja Sayajirao University of Baroda Vadodara-390 002, India
E-mailnalinipurohit22@gmail.com
| Date of Submission | 22 September 2010 |
| Date of Revision | 14 April 2011 |
| Date of Acceptance | 18 April 2011 |
| Indian J Pharm Sci, 2011, 73 (2): 171-178 |
Abstract
In this paper we report the synthesis of a new family of 4-alkyl isocoumarin derivatives having bromo carbonyl and amino carbonyl group at 3rd position of the heterocyclic ring. Synthesis, spectral analysis and bioactivity of new isocoumarin derivatives are discussed in this paper. Some of the synthesized compounds displayed comparable antibacterial activity and some of the new compounds showed an interesting inhibitory effect on the growth of four pathogen fungi involved in plant diseases. A fair number of compounds were found to have good analgesic property on comparing with standard drug analgin.
Keywords
Antibacterial, antifungal, analgesic activities, isocoumarin, o-acyl benzoic acids, substituted bromoacetophenones
Synthesis and biological properties of isocoumarin derivatives incorporated with biologically active heterocycles have been reported for the past several years [1-4]. These compounds are of intense interest because of their broad antibacterial spectrum against both gram positive and gram negative bacteria and has drawn considerable attention of a number of investigators due to their varied biological and physiological activities apart from activities such as blood pressure lowering [5], anticoagulant [6], antifungal [7], antimicrobial [8,9], antiinflammatory [10,11] and antiangiogenic [12].
Though many of our synthesized compounds showed promising pharmacological properties on preliminary evaluation, to reach definitive conclusions regarding their therapeutic potential, many more compounds needed to be synthesized and screened. Therefore, it was proposed to synthesize some new isocoumarin derivatives containing other biologically potent moieties such as piperidine, and morpholine, which were present in standard drugs being used in market for various indications. These biheterocyclic compounds would also help to increase the understanding of structural activity relationship and for which a lot of biological parameters need to be studied. Furthermore, there are no studies in the literature, which reported coupling of isocoumarin ring with another biologically active nucleus either directly or through a carbon bridge.
There are several examples reported in literature where the presence of nitrogen atom in compounds, in various forms, has shown tremendous therapeutic applications. In continuation of our efforts to adapt heterocyclization chemistry to a high-throughput format, we chose to introduce nitrogen atom in isocoumarin moiety in the form of an amino group, to see its effect on the remedial features of isocoumarin. The lone pair of electrons on nitrogen imparts it the unique feature to act as a proton acceptor, which makes it one of the largest acid scavengers used in the synthesis of pharmaceuticals [13]. Bacteriostatic activity of tertiary amines and quaternary ammonium salts, p-toludine moiety has been reported long back [14].
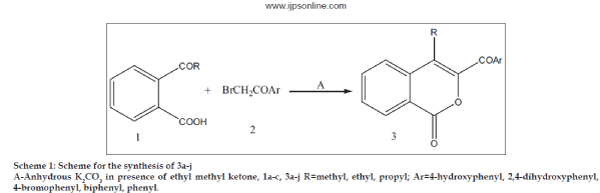
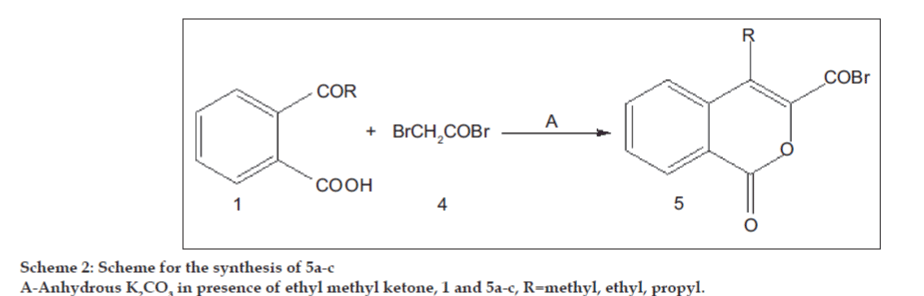
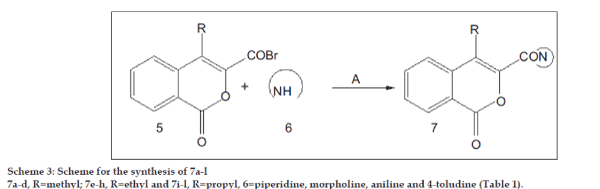
It was suggested that compounds exhibiting antimicrobial activity might act either by killing the microbes or by blocking their active site. The literature survey revealed that very little work has been done on the antimicrobial effect of aroyl substituted isocoumarins. Hence, this paper reports the synthesis, antibacterial, antifungal and analgesic activity of 4-alkyl-3-aroyl isocoumarin derivatives (Scheme 1 and 2) and 4-alkyl-3-amino carbonyl isocoumarin derivatives (Scheme 3).
Isocoumarin frame-work plays an essential role in making the compounds biologically active. In continuation to our previous work [5], we have disclosed an efficient synthesis of some new isocoumarin derivatives by condensing different o-acyl benzoic acids (1a-c) with bromoacetophenone derivatives (2a-j) (Scheme 1) and bromoacetylbromide (4) in presence of K2CO3 in ethylmethylketone for 10-12 h (Scheme 2). This was a convenient route to the target compounds. Some isocoumarins, which we have been reported earlier (3a-c) [5] were included in this paper to report their biological activity.
We report in this paper, synthesis and pharmacological investigation of novel bi-heterocyclics bridged via carbonyl group. Isocoumarin derivative were extended to 3-amino carbonyl-4-alkylisocoumarin (7a-l) by condensing 3-bromocarbonyl-4-alkylisocoumarin 5a-c (Scheme 2) with different primary and secondary amines (6a-d) (Scheme 3).
Materials and Methods
The reagents and the solvents used in this study were of analytical grade and were used without further purification. Melting points were determined in open capillaries and have been reported uncorrected (Table 1). The purity of the compounds was checked by TLC on silica gel GF254. IR were recorded on FTIR Perkin Elmer spectrophotometer and 1H NMR spectra on a Bruker spectrometer (400 MHz) using TMS as internal standard. Mass spectrums were recorded on Thermo Scientific Corporation, DSQ II Mass Spectrometer. All compounds gave satisfactory elemental analysis. O-acyl benzoic acid 1a [15], b-c [16] and bromo derivatives 2 [17] were synthesized according to the literature method. Formation of heterocycles was supported by IR, NMR and Mass spectra. Progress, purity of the reaction and intermediates were analyzed using pre-coated TLC plates and UV chamber.
| Code | R | Substitution at 3rd | MP0 | Mol. | % Yielda | C % | H % | N % |
|---|---|---|---|---|---|---|---|---|
| position | formula | (Cal) | (Cal) | (Cal.) | ||||
| 3a | Methyl | 4-hydroxy phenyl | 217 | C17H12O4 | 69.30 | 72.82 (72.85) | 4.30 (4.28) | - |
| 3b | Methyl | 2,4-dihydroxy phenyl | 110 | C17H12O5 | 76.05 | 68.95 (68.93) | 4.21 (4.05) | - |
| 3c | Methyl | biphenyl | 145 | C23H16O3 | 62.73 | 81.59 (81.11) | 4.83 (4.70) | - |
| 3d | Methyl | 4-bromo phenyl | 172 | C17H11O3Br | 57.80 | 59.07 (59.49) | 3.30 (3.20) | - |
| 3e | Ethyl | 4-bromo phenyl | 115 | C18H13O3Br | 49.00 | 60.50 (60.52) | 3.72 (3.64) | - |
| 3f | Ethyl | 4-methoxy phenyl | 140 | C18H16O4 | 45.00 | 72.90 (72.97) | 5.41 (5.40) | - |
| 3g | Propyl | 4-bromo phenyl | 132 | C19H15O3Br | 52.00 | 61.52 (61.47) | 4.08 (4.04) | - |
| 3h | Propyl | 4-hydroxy phenyl | 92 | C19H16O4 | 56.75 | 74.00 (74.02) | 5.21 (5.46) | - |
| 3i | Propyl | biphenyl | 110 | C25H20O3 | 72.14 | 81.49 (81.52) | 5.40 (5.43) | - |
| 3j | Propyl | phenyl | 121 | C17H12O3 | 73.09 | 77.30 (77.27) | 4.62 (4.54) | - |
| 5a | Methyl | bromo carbonyl | 94 | C11H7O3Br | 60.93 | 49.00 (49.45) | 2.50 (2.62) | - |
| 5b | Ethyl | bromo carbonyl | 52 | C12H9O3Br | 56.86 | 51.30 (51.26) | 3.41 (3.20) | - |
| 5c (líq.) | Propyl | bromo carbonyl | >200 | C13H11O3Br | 47.95 | 52.54 (52.89) | 3.70 (3.73) | - |
| 7a | Methyl | piperidine | 79 | C16H17O3N | 65.32 | 70.52 (70.84) | 6.38 (6.27) | 4.94 (5.16) |
| 7b | Methyl | morpholine | 61 | C15H15O4N | 65.02 | 65.46 (65.93) | 5.53 (5.49) | 4.97 (5.12) |
| 7c | methyl | aniline | Semi | C17H13O3N | 35.6 | 73.28 (73.11) | 4.72 (4.65) | 5.12 (5.01) |
| solid | ||||||||
| 7d | methyl | 4-toludine | 78 | C18H15NO3 | 35.00 | 73.86 (73.72) | 5.50 (5.11) | 4.48 (4.77) |
| 7e (líq.) | ethyl | piperidine | 130 | C17H19O3N | 52.27 | 71.12 (71.57) | 6.38 (6.66) | 4.94 (4.91) |
| 7f | ethyl | morpholine | 115 | C16H17O4N | 75.97 | 67.09 (66.89) | 7.09 (5.92) | 5.63 (4.87) |
| 7g | ethyl | aniline | 140 | C18H15O3N | 55.61 | 73.54 (73.72) | 5.42 (5.11) | 4.97 (4.77) |
| 7h | ethyl | 4-toludine | 145 | C19H17O3N | 37.82 | 74.02 (74.26) | 5.42 (5.53) | 4.92 (4.56) |
| 7i (líq.) | propyl | piperidine | > 220 | C18H21O3N | 63.27 | 72.12 (72.24) | 6.98 (7.02) | 4.90 (4.68) |
| 7k | propyl | aniline | 135 | C21H17O3N | 52.59 | 74.09 (74.26) | 5.46 (5.53) | 4.32 (4.56) |
| 7l | propyl | 4-toludine | 190 | C20H15O3N | 69.24 | 75.75 (74.76) | 6.78 (5.91) | 4.95 (4.36) |
Table 1 Physical data of the synthesized compounds.
Synthesis of 4-propyl-3-(4-phenyl benzoyl) isocoumarin (3i)
o-Butyric benzoic acid (2 g, 0.010 mole) (1c), p-phenyl bromoacetophenone (2.86 g, 0.010 mole) (2c), K2CO3 (3.017 g, 0.0218 mole) and ethyl methyl ketone were taken in a round bottom flask and was refluxed for 10-12 h using magnetic stirrer at 80-90º. Reaction mixture was monitored by TLC. Solvent was then removed, 20-30 ml water added and product was extracted with 100 ml ethyl acetate. Solvent layer was first washed with saturated sodium bicarbonate, then with water and it was dried over anhydrous Na2SO4. After removal of solvent the crude product was purified by column chromatography. Elution with solvent system petroleum ether (60-80º)-ethyl acetate (95:5) gave pure compound as yellow crystalline solid 3i. Same procedure was followed for compounds 3a-h and 3j.
Synthesis of 3-bromo carbonyl-4-methyl isocoumarin (5a)
o-Acetyl benzoic acid (2 g, 0.012 mole) (1a), bromoacetyl bromide (1.06 ml, 0.012 mole) (4), K2CO3 (3.53 g, 0.025 mole) and ethyl methyl ketone were taken in a round bottom flask and refluxed for 10-12 h at 80-90º using magnetic stirrer. The purity of the compound was tested with TLC using solvent system petroleum ether (60-80º) -ethyl acetate (98:2). Work up after solvent system gave pure white crystals. The same procedure was followed to yield compounds 5a-c respectively (Scheme 2).
Synthesis of 4-methyl-3-piperdinyl carbonyl isocoumarin (7a)
3-Bromo carbonyl-4-methyl isocoumarin (2.0 g, 0.0074 mole) (5a), piperidine (6a) (1.55 ml, 0.015 mole) and DMF was refluxed on sand bath for 3-5 h. The reaction mixture was monitored by TLC and after cooling reaction mixture was poured on crushed ice. The product was filtered and purified by column chromatography using petroleum ether (60-80º) and ethyl acetate as eluent to yield pure compound as white crystalline solid 7a-l (Scheme 3).
Characteristics of 3a-i
3a; IR (KBr) cm-1: 1730 (-C=O, aroyl), 1758 (-C=O, lactone) (3a-i), (5a-c), 1H NMR δ: 1.9 (s, 3H, CH3), 7.30-7.90 (m, 7H, aromatic protons), 8.20 (dd, 1H, C8-H), 12.5 (s, 1H, OH); ms m/z: 280 (M+), 265, 263, 187, 159, 146 and 121. 3b; 1H NMR δ: 2.5 (s, 3H, CH3), 6.3-7.9 (m, 6H, aromatic protons), 8.2 (d, 1H, C8-H), 12.4 (s, 1H, OH), 12.6 (s, 1H, OH); ms m/z: 295 (M+-1), 281, 236, 221, 185, 149, 121 and 110. 3c; 1H NMR δ: 2.2 (s, 3H, CH3), 7.20-7.90 (m, 12H, aromatic protons), 8.43-8.45 (dd, 1H, C8-H); ms m/z: 342 (M++2), 325, 312, 187, 159, 154 and 146. 3d; 1H NMR δ: 1.50 (s, 3H, CH3), 6.80-8.00 (m, 7H, aromatic protons), 8.35 (d, 1H, C8-H); ms: m/z: 343.9 (M++1), 263, 220, 183.9, 155.9, 105 and 77. 3e; 1H NMR δ: 1.1 (t, 3H, CH3), 1.7 (q, 2H, CH2), 7.60-8.00 (m, 7H, aromatic protons), 8.40 (dd, 1H, C8-H); ms, m/z: 357.9 (M++1), 341.9, 277, 262, 234, 185, 182.9, 173, 154.9, 145, 117 and 76. 3f: 1H NMR δ: 1.3 (t, 3H, CH3), 2.8 (q, 2H, CH2), 4.0 (s, 3H, OCH3), 6.95- 8.05 (m, 7H, aromatic protons), 8.41-8.43 (dd, 1H, C8-H); ms m/z 308 (M+), 262, 187, 146, 135 and 108. 3g: 1H NMR δ: 1.0 (t, 3H, CH3), 1.7 (m, 2H, CH2), 2.8 (t, 2H, CH2), 7.60-7.95 (m, 7H, aromatic protons), 8.40-8.45 (dd, 1H, C8-H); ms m/z: 370.9 (M+), 300, 276, 262, 214, 187, 157, and 146. 3h; 1H NMR δ: 1.1 (t, 3H, CH3), 1.7 (m, 2H, CH2), 2.7 (q, 2H, CH2), 6.87 (s, 1H, OH), 7.50-7.90 (m, 7H, aromatic protons), 8.0 (d, 1H, C8-H); ms m/z 308 (M+), 294, 280, 252, 236, 215, 186,172, 157, 146 and 121. 3i; 1H NMR δ: 1.0 (t, 3H, CH3), 1.7 (m, 2H, CH2), 2.8 (t, 2H, CH2), 7.40-7.95 (m, 12H, aromatic protons), 8.42-8.44 (d, 1H, C8-H); ms m/z 369 (M++1), 325, 297, 214, 154 and 146.
Characteristics of 7a-l
7a; IR (KBr) cm-1: 1728 (-C=O, aroyl), 1760 (-C=O, lactone), 1654 (-CON-) (7a-l), 1H NMR δ: 1.9 (s, 3H, CH3), 1.5 (m, 6H, CH2-CH2-CH2), 3.4 (t, 4H, CH2-N-CH2) 7.2-7.6 (m, 3H, aromatic protons), 7.9 (d, 1H, C8-H); ms m/z: 271 (M+), 256, 186, 160, 146 and 118. 7b; 1H NMR δ: 2.1 (s, 3H, CH3), 3.2 (t, 4H, CH2-N-CH2), 3.6 (t, 4H, CH2-O-CH2), 7.3-7.6 (m, 3H, aromatic protons), 7.8-7.9 (dd, 1H, C8-H); ms m/z: 273 (M+), 258, 245, 187, 159 and 146. 7c; 1H NMR δ: 1.8 (s, 3H, CH3), 7.3-7.6 (m, 8H, aromatic protons), 7.9 (d, 1H, C8-H), 9.5 (s, 1H, NH); ms m/z: 279 (M+), 264, 188, 187, 159 and 146. 7d; 1H NMR δ: 1.9 (s, 3H, CH3), 2.4 (s, 3H, CH3), 7.1-7.6 (m, 7H, aromatic protons), 8.0 (d, 1H, C8-H), 13.1 (s, 1H, NH); ms m/z: 293 (M+), 265, 263, 203, 159, 146, 120 and 77. 7e; 1H NMR δ: 1.3 (t, 3H, CH3), 2.2 (q, 2H, CH2), 1.6 (m, 6H, CH2-CH2-CH2), 3.4 (t, 4H, CH2-N-CH2) 7.3-7.5 (m, 3H, aromatic protons), 8.0 (d, 1H, C8-H); ms m/z: 28 (M+-1), 257, 256, 201, 173 and 146. 7f; 1H NMR δ: 1.2 (t, 3H, CH3), 2.9 (q, 2H, CH2), 3.2 (t, 4H, CH2-N-CH2), 3.6 (t, 4H, CH2-O-CH2), 7.3-7.6 (m, 3H, aromatic protons), 7.8-7.9 (dd, 1H, C8-H); ms m/z: 287 (M+), 272, 258, 201, 187 and 146. 7g; 1H NMR δ: 1.1 (t, 3H, CH3), 1.9 (q, 2H, CH2), 7.3-7.7 (m, 8H, aromatic protons), 8.0 (d, 1H, C8-H), 11.0 (s, 1H, NH); ms m/z: 293 (M+), 251, 216, 161, 118 and 77. 7h; 1H NMR δ: 1.0 (t, 3H, CH3), 2.1 (q, 2H, CH2), 2.4 (s, 3H, CH3), 7.1-7.5 (m, 7H, aromatic protons), 8.0 (d, 1H, C8-H), 9.8 (s, 1H, NH); ms m/z: 307 (M+), 292, 279, 277, 173, 146 and 134. 7i; 1H NMR δ: 1.0 (t, 3H, CH3), 1.5 (m, 6H, CH2-CH2-CH2), 1.7 (m, 2H, CH2), 2.1 (t, 2H, CH2), 3.6 (t, 4H, CH2-N-CH2) 7.3-7.5 (m, 3H, aromatic protons), 8.1 (d, 1H, C8-H); ms m/z: 298 (M+-1), 265, 256, 208, 146 and 86. 7j; 1H NMR δ: 1.0 (t, 3H, CH3), 1.8 (m, 2H, CH2), 2.4 (q, 2H, CH2), 3.4 (t, 4H, CH2-N-CH2), 3.7 (t, 4H, CH2-O-CH2), 7.4-7.6 (m, 3H, aromatic protons), 7.9 (d, 1H, C8-H); ms m/z: 301 (M+), 258, 215, 173, 146, 86 and 77. 7k; 1H NMR δ: 1.0 (t, 3H, CH3), 1.7 (m, 2H, CH2), 2.0 (q, 2H, CH2), 7.2-7.7 (m, 8H, aromatic protons), 8.0 (d, 1H, C8-H), 9.0 (s, 1H, NH); ms m/z: 307 (M+), 264, 236, 173, 145 and 77. 7l; 1H NMR δ: 0.7 (t, 3H, CH3), 1.0 (m, 2H, CH2), 2.0 (m, 2H, CH2), 2.4 (s, 3H, C4’-CH3), 3.4 (s, 1H, NH), 7.1-7.7 (m, 7H, aromatic protons), 7.8-7.9 (dd, 1H, C8-H); ms m/z: 322 (M++1), 306, 293, 278, 264 and 173.
Antimicrobial and Analgesic Activity
Antibacterial and antifungal activity of new compounds were tested in vitro in bacterial strains, Staphylococcus aureus and Escherichia coli, fungal strains of Thielaviopsis paradoxa, Phomopsis mangiferae, Fusarium pallidoroseum, Colletotrichum capsici using serial agar dilution (cup plate method) [18], Potato Dextrose Agar medium (Poisoned Food Technique) [19] respectively, analgesic activity in mice (both male and female) by tail flick method [20].
The two microorganisms were cultured in dishes containing agar medium, four cups (8 mm) were put onto the dishes and each tested compound (0.1 ml of 2 mg/ml) was added into the cups under aseptic condition. Then the dishes were incubated at 370 for 24 h. The zone of inhibition of the growth of the bacteria, which were produced by diffusion of the compounds from the cup into the surrounding medium, was measured to evaluate the antibacterial activity. Each experiment was repeated twice. DMF was used as a positive control for the experiments and the results were compared against standard drug ampicillin (Table 2).
The standard fungal culture T. paradoxa, P. mangiferae, F. pallidoroseum and C. capsici were grown on PDA slants at room temperature. Mycelial growth inhibition of T. paradoxa, P. mangiferae, F. pallidoroseum and C. capsici was evaluated by the poisoned food technique [19], where the inhibition in growth of the fungal strain was observed on PDA. The stock solutions (1000 ppm) were made from each of the test compounds. The required % concentrations of the compounds (mg/ml) were obtained by mixing the appropriate amount of the stock solution with 20 ml of molten PDA. The amended PDA was poured into Petri dishes and allowed to set.
An inoculum of the fungus obtained from 7 days old axenic culture, grown as above, was placed at the centre of the amended agar medium. Each experiment was performed in triplicate. The diameter of the fungal colony was measured after 4 days and then 7 days at 26±10 and the % inhibition was calculated using the Eqn, % inhibition= (growth area in reference-growth area in sample)/growth area in reference×100 (Table 3).
Analgesic activity of the compounds was determined by tail flick method [20]. One hundred and eight mice of either sex weighing between 20-25 g, which shows positive response were selected and divided into 10 groups with four mice in each group. The first group served as control, which received 2% gum acacia. Second group served as standard, which received analgin at a dose of 50 mg/kg body weight orally. Groups 3-10 received 8 test compounds at a dose of 50 mg/kg body weight of mouse, orally.
| Code | R | Ar/Amine/substitution at 3 rd position | S. aureus | E. coli |
|---|---|---|---|---|
| 3a | methyl | 4-hydroxy phenyl | 12 | 14 |
| 3b | methyl | 2,4-dihydroxy phenyl | 13 | 14 |
| 3c | ethyl | biphenyl | 13 | 13 |
| 3f | ethyl | 4-methoxy phenyl | 14 | 14 |
| 3g | propyl | 4-bromo phenyl | 11 | 13 |
| 5b | ethyl | bromo carbonyl | 0 | 11 |
| 5c | propyl | bromo carbonyl | 16 | 14 |
| 7e | ethyl | piperidine | 12 | 11 |
| 7f | ethyl | morpholine | 0 | 15 |
| 7g | ethyl | aniline | 11 | 11 |
| 7j | propyl | morpholine | 11 | 15 |
| 7k | propyl | aniline | 11 | 11 |
| 7l | propyl | 4-toludine | 0 | 17 |
| Control (DMF) | - | - | 0 | 11 |
| Standard | - | - | 15 | 5 |
Table 2Antibacterial activity
| Code | R | Ar/Amine/substitution at 3rd position | T. paradoxa | P. mangifera | F. pallidoroseum | C. capsci |
|---|---|---|---|---|---|---|
| 3a | methyl | 4-hydroxy phenyl | - | - | 70.00 | - |
| 3b | methyl | 2,4-dihydroxy phenyl | - | - | 70.60 | 46.20 |
| 3f | ethyl | 4-methoxy phenyl | 26.38 | 66.66 | - | - |
| 3g | propyl | 4-bromo phenyl | 21.21 | 20.58 | - | - |
| 3j | propyl | phenyl | - | - | 86.67 | - |
| 5c | propyl | bromo carbonyl | - | 80.53 | - | - |
| 7j | propyl | 4-toludine | 37.57 | 57.27 | - | - |
| Standard | - | - | 44.70 | 45.00 |
Table 3Antifungal activity
| Code | R | Ar/Amine/substitution | Dose (mg/kg) | Average±SE reaction time (s) Time after drug treatment | ||||
|---|---|---|---|---|---|---|---|---|
| at 3rd position | body weight | |||||||
| 0 | 30 | 60 | 90 | |||||
| Control | - | - | - | 3.01±0.358 | 3.20±0.288 | 3.10±0.358 | 3.02±0.00 | |
| Standard | - | - | 50 | 3.09±0.408 | 5.25±0.249 | 7.75±0.249 | 9.00 ±0.000 | |
| 3a | methyl | 4-hydroxy phenyl | 50 | 3.01±0.00 | 4.08±0.408 | 4.02±0.408 | 4.26±0.408 | |
| 3b | methyl | 2,4-dihydroxy phenyl | 50 | 4.04±0.408 | 4.58±5.77 | 6.35±0.50 | 7.70±0.249 | |
| 3d | methyl | 4-bromo phenyl | 50 | 3.69±0.408 | 5.34±0.249 | 6.51±0.408 | 5.56±0.408 | |
| 3e | ethyl | 4-bromo phenyl | 50 | 2.71±0.245 | 3.72±0.245 | 4.78±0.381 | 6.06±0.577 | |
| 3f | ethyl | 4-methoxy phenyl | 50 | 3.00±0.408 | 4.50±0.408 | 5.50±0.577 | 6.25±0.249 | |
| 3h | propyl | 4-hydroxy phenyl | 50 | 2.72±0.249 | 5.33±0.577 | 8.35±0.456 | 8.22±0.456 | |
| 7f | ethyl | morpholine | 50 | 3.09±0.408 | 4.40±0.408 | 7.77±6.249 | 8.65±0.249 | |
| 7g | ethyl | aniline | 50 | 4.00±0.408 | 4.75±0.50 | 6.25±0.353 | 7.25±0.50 | |
Table 4 Analgesic activity
The tail of the mouse was dipped (up to 5 cm) in a water bath at 55±0.7º. The time taken to withdraw the tail clearly out of water was considered as the reaction time with the cut-off time being 60 s. The first reading was taken immediately after administration of the standard drug and test compounds and afterwards at the intervals of 30 min. The response time was recorded and the results are described in (Table 4).
Results and Discussion
The required starting materials bromoacetophenone derivatives and o-acyl benzoic acid to accomplish the synthesis of title compounds, 4-alkyl-3-aroyl isocoumarins, 4-alkyl-3-bromocarbonyl isocoumarins and 4-alkyl-3-amino carbonyl isocoumarins, was refluxed in presence of anhydrous K2CO3 in ethyl methyl ketone at 80-90º for 8-10 h (Scheme 1 and 2). Both condensation as well as cyclization occurs in single step and in good yield. The reaction of 4-alkyl-3-bromocarbonyl isocoumarin (Scheme 2) with aniline/p-toludine as primary amine and morpholine/piperidine as secondary amine in DMF for 5 h resulted in 4-alkyl-3-amino carbonyl isocoumarins in moderate yield. The synthetic route is shown in Scheme 3. However, the desired condensation and yield was successful only when the reaction was carried out with secondary amine (60–70%), condensation with primary amines in most of the compounds resulted 45% yield. The selection of substituted bromoacetophenone and amines was based on presence of electron withdrawing and electron releasing groups, which would assist in later studies as structure activity relationship.
All compounds (3a-l) (Scheme 1) showed absorption at 1730 cm-1 for aroyl carbonyl and 1758 cm-1 for lactonic carbonyl as functional groups. A singlet of methyl group of isocoumarin moiety at d 2.5, quartet and triplet d 2.89 and 1.4 for ethyl group and triplet, multiplet and triplet d 2.8, 1.7 and 1.0 for propyl group confirms the CH3, CH2CH3 and CH2CH2CH3, respectively at 4th position of isocoumarin ring. All aromatic protons shows signals between d 7.2-7.9 and the proton at 8th position of isocoumarin ring show a characteristic doublet at d 8.4 and the presence of the hydroxy of aroyl group situated at 4th position of phenyl ring in compounds 3a, 3b and 3h is confirmed by the IR absorption at 3182 cm-1. In NMR spectra the hydroxy proton shows signal along with the aromatic protons. The singlet for methoxy group in 3f is obtained at d 3.1. Mass spectra of compound 3c (molecular mass=354) shows molecular ion peak M/Z at 355 (M++1).
In (Scheme 2) 5a-c, IR spectra show absorptions at 1750 cm-1 for lactonic carbonyl and 1850 cm-1 for bromocarbonyl group. All these compounds in Scheme 2 were characterized by IR and elemental analysis only due to their instability.
IR absorptions of compounds in (Scheme 3), shows signals at 1728 cm-1 for aroyl carbonyl, 1760 cm-1 for lactonic carbonyl and 1654 cm-1 for –CON-, for all compounds. NMR spectrum of Compounds 7a, b, e, f and j having secondary amine moiety as morpholine and piperidine ring show signals at d 2.67 (s) for CH3, d 1.1-1.3 (t), d 3.0 (q) for CH2CH3 and d 2.9 (t), d 1.6 (m), d 0.9 (t) for CH2CH2CH3 at 4th position and, d 3.6 (q, 4H, -CH2-O-CH2), d 3.2 (q, 4H, -CH2-N-CH2) for morpholine ring, d 7.3-7.9 (m, 4H, aromatic protons) of isocoumarin ring . Compounds 7d, h and l having primary amine moiety as p-toludine show NMR signals at d 2.7 (s) for CH3 d 2.9 (q), d 1.5 (t) for CH2CH3 and d 0.7-0.8 (q), d 1.2 (m), d 2.0 (q) for CH2CH2CH3 at 4th position of isocoumarin ring and for CH3 substituted with phenyl ring at 3rd position shows d 2.4 (s, 3H, -CH3), d 7.1- 7.7 (m, 8H, aromatic protons), and the characteristic singlet of the –NH- at d 3.4 (Scheme 3).
In addition to this the mass spectra of compound 7l (molecular mass 321) shows base peak at 279 for (M+-CH2CH2CH3). The other peaks obtained in mass spectra are 263 and 175 for (M+-CH2CH2CH3, CH3) and (M+-CH2CH2CH3, CONHC6H5CH3) for the same compound.
All compounds were screened for antibacterial activity towards different strains of S. aureus and E. coli at concentration 0.1 mg/ml compared to standard drug ampicillin. All compounds show good zone of inhibition against gram -ve bacteria than gram +ve bacteria. Few compounds were screened for antifungal activity towards different fungal species at concentration 1 mg/ml. Based on the structure activity relationship it can be concluded that length of alkyl chain at 4th position of isocoumarin ring does make a difference. With increase in carbon chain, activity increases, which is found with compound 3j and 5c (Table 3) where 3-carbon alkyl chain (propyl group) showed excellent activity against Fusarium pallidoroseum and Phomopsis mangiferae.
The potential antimicrobial activity of compounds 3a-c, g, 5b, c, 7e-g and 7j-l towards S. aureus (gram +ve) bacteria and E. coli (gram –ve) bacteria, antifungal activity towards Thielaviopsis paradoxa, Phomopsis mangiferae, Fusarium pallidoroseum and Colletotrichum capsici was investigated (Table 2). The experiments have revealed that in isocoumarin, aroyl group substituted at 3rd position of isocoumarin moiety with electron releasing groups as well as bromo carbonyl group gives much better results against S. aureus bacteria and the zone of inhibition was found to be maximum with bromocarbonyl group rather than aroyl group substituted with electron releasing group. Length of alkyl chain at 4th position does not make any difference in isocoumarin derivatives (Scheme 2), however, alkyl chain length does affect the activity in isocoumarins having amide linkage at 3rd position (Scheme 3). Antibacterial activity against E. coli (gram –ve) was found to be maximum with 7j (Table 1) having long alkyl chain (propyl) and secondary amide group and activity was found to be moderate with 7e and 7f having two carbon chain (ethyl) at 4th position. Isocoumarins having amino carbonyl at 3rd position, (7f and 7j) (Scheme 3) had no inhibitory activity. But all the isocoumarins were found to be active against E. coli bacteria (Table 2).
3a-b, f, g, i, j, 5b, 7f and 7k were tested for their microbial activity using four fungal species. All isocoumarins shows moderate inhibition against Thielaviopsis paradoxa and good inhibition against Phomopsis mangiferae. With Fusarium pallidoroseum compounds 3a, 3b and 3j shows excellent inhibition, while 3b was found to inhibit Colletotrichum capsici. (Table 3).
3a-c, d-f, h, 7f, g were tested for analgesic activity and the results are presented in (Table 4). Here also like antimicrobial activity isocoumarin derivatives with electron releasing groups (3a, 3f, 3h) shows better activity than having electron-withdrawing group (3d, 3e). However, presence of two electronreleasing groups in single moiety drastically reduces the response time (3b) as compared to those having single electron releasing group. Isocoumarins with amide linkage (both secondary and tertiary) shows very good response time, almost comparable to the standard drug.
Acknowledgements
Authors are grateful to Sun Pharmaceutical Industries Ltd. Vadodara and SAIF, Punjab University for providing facility of NMR spectral analysis; to Prof. Anjana Desai, Head and Mrs. Aparna S of Microbiology Department, The M. S. University Baroda for antibacterial screening; to Prof. Arun Arya, Head, Department of Botany, The M. S. University, Baroda for antifungal screening; to Mr. G. Paramesh, Department of Chemistry, Gulberga University for analgesic activity and University Grants Commission, New Delhi for financial support.
References
- Heynekamp JJ, Hunsaker LA, Vander Jagt TA, Royer RE, Decka LM, Vander Jagt DL. Isocoumarin-based inhibitors of pancreatic cholesterol esterase. Bioorg Med Chem 2008;16:5285-94.
- Baojian L, Binhua Z, Hailiang L, Lin M, Ai-Yun P. Phosphaisocoumarins as a new class of potent inhibitors for pancreatic cholesterol esterase. Eur J Med Chem 2010;45:1955-63.
- Devienne KF, Ca´lgaro-Helena AF, Dorta DJ, Prado MR, Raddi MG, Uyemura WA, et al. Antioxidant activity of isocoumarins isolated from Paepalanthus bromelioides on mitochondria. Phytochemistry 2007;68:1075-80.
- Bihel F, Gilles Q, Lelouard H, Petit A, Costa CA, Pourquie´ O, et al. Synthesis of new 3-alkoxy-7-amino-4-chloro-isocoumarin derivatives as new ß-amyloid peptide production inhibitors and their activities on various classes of protease. Bioorg Med Chem 2003;11:3141-52.
- Purohit NV. Synthesis and studies on biological activities of some substituted 2-benzopyran-1H-2-oxo-benzopyran-3-carboxylic acids and 2-benzofuran-1H-one. Indian J Chem 2001;40B:222-7.
- Kam CM, Kerrigan JE, Plaskon RR, Duffy EJ, Lollar P, Suddath FL, et al. Mechanism-based isocoumarin inhibitors for blood coagulationserine proteases. Effect of the 7-substituent in 7-Amino-4-chloro-3-(isothioureidoalkoxy) isocoumarins on inhibitory and anticoagulant potency. J Med Chem 1994;37:1298-306.
- Hussain MT, Rama NH, Hameed S, Malik A, Khan KM. Chemistry of isocoumarins: Synthesis and biological screenings of homalicine and dihydrohomalicine. Nat Prod Res 2005;19:41-51.
- Pinchuk IV, Bressolier P, Verneuil B, Urdaci MC. in vitroanti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Chemother2001;45:3156-61.
- Zhang W, Krohn K, Draeger S, Schulz B. Bioactive isocoumarins isolated from the endophytic fungus Microdochium bolleyi. J Nat Prod 2008;71:1078-81.
- Stasi LC, Camuesco D, Nieto D, Vilegas W, Zarzuelo A, Galvez J. Intestinal antiinflammatory activity of paepalantine, an isocoumarin isolated from the capitula of Paepalanthus bromelioides, in the trinitrobenzenesulphonic acid model of rat colitis. Plant Medica 2004;70:315-20.
- Chinworrungsee M, Kittakoop P, Isaka M, Chanphen R, Tanticharoen M, Thebtaranonth Y. Halorosellins A and B, unique isocoumarin glucosides from the marine fungus Halorosellinia oceanic. J Chem Soc Perkin Transition 1 2002;2473-6.
- Yuan H, Junker B, Helquist P, Taylor RE. Synthesis of Anti-Angiogenic isocoumarins. Curr Org Synt 2004;1:1-9.
- Tripathi RP, Saxena N, Tiwari VK, Verma SS, Chaturvedi V, Manju YK, et al. Synthesis and antitubercular activity of substituted phenylmethyl- and pyridylmethyl amines. Bioorg Med Chem; 2006;14;8186-96.
- Linnell WH, Vora SV. Chemotherapeutic studies in Bacteriostatis Part II. Tertiary amines and Quaternary Ammonium Salts containing the skeleton of p–toluidine. J Pharm Pharmacol 1952;4;55-65.
- Newman MS. Synthesis of o-acetylbenzoic acid. An experiment for an honors organic laboratory course. J Chem Edu 1977;54:191.
- Bonneville PL. The synthesis of keto acids and ketones by the reaction of acid anhydrides with cadmium alkyls. J Org Chem 1941;6:462-6.
- Vogel AI, Tatchell AR, Furnis BS. Hannaford AJ, Smith PW. Vogel’s. Textbook of Practical Organic Chemistry. 5th ed. London: ELBS Longman; 1996. p. 1016.
- Indu MN, Hatha AA, Abirosh C, Harsha U, Vivekanandan G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeriamonocytogenes and Aeromonas hydrophila. Braz J Microbiol2006;37;153-8.
- Nene YL, Thapliyal PL. Fungicide in Plant Disease Control, New Delhi; Oxford and IBH Publications Co; 1979. p. 507.
- Kulkarni SK. Handbook of Experimental Pharmacology. Delhi: Vallabh Prakashan; 1993.