- *Corresponding Author:
- A. K. Gupta
Food Safety Laboratory (Drug and Chemical Residues), Indian Veterinary Research Institute, Izatnagar-243 122 India
E-mail: alpanakr68@gmail.com
| Date of Submission | 10-Nov-2011 |
| Date of Decision | 11-Oct-2012 |
| Date of Acceptance | 25-Oct-2012 |
| Indian J Pharm Sci, 2012, 74 (5): 481-486 |
Abstract
A series of new 1,3-dihydro-3-hydroxy-3-(2-phenyl-2-oxoethyl)-2H-indol-2-ones (1a-g) and 1,3-dihydro-3-(2-phenyl-2-oxoethylidene)-2H-indol-2-ones (2a-g) were synthesised by Knoevenagel condensation of substituted indole-2,3-diones (isatins) with various acetophenones. The synthesised compounds were characterised by their physical data, elemental, IR, 1H NMR, 13C NMR and mass spectral analyses and their in vitro antioxidant activity was determined by 2,2-diphenyl-1-picrylhydrazyl free radical scavenging assay. These compounds showed moderate to good antioxidant activities as compared with the standard, ascorbic acid. The antioxidant potential of 3-hydroxy-3-substituted oxindoles (1a-g) increased in a concentration-dependent manner from 10 to 500 μg/ml with 5-fluoro and 5-methyl analogues showing maximum activity. Of 3-aroyl methylene indol-2-ones (2a-g), majority of compounds with halogen substitution at position 5 of isatin ring exhibited good antioxidant activity within a concentration range of 5-100 μg/ml and the maximum activity was observed at 20 and 25 μg/ml concentrations. Thus, our study provides evidence that some newly synthesised isatin derivatives exhibit substantial antioxidant activity at low concentrations.
Keywords
Antioxidant activity, free radical scavenging potential, 3-substituted-2-oxindoles, synthesis
Introduction
Antioxidant compounds play a pivotal role as a health protecting factor. Scientific evidence suggests that antioxidants reduce the risk of chronic diseases including cancer and heart disease [1]. Antioxidants derived from plants include vitamin C, vitamin E, carotenes, phytates and phytoestrogens [2]. However, due to the complexity of the composition of foods, separating the active component individually from food is costly and inefficient because of the possible synergistic interactions between the antioxidant compounds in the food mixture [3]. Thus, the development of new synthetic potent antioxidant compounds is of paramount significance for quick quantification of antioxidant effectiveness in preventing diseases.
The indole core is the building block for numerous alkaloids such as psilobin, harmine, strychnine, vinca, ibogo, aspidosperma which are used as drugs [4]. Plant hormone, indole 3-acetic acid and the animal hormone melatonin, a potent antioxidant, are the most important indole derivatives [5]. 3-Hydroxyoxindoles, the natural antioxidants, which are the metabolites of indole 3-acetic acid, are isolated from rice bran, grasses and other plants [6]. Some of 3-hydroxyoxindoles found in marine fish and shellfish are reported to exhibit radical scavenging activity comparable to known powerful antioxidants like uric acid and indoles in humans [7].
Chalcones, a class of compounds present in dietary flavonoids, have been shown to possess antioxidant, oxygen scavenging and antiinflammatory properties in a variety of experimental models and can trigger the intracellular cascade of protective pathways offering a promising strategy for their therapeutic applications [8]. Although studies on the bioavailability of chalcones from food sources are limited [3], a number of synthetic chalcones are known to exhibit a wide range of biological properties including cytotoxicity and cancer chemopreventive activity [9,10]. Recent emergence of 3-methylene-2-oxindoles as a new class of antineoplastic agents has aroused considerable interest in designing 2-oxindoles as drugs in medicinal chemistry [11,12].
In design of new drugs, the development of hybrid molecules through the combination of different pharmacophores in one frame may lead to compounds with interesting pharmacological properties. Keeping in view the wide spectrum of biological activities associated with 2-oxindole derivatives [13] and the chalcones, the combination of these two moieties in the same molecule is an interesting challenge for the development of new pharmacologically active antioxidants. Since isatins and related compounds, cyclic amide (lactam), have very close structural similarity with vitamin C which is a cyclic ester (lactone), it would be interesting to study the antioxidant properties of its chalcone derivatives. In this paper, we report for the first time In vitro antioxidant activity of a new series of compounds synthesised by Knoevenagel reaction of substituted isatins with various acetophenones [14] using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay.
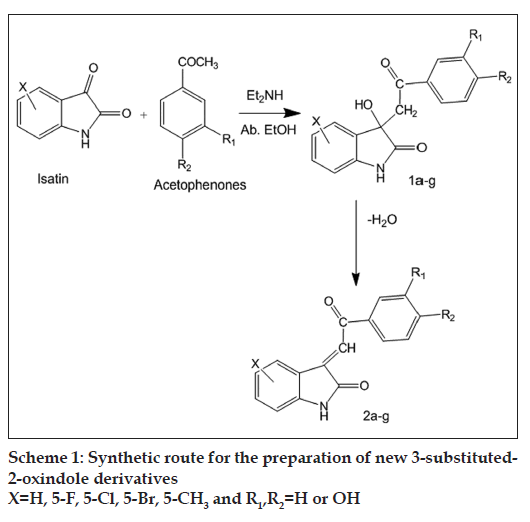
All chemicals and solvents used in this study were of analytical grade. All isatins were purchased from Sigma Chemicals, MO, USA. Other chemicals used were obtained from Himedia Chemicals, Mumbai, India. Scheme 1 illustrates the preparation of new 3-substituted-2-oxindole derivatives. Characterization of the synthesised compounds was done on the basis of their elemental studies, IR, 1H NMR, 13C NMR and mass spectral analysis. The progress of the reaction was monitored by thin layer chromatography (TLC) by using Merck silica gel coated alumina plates and UV chamber. Melting points of newly synthesised compounds were determined by using open capillary tubes in scientific melting point apparatus and are uncorrected. All spectral studies were conducted at Central Drug Research Institute, Lucknow. IR spectra were obtained on a Perkin-Elmer model 557 grating infrared spectrophotometer using KBr pellet. NMR and mass spectra of the synthesised compounds were recorded on Bruker Avance 400 spectrophotometer and Jeol SX-102 mass spectrometer, respectively.
For synthesis of 1,3-dihydro-3-hydroxy-3-(2-phenyl- 2-oxoethyl)-2H-indol-2-ones (1a-g), equimolar quantities (0.01 mol) of indole 2,3-dione (isatin) and acetophenone were refluxed on a steam bath in the presence of base, diethyl amine (0.5 ml), as catalyst in absolute ethanol (25-30 ml) for 40 min. The progress of reaction was monitored on TLC. After completion of reaction, mixture was left at room temperature for 2-3 days when light yellow shining crystals precipitated (1) out, which were filtered, dried and purified by recrystallization from ethanol. Compound Ia: Cream coloured shining flakes; Yield 90%; melting point (MP) 172°; IR (KBr, ν/cm): 3420 (OH), 3260 (NHCO), 1715 (CO-Ar), 1685 (NHCO), 1617, 1475, 1363, 1216; 1H NMR (DMSO-d6+CDCl3, δ ppm): 3.56 (s, 2H, CH2), 4.02 (s, 1H, OH), 6.12-7.9 (m, 9H, ring proton), 10.23 (s, 1H, NH); 13C NMR (δ ppm): 190.3 (s, CO-Ar), 167. 3 (CONH), 142, 136, 136, 136, 133.5, 132, 128, 126.7, 120, 118.4, 117 (aromatic carbons), 111.4 (C-3 carbon), 110.3 (CH2); MS (m/z); 267 M+. All compounds (1a-g) were synthesised in high yield and sufficient purity in a similar manner and their structural and analytical data are presented in Table 1.
| Compds | X | R1 | R2 | M.P. | Yield | Molecular | Nitrogen | |
|---|---|---|---|---|---|---|---|---|
| (0C) | (%) | Formula | Found (%) | |||||
| [Calcd %]* | ||||||||
| 1a | H | H | H | 172 | 90 | C16 H13NO3 | 5.18 [5.24] | |
| 1b | 5-F | H | H | 170 | 78 | C16 H12NO3F | 4.90 [4.91] | |
| 1c | 5-Cl | H | H | 207 | 87 | C16 H12NO3Cl | 4.60 [4.65] | |
| 1d | 5-Br | H | H | 220 | 86 | C16 H12NO3Br | 4.0 [4.04] | |
| 1e | 5-CH3 | H | H | 189 | 92 | C17 H15NO3 | 4.97 [5.02] | |
| 1f | H | H | OH | 146 | 68 | C16 H13NO4 | 4.87 [4.94] | |
| 1g | H | OH | H | 149 | 70 | C16 H13NO4 | 4.88 [4.94] | |
| 2a | H | H | H | 189 | 83 | C16 | H11NO2 | 5.48 [5.62] |
| 2b | 5-F | H | H | 187 | 81 | C16 H10 NO2F | 5.19 [5.24] | |
| 2c | 5-Cl | H | H | 154 | 88 | C16 H10 NO2Cl | 4.9 [4.94] | |
| 2d | 5-Br | H | H | 160 | 86 | C16 H10 NO2Br | 4.24 [4.26] | |
| 2e | 5-CH3 | H | H | 173 | 90 | C17 | H13NO2 | 5.23 [5.32] |
| 2f | H | H | OH | 150 | 82 | C16 | H11NO3 | 5.17 [5.28] |
| 2g | H | OH | H | 178 | 79 | C16 | H11NO3 | 5.27 [5.28] |
Table 1: Characterization Data of The Synthesized Compounds (1a-g And 2a-g)
For synthesis of 1,3-dihydro-3-(2-phenyl-2 -oxoethylidene)-2H-indol-2-ones (2a-g), a mixture of 1,3-dihydro-3-hydroxy-3-(2-phenyl- 2-oxoethyl)-2H-indol-2-one (0.01 mol, 1a), concentrated hydrochloric acid (0.5 ml) and glacial acetic acid (10 ml) were heated at 95° on a steam bath for 20-30 min. The progress of reaction was monitored on TLC using several solvent systems of different polarity. On cooling the reaction mixture, red needles were obtained which were filtered, dried and purified by recrystallization from ethanol. Compound 2a: Bright red needles; yield: 83%; MP 189°; IR (KBr, ν/cm): 3280 (NHCO), 1710 (CO-Ar), 1654 (NHCO), 1600, 1462, 1330, 1230, 1089, 1011; 1H NMR (DMSO-d6+CDCl3, δ ppm): 6.72 (s,=CH), 6.84-8.23 (m, 9H, ring proton) 10.83 (s, 1H, NH); 13C NMR (d ppm): 191.39 (s, CO-Ar), 169.81 (CONH), 141.2, 138, 137.2, 133.5, 132.5, 129.7, 129.1, 126.2, 120.9, 117.4 (aromatic carbons), 110 (C-3 carbon), 104.4 (=CH); MS (m/z): 249 M+. All the compounds (2a-g) were synthesised in high yield and sufficient purity in a similar manner and their structural and analytical data are presented in Table 1. Spectral data of all the synthesised compounds are given in Table 2.
| Compd. No. | IR ( cm-1) | 1H NMR (δ ppm) | ||
|---|---|---|---|---|
| 1a | 3420(OH), 3260(NHCO), 1715(CO), 1685(NHCO), | 3.56(s,2H,CH2), | 4.02 (s,1H,OH), 6.12-7.9(m,9H,ring | |
| 1617, 1520, 1475, 1216,1080,830,770 | protons), 10.23(s,1H,NH), | |||
| 1b | 3400(OH), 3280(NHCO), 1720(CO), 1672(NHCO), 1609, | 3.75(s,2H,CH2), | 4.20 (s,1H,OH), 6.22-8.88(m,8H,ring | |
| 1508, 1427, 1215, 929, 766 | protons), 10.46(s,1H,NH), | |||
| 1c | 3450(OH), 3230(NHCO), 1726(CO), 1675(NHCO), | 3.55(s,2H,CH2), | 4.10 (s,1H,OH), 6.72-8.38(m,8H,ring | |
| 1610, 1500, 1495 , 1210, 940, 770 | protons), 10.30 (s,1H,NH), | |||
| 1d | 3410(OH), 3300(NHCO), 1725 (CO), 1670(NHCO) | 3.3(s,2H,CH2), 4.08 (s,1H,OH), 6.62-8.43(m,8H,ring | ||
| 1607, 1510, 1420, 1216,1080,930, 770 | protons), 10.23 (s,1H,NH), | |||
| 1e | 3400(OH), 3280(NHCO), 1715(CO), 1680(NHCO) | 2.29 | (s,3H,CH3), 3.68 (s,2H,CH2), 4.15 (s,1H,OH), 6.12- | |
| 1608,1520, 1440, 1190,1040,920,766 | 8.72 | (m,8H,ring protons), 9.95 (s,1H,NH), | ||
| 1f | 3420-3310(OH), 3250(NHCO), 1720 (CO), 1670(NHCO), | 3.50 | (s,2H,CH2), 4.24 (s,1H,OH), 5.85 (s,1H,Ar-OH), | |
| 1590, 1620, 1522, 1210, 940, 765 | 6.22-8.88 (m,7H,ring protons), 10.16(s,1H,NH), | |||
| 1g | 3400-3360(OH), 3300(NHCO), 1725(CO), 1670 (NHCO), | 3.65 | (s,2H,CH2), 4.18 (s,1H,OH), 5.90 (s,1H,Ar-OH), | |
| 1609, 1508, 1427, 1210, 929, 760 | 6.62-8.43 (m,8H,ring protons), 9.96 (s,1H,NH), | |||
| 2a | 3280(NHCO), 1710(CO), 1654 (NHCO),1600(C=C) | 6.72 | (s,1H,CH), | 6.84-8.23 (m,9H, ring protons), 10.83 |
| 1462, 1330, 1230, 1089, 1011, 830, 740 | (s,1H,NH), | |||
| 2b | 3260(NHCO), 1700(CO), 1670 (NHCO),1620(C=C) 1450, | 6.68 | (s,1H,CH), | 6.8-8.79 (m,8H,ring protons), 10.70 |
| 1300, 1220, 1055, 850, 750 | (s,1H,NH), | |||
| 2c | 3200(NHCO), 1690(CO), 1660 (NHCO),1590(C=C) | 6.72 | (s,1H,CH), | 7.4-8.64(m,8H,ring protons), 10.70 |
| 1445,1300, 1250, 1230, 1089, 1011 | (s,1H,NH) | |||
| 2d | 3220(NHCO), 1700(CO), 1685 (NHCO),1610(C=C) | 6.66 | (s,1H,CH), | 7.4-8.71(m,8H,ring protons), 10.68 |
| 1450,1310,1190,1010, 830,735 | (s,1H,NH) | |||
| 2e | 3190(NHCO), 1695(CO), 1675 (NHCO),1610(C=C) | 2.27(s,3H,CH3), | 6.86 (s,1H,CH), 7.3-8.83(m,8H,ring | |
| 1460, 1310, 1200,1025,840,760 | protons), 10.68 (s,1H,NH) | |||
| 2f | 3370(OH), 3300(NHCO), 1705(CO), 1670(NHCO) | 5.65(s,1H,CH), 6.18 (s,1H,OH), 6.62-8.43(m,8H,ring | ||
| 1620(C=C) 1450,1320,1210, 1020,840,755 | proton), 9.86 (s,1H,NH) | |||
| 2g | 3180(NHCO), 1705(CO), 1640 (NHCO),1590(C=C) | 6.42 | (s,1H,CH), | 6.10 (s,1H,OH), 7.2-8.81(m,8H,ring |
| 1610(C=C), 1440,1320,1210,1005, 830,760 | proton), 10.73 (s,1H,NH) | |||
Table 2: Spectral Data Of 1,3-Dihydro-3-Hydroxy-3-(2-Phenyl-2-Oxoethyl)-2H-Indol-2-Ones (Ia-g)
In vitro antioxidant activity of the synthesised compounds was quantitatively measured by DPPH radical scavenging assay [15]. DPPH is a stable free radical at room temperature and accepts an electron or hydrogen radical to become stable diamagnetic molecule. DPPH radical is scavenged by antioxidants through the donation of proton forming the reduced DPPH. Solutions of synthesised 3-substituted oxindoles were prepared in absolute ethanol at concentrations ranging from 10 to 500 μg/ml. A DPPH blank was prepared without compound, and ethanol was used for the baseline correction. The well-known antioxidant, ascorbic acid was used for comparison or as a positive control. DPPH solution was freshly prepared daily and was kept in dark at 4° between the measurements. Briefly, 2 ml of each compound solution having different concentrations (10-500 μg/ml) were taken in different test tubes and 2 ml of 0.1 mM ethanol solution of DPPH was added and shaken vigorously. The tubes were then incubated at 37° for 30 min. Changes in absorbance were measured at 517 nm using a UV/Vis spectrophotometer and the remaining DPPH was calculated. Measurement was performed in triplicate. The radical scavenging activity was expressed as percentage inhibition of DPPH and was calculated using the equation: Radical scavenging activity (%) [(A0 - A1)/A0] ×100. Where, A0 is the absorbance of the control (blank, without compound) and A1 is the absorbance of the compound. The radical scavenging activity of ascorbic acid at various concentrations was also measured and compared with those of the newly synthesised compounds.
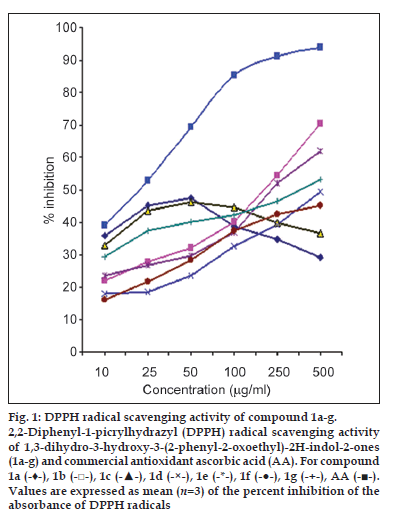
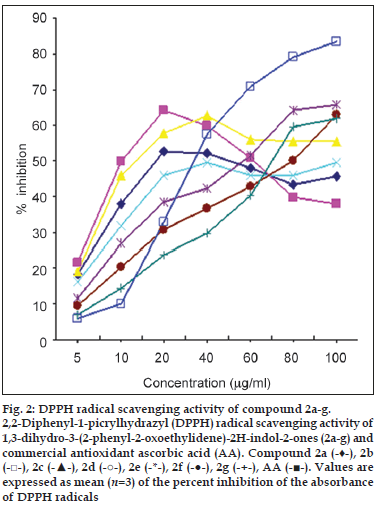
Radical scavenging activities are of great significance due to the deleterious role of free radicals in biological systems. The In vitro antioxidant properties of the newly synthesised compounds at different concentrations were examined by a well-documented assay like DPPH free radical scavenging assay. The effect of antioxidants on DPPH radicals is considered due to their hydrogen donating ability [16]. Antioxidant molecule can quench DPPH free radicals and convert them to a colourless/bleached product ultimately resulting in a decrease in the absorbance. The In vitro antioxidant activity of the synthesised compounds Ia-g and compounds 2a-g compared to ascorbic acid as standard are shown in figs. 1 and 2, respectively. Our results indicate that newly synthesised compounds showed moderate to good antioxidant activity at low concentrations as compared to ascorbic acid.
Fig. 1: DPPH radical scavenging activity of compound 1a-g. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of 1,3-dihydro-3-hydroxy-3-(2-phenyl-2-oxoethyl)-2H-indol-2-ones (1a-g) and commercial antioxidant ascorbic acid (AA). For compound 1a (-♦-), 1b (-□-), 1c (-▲-), 1d (-×-), 1e (-*-), 1f (-●-), 1g (-+-), AA (-■-). Values are expressed as mean (n=3) of the percent inhibition of the absorbance of DPPH radicals
Fig. 2: DPPH radical scavenging activity of compound 2a-g. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of 1,3-dihydro-3-(2-phenyl-2-oxoethylidene)-2H-indol-2-ones (2a-g) and commercial antioxidant ascorbic acid (AA). Compound 2a (-♦-), 2b (-□-), 2c (-▲-), 2d (-○-), 2e (-*-), 2f (-●-), 2g (-+-), AA (-■-). Values are expressed as mean (n=3) of the percent inhibition of the absorbance of DPPH radicals
In an attempt to establish some structure activity relationship based on the position and presence of different substituents and to understand as to how different functionalities have an effect on the antioxidant properties, a series of new 1,3-dihydro- 3-hydroxy-3-(2-phenyl-2-oxoethyl)-2H-indol-2-ones (1a-g) and 1,3-dihydro-3-(2-phenyl-2-oxoethyli dene)-2H-indol-2-ones (2a-g) were synthesised. These compounds were characterised by various physicochemical and spectroscopic techniques. As per chemical structural features, two different types of compounds were synthesised under the study area. It was observed that small structural modification of the molecule can greatly alter its biological activity. Based on the structure activity relationships, it is indicated that the presence of substitution on the isatin ring and on the side chain phenyl ring influences the antioxidant potency of the molecule.
The DPPH radical scavenging efficacy of 3-hydroxy-3-substituted oxindoles (1a-g), synthetic precursors of isatin chalcone; did not show a regular trend. The scavenging of DPPH radicals by most of these compounds occurred in a concentration-dependent manner from 10 to 500 μg/ml with 5-fluoro and 5-methyl analogues showing maximum effect of 70 and 62%, respectively. Whereas for its unsubstituted counterpart (1a) and 5-chloro analogue (1c), the maximum free radical scavenging activity was 46 and 44%, at a concentration of 50 μg/ml, respectively.
Among 3-aroyl methylene indol-2-ones (2a-g), the majority of compounds under study showed good antioxidant activity within a concentration range of 5-100 μg/ml. The concentration?inhibition curves for unsubstituted and 5-halogenated derivatives (2a-d) were bell shaped with higher concentrations being less effective (fig. 2). The maximum inhibition for this class of compounds was observed between 20 and 25 μg/ml concentrations which were found to be much higher than that for ascorbic acid at similar concentrations. Compounds with methyl substitution on isatin ring and hydroxyl group on side chain phenyl ring (2e-g) were found to exhibit moderate antioxidant activity with increase in concentration.
Our findings indicate that heterocyclic systems with oxindole nucleus possess moderate to good antioxidant activity at low concentrations. It may be due to keto lactam ring being responsible to initiate the free radical scavenging activity due to its N-H and C=O moieties. The strategies to design and develop new isatin derivatives and other related compounds and their in vivo screening would be the purpose of further investigations.
Acknowledgements
Financial assistance to the work by DST, Delhi under Women Scientist Scheme (WOS-A) is gratefully acknowledged by the corresponding author. The authors express their gratitude to the Director, Indian Veterinary Research Institute, Izatnagar for providing necessary facilities for the study.
References
- Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy:
- A new pharmacological approach in shock, inflammation, and ischemia/ reperfusion injury. Pharmacol Rev 2001;53:135-59.
- Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition 2002;18:872-9.
- Hijova E. Bioavailability of chalcones. BratislLekListy 2006;107:80-4.
- deSá Alves FR, Barreiro EJ, Fraga CA. From nature to drug discovery: The indole scaffold as a ‘privileged structure’. Mini Rev Med Chem 2009;9:782-93.
- Estevao MS, Carvalho LC, Ferreira LM, Fernandes E, Marques MM. Analysis of the antioxidant activity of an indole library: Cyclic voltammetry versus ROS scavenging activity. Tetrahedron Lett 2011;52:101-6.
- Satyamaheshwar P. 3-Substituted-3-hydroxyl-2-oxindole, an emerging new scaffold for drug discovery with potential anticancer and other biological activities. CurrBioactCompd 2009;5:20-38.
- TakaoT, Kitatani F, Watanabe N, Yagi A, Sakata K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. BiosciBiotechnolBiochem 1994;58:1780-3.
- Gacche RN, Dhole NA, Kamble SG, Bandgar BP. In vitro evaluation of selected chalcones for antioxidant activity. J Enzyme Inhib Med Chem 2008;23:28-31.
- Won SJ, Liu CT, Tsao LT, Weng JR, Ko HH, Wang JP, et al. Synthetic chalcones as potential antiinflammatory and cancer chemopreventiveagents. Eur J Med Chem 2005;40:103-12.
- Cheng JH, Hung CF, Yang SC, Wang JP, Won SJ, Lin CN. Synthesis and cytotoxic, antiinflammatory, and antioxidant activities of 2’, 5’-dialkoxylchalcones as cancer chemopreventive agents. Bioorg Med Chem 2008;16:7270-6.
- Vine KL, Matesic L, Locke JM, Ranson M, Skropeta D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000-2008. Anticancer Agents Med Chem 2009;9: 397-414.
- Abadi AH, Abou-Seri SM, Abdel-Rahman DE, Klein C, Lozach O, Meijer L. Synthesis of 3-substituted-2-oxoindole analogues and their evaluation as kinase inhibitors, anticancer and antiangiogenic agents. Eur J Med Chem 2006;41:296-305.
- Pandeya SN, Smitha S, Jyoti M, Sridhar SK. Biological activities of isatin and its derivatives. Acta Pharm 2005;55:27-46.
- Popp FD, Donigan BE. Synthesis of 3-hydroxy-3-phenacyloxindole analogs. J Pharm Sci 1979;68:519-20.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LebensmWissTechnol 1995;28:25-30.
- Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213-20.