- *Corresponding Author:
- Shobha R. Desai
Department of Chemistry, SS Arts College and TP Science Institute, Sankeshwar-591 313
E-mail: dr.shobha.desai@gmail.com
| Date of Submission | 10 January 2011 |
| Date of Revision | 3 October 2011 |
| Date of Acceptance | 17 October 2011 |
| Indian J Pharm Sci, 2011, 73 (5): 593-596 |
Abstract
In this study, various 5-β-[(N-benzenesulphony/tosyl)-4-(un) substituted anilino]ethyl-2-mercapto-1,3,4-oxadiazole (4a-f), with sulphonamide moiety at the side chain have been synthesised. The structures of the newly synthesised compounds have been established on the basis of their spectral data and elemental analysis. All the compounds were screened for antimicrobial activities against Escherichia coli, Bacillus cirroflagellosus, Aspergillus niger. Colletotrichum capsici and antituberclosis activity against Mycobacterium tuberculosis H37Rv strain. Only two compounds 4b (73%) and 4e (54%), have shown moderate antituberculosis activity. All the compounds have shown moderate antiinflamatory activity and least ulcerogenecity. Most of the compounds have shown significant analgesic activity (64.20-120.72%) in comparison with the standard, Aspirin (49.39%) In the MES method, however only compound 4a, exhibited a protection of 33.33%, and others failed to protect.
Keywords
Antimicrobial, analgesic, antiinflamatory, antitubercular, anticonvulsant, 1,3,4-oxadiazole
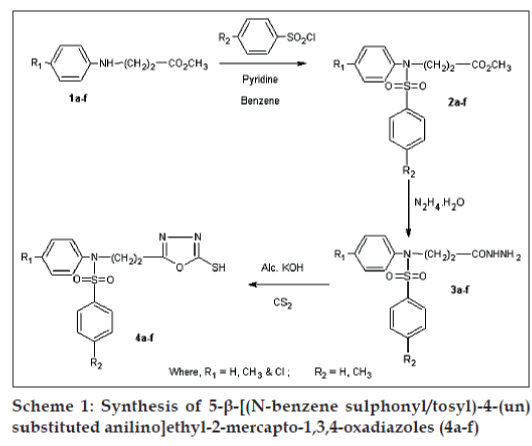
Various 5-substituted-2-mercapto-1,3,4-oxadiazoles posses significant antimicrobial [1-3], antiinflammatory [4-6], analgesic [4-6], anticonvulsant [7,8], antitubercular [9,10] and hypoglycemic [11] activities. In view of the established pharmacological activities of 1,3,4-oxadiazoles and in continuation [12-14] of our quest for 1,3,4-oxadiazoles with better pharmacological activities, we report herein the synthesis, pharmacological and antimicrobial activities of some new 5-b- [(Nbenzenesulphonyl/ tosyl)-4-(un) substituted anilino] ethyl-2-mercapto-1,3,4-oxadiazoles (4a-f) with "sulphonamide isostere" a pharmacologically active moiety at the 5th position of 1,3,4-oxadiazole nucleus. All the compounds were screened for antituberculosis activity against Mycobacterium tuberculosis H37Rv strain and antimicrobial activities against Escherichia coli, Bacillus cirroflagellosus, Aspergillus niger and Colletotrichum capsici. Same compounds were also screened for pharmacological activities such as antiinflammatory, analgesic, anticonvulsant, change in adrenal gland weight and ulcerogenic activity. Synthetic route is, depicted as Scheme 1.
Melting points were determined in open capillaries and are uncorrected. IR spectra in KBr, were recorded on a Perkin Elmer Spectrophotometer and 1H-NMR spectra on a Varian 300 MHz NMR spectrometer using TMS as an internal standard (chemical shifts in δ ppm). Mass spectra were recorded on Finnigan Mat 8230 spectrophotometer.
Methyl-β- [(4-H/CH 3/Cl)anilino]propionates, (1a- f), were prepared according to the literature methods [15-17]. Esters were sulphonylated/tosylated with benzenesulphonyl/tosyl chloride to methyl-β- [(Nbenzenesulphonyl/ tosyl)anilino]propionates) (2a-f) [18]. β- [(N-Benzenesulphonyl/tosyl)-4-(un)substituted anilino]propionic acid hydrazides (3a-f) [19], were prepared by refluxing a mixture of methyl-β- [(Nbenzenesulphonyl/ tosyl)anilino]propionates (2a-f) in ethanol with hydrazine hydrate. Hydrazides (3a-f), when refluxed with an alcoholic solution of potassium hydroxide and carbon disulphide yielded the title compounds. Title compounds were characterised by IR, 1H-NMR and mass spectral studies.
General procedure for preparation of 5-b- [(N-benzene sulphonyl/tosyl)-4-un(substituted anilino]ethyl-2- mercapto-1,3,4-oxadiazoles (4a-f), involved the addition of carbon disulphide (2.43g, 0.02 mole) dropwise to a clear solution of potassium hydroxide (1.12 g, 0.02 mol) in water (10 ml) and β- [(Nbenzenesulphonyl/ tosyl)-4-(un)substituted anilino] propionic acid hydrazide (3a-f) (0.02 mol) in 15 ml ethanol with stirring and cooling in ice. Reaction mixture was refluxed on water bath till the evolution of hydrogen sulphide gas ceased (8-10 h). The reaction mixture was concentrated in vacuo, residual mass was poured on crushed ice and neutralised with acetic acid. The precipitated oxadiazole was separated and crystallised from ethyl alcohol. 4d: IR (KBr) υ cm-1: 3500(b) (NH), 3000(s) (CH2), 1680(s), 1540 (m) (>C=C< and >C=N-), 1190 (s) (-C-O-C-), 1420 (m) (>C-N-), 1200(s), 1150 (m) (>C=S), 1380 (s) (SO2). 4d: 1H-NMR (DMSO-d6): δ 2.34 (s, 3H, CH3), 2.40 (s, 3H, CH3), 2.91 (t, 2H, -CH2-), 3.89 (t, 2H, -NCH 2), 6.89-7.63 (m, 8H, ArH), 10.68 (br.s. 1H, -NHC= S, D2O-exchangeable). 4d: MS; (m/z, Rel.Abund): 274 (99), 155 (99), 119 (78), 105 (24), 76 (10), 66 (16). 4e: IR (KBr) υ cm-1:3400(b) (NH), 3000(s) (CH2), 1660(s), 1540 (m) (>C=C< and >C=N-), 1190 (s) (-C-O-C-), 1430 (m) (>C-N), 1220(s), 1120(m) (>C=S), 1370 (s) (SO2). 4e: 1H-NMR (DMSO-d6): δ, 2.90 (t, 2H, CH2), 3.90 (t, 2H, -N-CH2), 7.01-7.81 (m, 9H, ArH) 13.98 (br.s., 1H, -NH-C=S – D2O exchangeable). Physicochemical properties of title oxadiazoles are enumerated in Table 1.
| Compd* No | R1 | R2 | M.P, (º) | Yield (%) | Antimicrobial activity , (RI) | Antituberculosis activity** M. tuberculosis (Percent inhibition) | |||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | B. cirro | A. niger | C. capsici | ||||||
| 4a | H | H | 133-35 | 45 | 20.68 | ------ | 38.22 | 100.00 | 30 |
| 4b | H | CH3 | 80-81 | 40 | 20.68 | ------ | 20.72 | 64.65 | 73 |
| 4c | CH3 | H | 130-31 | 46 | ---- | 36.99 | 20.72 | 126.93 | 34 |
| 4d | CH3 | CH3 | 140-41 | 47 | 46.06 | ------ | 78.76 | 223.90 | 36 |
| 4e | Cl | H | 100-01 | 45 | 20.68 | 4.34 | 48.52 | 126.93 | 54 |
| 4f | Cl | CH3 | 90-91 | 46 | ----- | 72.37 | 48.51 | 206.06 | ---- |
*All the compounds were analysed for C, H and N. The experimental values were within ± 0.04% of the calculated value. ** - Rifampicin as standard (97%, at 12.5 µg/ml), Concentration (antituberculosis activity) - MIC: 12.5 µg/ml (Bactec 460 radiometric system). Concentration (antimicrobial activity)- 100 µg/ml (Cup plate method). DMF as solvent control; Cotrimoxazole (Trimethoprim 500 mg and Sulphamethoxazole 800 mg) and Flucanazole as standards for antibacterial and antifungal respectively. Relative percent inhibition, RI = [100 x (X-Y)/(Z-Y)]; where X , Y and Z are total area of inhibitions in test, solvent (DMF) and standard respectively; Area = πr2; where r = Radius of zone of inhibition.
Table 1: Physicochemical Properties, Antimicrobial And Anti-Tubercular Activities Of 1,3,4-Oxadiazoles (4a-F)
After establishing the physicochemical and spectral properties, the newly synthesised oxadiazoles were tested for their antituberculosis activity against M. Tuberculosis H37Rv by Bactec 460 radiometric system at Southern Research Institute, Frederic Research Centre, Frederic MD. All the compounds were tested at a concentration of 12.5 μg/ml. Rifampicin was used as a standard (97% at 12.5 μg/ml).
Newly synthesised oxadiazoles, (4a-f) were first subjected to acute toxicity study by Miller and Tainter [20] method, in order to determine their LD50. The graphically calculated LD50 values of the newly synthesised compounds were found to be 2291 (4e), 1405±17.04 (4d and 4a), 1086±17.04 (4b and 4c), 816±13.08 (4f).
Antiinflammatory activity of 1,3,4-oxadiazoles (4a-f) was studied by carrageenan induced rat paw edema method (acute) due to Wilhelmi et al [21], cotton pallet induced granulation tissue formation method (chronic) due to Meir et al. [22] and adrenal weight change methods. An arbitrary scoring system [23] was followed to determine the severity of the ulcers. Analgesic activity of newly synthesised oxadiazoles (4a-f) was carried out by radiant heat induced rat tail flick method [24] using aspirin as a standard. Anticonvulsant activity was studied by MES method [25,26]. The percentage protections were then assessed by Fischer’s exact test [27].
All the compounds were screened for antimicrobial activities against Gram negative bacterium Escherichia coli, Gram positive bacterium Bacillus cirroflagellosus and fungi Aspergillus niger and Colletotrichum capsici by cup plate method [28]. Antimicrobial activity is calculated as relative percent inhibition, RI with reference to the standard. DMF was used as solvent control. Cotrimoxazole (trimethoprim 500 mg and sulphamethoxazole 800 mg) and Diflucan (fluconazole) are used as standards for antibacterial and antifungal respectively.
In the carrageenan induced rat paw edema method, all the compounds exhibited moderate to minimum antiinflammatory activity, 4a (19.36% , 43.35%, 40.15%), 4b (--, 39.26%, 59.11%), 4c (--, 43.39%, 57.61%), 4d (--, 43.31%, 72.31%), 4e (51.59%, 47.37%, 60.10%) 4f (19.36%, 79.76%, 75.07%), in comparison with the standard aspirin (54.84%, 55.05%, 73.81%) after 1, 3, 5 h respectively. However the same compounds exhibited better antiinflammatory activity 4a (57.84%), 4b (53.72%), 4c (46.14%), 4d (61.57%), 4e (50.97%), 4f (58.69%), in comparison to aspirin (41.886%) in the cotton pellet method. This has been further substantiated by the suppression of adrenal gland weight of the compounds (58.27-45.09) in comparison with that of the standard aspirin (50.13%) except for compounds 4c and 4d. All the synthetic 1,3,4-oxadiazoles have shown lesser degree (20.00-40.00) of ulcerogenecity, as compared to standard aspirin (43.33) Table 2.
| Compno | Dose(mg/Kgb.w.,) | Ulcerindex | Anti inflammatory activitya Percentage inhibition | Analgesicactivityapercentprotection(P<0.05) | Anticonvulsantbactivity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Carrageenan method | Cottonpelletmethod | Adrenalweightmethod | |||||||
| 1 h | 3 h | 5 h | |||||||
| MESprotection | |||||||||
| Rat tail flicK method | |||||||||
| Std | 200 | 43.33 | 54.84 | 55.05 | 73.81 | 41.89 | 50.13 | 49.39 | 100 >1(s) |
| (a/b) | <0.02 | <0.01 | <0.001 | <0.001 | <0.01 | <0.001 | |||
| 4a | 100 | 33.33 | 19.36 | 43.35>0.02 | 40.15 | 57.84 | 48.28 | 68.24 | 33.33 <0.03 |
| >0.1 | >0.5 | <0.001 | <0.001 | <0.001 | (ns) | ||||
| 4b | 100 | 36.66 | ---- | 39.26 | 59.11 | 53.72 | 45.09 | 83.04 | 0 --- (ns) |
| <0.05 | >0.1 | <0.01 | <0.001 | <0.001 | |||||
| 4c | 100 | 20.00 | ---- | 43.39 | 57.61 | 46.14 | 28.61 | 64.20 | 0 ---(ns) |
| >0.5 | <0.1 | <0.01 | <0.001 | <0.001 | |||||
| 4d | 100 | 40.00 | ---- | 43.31 | 72.31 | 61.57 | 30.43 | 76.31 | 0 ---(ns) |
| <0.02 | <0.01 | <0.001 | <0.001 | <0.001 | |||||
| 4e | 200 | 40.00 | 51.59 | 47.37 | 60.10 | 50.97 | 58.27 | 120.72 | 0 ---(ns) |
| <0.02 | >0.1 | <0.02 | <0.001 | <0.001 | <0.001 | ||||
| 4f | 75 | 36.66 | 19.36 | 79.76 | 75.07 | 58.69 | 50.31 | 109.95 | 0 ---(ns) |
| <0.05 | >0.5 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
Std (Standard) a- Aspirin and b- Phenytoin sodium. LD50 ± D (Miller and Tainter method): 2291 (4e), 1405±17.04 (4d), 1405±12.23 (4a), 1086±17.04 (4b,4c), 816±13.08 (4f); a-standard used for antiinflamatory and analgesic activities; b-standard used for anticonvulsant activity; statistical differences between the treatment and the control group of animals were evaluated by student’s t test for evaluation of the analgesic and antiinflammatory activity; s- Significant, ns-Non significant Dose for anticonvulsant activity; Std Phenytoin sodium(18mg/kg b.w), 4a-4d (200 mg/kg b.w), 4e and 4f (150mg/kg b.w)
Table 2: Pharmacological Activities Of 5-Β-[(N-Benzenesulphonyl/Tosyl)-4-(Un)Substituted Anilino]Ethyl-2-Mercapto-1,3,4-Oxadiazoles (4a-F)
In the radiant heat induced rat tail flick method, all the compounds exhibited most significant analgesic activity in comparison with aspirin (49.39%, < 0.01). The activity is in the order 4e (120.72%), 4f (109.95%), 4b (83.04%), 4d (76.31%), 4a (68.24%), 4c (64.20%) (Table 2).
In the maximum electroshock seizure (MES) method only compound 4a has shown a protection of (33.33%) in comparison with the standard Phenytoin (100%) (Table 2). All the compounds have shown greater antifungal activity against Colletotrichum capsici. The activity expressed as RI, enumerated in the decreasing order is 4f (206.06%), 4d (223.90%), 4c and 4e (126.93%), 4a (100.00%) and 4b (64.65%). However the same compounds exhibited minimum antifungal activity (20.72-48.52%) against Aspergillus niger, except compound 4d (78.76%). The compounds have shown moderate to minimum antibacterial activity, 4a, 4b, 4e (20.68%), 4d (46.06) against Escherichia coli and 4c (36.99%), 4e (4.34%) and 4f (72.37%) against Bacillus cirroflagellosus respectively (Table 1). Only compound 4b (73%) has shown moderate antituberculosis activity in comparison with the standard Rifampicin which has 97% at 12.5 μg/ ml, other compounds exhibited lesser activity (30- 54%) against, Mycobacterium tuberculosis H37Rv (Table 1).
Acknowledgements
Authors thank the Chairman, Department of Chemistry, Karnataka University, Dharwad, for providing the necessary facilities. Authors also thank the Heads of RSIC-CDRI, Lucknow, TIFR-Bombay, RSIC-IIT, Bombay for spectral analysis and the authorities of TAACF, Southern Research Institute, Birmingham for screening of the compounds for antituberculosis activity. Authors UVL and SRD are thankful to CSIR and UGC, New Delhi respectively for the financial assistance in the form of SRF and FIP.
References
- Naveena CS, Boja P, Kumari NS. Synthesis, characterization and antimicrobial activity of some disubstituted 1,3,4-oxadiazoles carrying 2-(aryloxymethyl)phenyl moiety. Eur J Med Chem 2010;45:4708-19.
- Chawla R, Arora A, Parameswaran MK, Chan P, Sharma D, Michael S, et al. Synthesis of novel 1,3,4-oxadiazole derivatives as potentialantimicrobial agents. Acta Pol Pharm 2010;67:247-53.
- Farshori NN, Banday MR, Ahmad A, Khan AU, Rauf A. Synthesis, characterization, and in vitro antimicrobial activities of 5-alkenyl/ hydroxyalkenyl-2-phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg Med ChemLett 2010;20:1933-8.
- Akhter M, Husain A, Azad B, Ajmal M. Aroylpropionic acid based 2,5-disubstituted-1,3,4-oxadiazoles: Synthesis and their antiinflammatory and analgesic activities. Eur J Med Chem 2009;44:2372-8.
- Bhandari SV, Bothara KG, Raut MK, Patil AA, Sarkate AP, Mokale VJ. Design, synthesis and evaluation of antiinflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg Med Chem 2008;16:1822-31.
- Amir M, Kumar S. Synthesis and evaluation of antiinflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm 2007;57:31-45.
- Rajak H, Deshmukh R, Veerasamy R, Sharma AK, Mishra P, Kharya MD. Novel semicarbazones based 2,5-disubstituted-1,3,4-oxadiazoles: One more step towards establishing four binding site pharmacophoric model hypothesis for anticonvulsant activity. Bioorg Med ChemLett 2010;20:4168-72.
- Zarghi A, Hajimahdi Z, Mohebbi S, Rashidi H, Mozaffari S, Sarraf S, et al. Design and synthesis of new 2-substituted-5- [2-(2-halobenzyloxy)phenyl]-1,3,4-oxadiazoles as anticonvulsant agents. Chem Pharm Bull 2008;56:509-12.
- Suresh Kumar GV, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C. Synthesis of some novel 2-substituted-5- [isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur J Med Chem 2010;45:2063-74.
- Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 2008;43: 1989-96.
- Shingalapur RV, Hosamani KM, Keri RS, Hugar MH. Derivatives of benzimidazolepharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur J Med Chem 2010;45:1753-9.
- Hosur MC, Talawar MB, Laddi UV, Bennur RS, Bennur SC. Synthesis and antimicrobial activities of some new 1,3,4-oxadiazoles. Indian J Heterocyclic Chem 1994;3:237-42.
- Laddi UV, Desai SR, Somannavar YS, Bennur RS, Bennur SC. Synthesis, anti inflammatory and biological activities of some new 2-mercapto-5-substituted-1,3,4-oxadiazoles. Indian Drugs 1997;34:666-73.
- Laddi UV, Desai SR, Bennur RS, Bennur SC. Some new 1,3,4-oxadiazoles as antimicrobial agents. Indian J Heterocyclic Chem 2002;11:319-22.
- Elderfield RC, Gensler, WJ, Bembry TH, Kremer CB, Brody F, Hageman HA, et al. Experiments on the Synthesis of 4-Hydroxy- and 4-Chloroquinolines from p-Anilinopropionic Acids. J Am ChemSoc 1946;68:1259.
- Baltrusis R, Mariosius IK. Synthesis of 1-aryldihydrouracils and 1-aryl-2-thiodihydrouracils and their transformations. KhimiyaGeterotsiklicheskikhSoedinenii 1969;5:904-7. ChemAbstr 1970;72:111413t..
- Lietuvos TS. AuksutjuMokykluMoksloDarbai. ChemIrChem Tech 1963;3:69. ChemAbstr 1963;59:8858.
- Sangwan NK, Kelkar PM, Rastogi SN, Anand N, Antifertility agents: Part XLIII-Synthesis of 3-aryl-4,5-dihydro-2-substituted-5-tosyl-2H-pyrazolo(4,3-c)quinolines and 2,4-dihydro-3-phenyl(1) benzopyrano(4,3-c)pyrazoles and their derivatives. Indian J Chem 1985;24B:639.
- Desai SR, Laddi UV, Bennur RS, Patil PA, Bennur S. Synthesis and Pharmacological Activities of Some New 3-Substituted-4-Amino-5-Mercapto-1,2,4Triazoles. Indian J Pharm Sci 2011;73:115-20.
- Miller LC, Tainter ML. Graphical method for determination of LD50. ProcSocExptBiol Med 1944;57:261-71.
- Wilhelmi G, Domenjoz RJ. Influence of pyrazoles, cortisone, and adrenocorticotropic hormone upon the chicken-albumin edema of the rat paw. Arzneimittel-Forschung 1951;1:151-4.
- Meir R, Schuler W, Desaulles P. ZurFrage des Mechanismus der Hemmung des Bindegewebswachstumsdurch Cortisone. Experientia 1950;6:469-71.
- Gupta MB, Nath R, Gupta GP, Bhargava KP. A study of the anti-ulcer activity of diazepam and other tranquilosedatives in albino rats. ClinExpPharmacolPhysiol 1985;12:61-6.
- Davies OL, Reventos J, Welpole AL. A method for the evaluation of analgesic activity using rats. Brit J Pharmacol 1946;1:255-64.
- Holland GF, Jaeger DA, Wagner RL, Laubach GD, McLamore, Pan SY. Hypoglycemic activity in a series of 1-aryl-3-arylsulfonylureas. J Med Pharm Chem 1961;3:99-110.
- Kadlimatti SH, Thungum J. Influence of lithium on the anticonvulsant activity of carbamazepine. Indian J PhysiolPharmacol 1987;31:35-41.
- Statistical methods in medicinal Research. In: Armitage p, editor. Vol. 4. New Jersey: Black Well Scientific Publications; 1977. p. 135-8.
- Seeley HW, Denmark PV. Microbes in action editors. A Lab Manual of Microbiology. New York: W.H. Freeman and Co.; 1972.